Pancreatic Juice Pathological Microenvironment Induces Apoptosis of Human Pancreatic Duct Epithelial Cells through Autophagy by Activating p38 MAPK Signaling Pathway
Received: 18-Jul-2022 / Manuscript No. cmb-22-69661 / Editor assigned: 19-Jul-2022 / PreQC No. cmb-22-69661(PQ) / Reviewed: 01-Aug-2022 / QC No. cmb-22- 69661 / Revised: 04-Aug-2022 / Manuscript No. cmb-22-69661(R) / Accepted Date: 11-Aug-2022 / Published Date: 11-Aug-2022 DOI: 10.4172/1165-158X.1000240
Abstract
Background: Acute pancreatitis is a common acute abdominal disease in clinical practice, the essence of which is the inflammatory reaction to the pancreas. Patients with rapid progression of the disease may have local or systemic inflammatory reaction. In this pathological process, the pancreatic duct mucosal barrier function is impaired. However, the specific mechanism is still unclear.
Methods: Naso-pancreatic tube drainage was performed in the early stage of severe acute pancreatitis, pancreatic juice was extracted from drainage bag of patients within 24 hours after operation. Pancreatic juice extracted was used to stimulate Human Pancreatic Ductal Epithelial (HPNE) cells, and the proliferation of HPNE cells was detected by Cell Counting Kit-8 (CCK8) and 5-Ethynyl-2’-Deoxy Uridine (EDU) methods. Annexin-V/PI apoptosis detection kit was used to detect cells apoptosis. Fluorescence microscope was used to observe the occurrence of autophagy flux in pancreatic juice pathological microenvironment. After HPNE cells were treated with 3-Methyl Adenine (3-MA), and detected the expression of apoptotic proteins by Western blotting. HPNE cells were treated with p38 MAPK signaling pathway inhibitors (SB203580) for 4 hours, and then detected the expression of apoptosis and autophagy proteins by western blotting.
Results: The proliferation of HPNE cells was inhibited, and apoptosis of HPNE cells occurred in pancreatic juice pathological microenvironment. Besides, the pancreatic juice pathological microenvironment induced autophagy in HPNE cells. Interference with autophagy reduced the apoptosis of HPNE cells. Interference of p38 MAPK signaling pathway with SB203580 inhibited autophagy and reduced the apoptosis of HPNE cells.
Conclusion: In the pancreatic juice microenvironment of severe acute pancreatitis, the activation of p38 MAPK signaling pathway induced excessive autophagy and apoptosis of HPNE cells, and inhibition of p38 MAPK signaling pathway reduced the apoptosis of HPNE cells.
Keywords
Pancreatic Juice; p38 MAPK; Autophagy; Apoptosis
Introduction
Approximately 20% of patients with Acute Pancreatitis (AP) continue to worsen to Severe Acute Pancreatitis (SAP), resulting in mortality up to 20-30% [1]. HPNE cells and mucus secreted form the Pancreatic Ductal Mucosa Barrier (PDMB), which played an important role in the biological function of the pancreas by preventing bile and digestive enzymes from flowing back into the pancreatic parenchyma under normal physiological conditions [2]. During the occurrence and development of acute pancreatitis, the integrity of the pancreatic duct mucosal barrier is easily destroyed, resulting in pancreatic juice leakage, which can accelerate the deterioration of the disease. Therefore, it is urgent to clarify the regulatory mechanism of pancreatic duct mucosal barrier impaired in the pancreatic juice pathological microenvironment, so as to provide a theoretical basis for improving pancreatic duct permeability.
It has been reported that the pancreatic juice in the pancreatic duct carried a variety of inflammatory factors affecting the entire pancreas, and the pancreatic juice microenvironment may be a reliable indicator to evaluate the degree of local pancreatic inflammation [3]. Therefore, we speculate that the pancreatic juice pathological microenvironment during SAP may cause damage to HPNE cells. However, the mechanism of the damage is still unclear. The purpose of this study was to explore the mechanism of HPNE cells damaged in the pancreatic juice pathological microenvironment pancreatic juice, and to provide a potential therapeutic target for the improvement of pancreatic duct permeability in the pathological process of acute pancreatitis.
Macroautophagy (hereafter referred to as autophagy) is a mediated by lysosomes to maintain cellular homeostasis of self-protection mechanism, and to gobble up their surplus material and wasted organelles and fusion with lysosomes form autophagy-lysosome, finally lysosomal enzyme digestion decomposed into small molecules, to achieve the stability of the cell itself and update [4]. During the development of SAP, it is found that autophagy played an important role in protecting cells from injury [5], and involved in the destruction of intestinal mucosal barrier during SAP [6]. Besides, autophagy is also involved in lung injury related to severe acute pancreatitis, and inhibition of excessive autophagy could reduce the severity of the disease [7], it is reported that inhibition of autophagy reduced apoptosis of the neurons. Therefore, we speculate that intervention of autophagy may be a potential therapeutic strategy for alleviating the apoptosis of HPNE cells.
Mitogen-Activated Protein Kinase (MAPK) is evolutionarily highly conserved and ubiquitous serine/threonine kinases in eukaryotes, consisting of conserved tertiary kinase cascades [8]. MAPK signaling pathway is closely related to autophagy triggered by many external factors and plays an important regulatory role in aging and apoptosis [9, 10]. Zhuoan Chen showed that inhibition of p38 MAPK signaling pathway reduced the risk of acute pancreatitis developing from local pathological changes to systemic inflammatory response syndrome and multiple organ failure [11]. Si Dong Wei found that taurine reduced liver injury in SAP rats by inhibiting the p38 MAPK signaling pathway [12]. Therefore, we tried to inhibit p38 MAPK signaling pathway to detect the apoptosis of HPNE cells.
In this study, we found that HPNE cells existed apoptosis in the pancreatic juice pathological microenvironment, and inhibition of autophagy could reduce the occurrence of apoptosis. It was confirmed that the damage of pancreatic juice pathological microenvironment to HPNE cells may be realized through the p38 MAPK signaling pathway, we provided a potential therapeutic target for further understanding the mechanism of pancreatic duct mucosal barrier destruction and improving pancreatic duct permeability.
Materials and Methods
Pancreatic juice was collected and pretreated
Patients with Acute Physiology and Chronic Health Score (APACHE-Ⅱ score) ≥ 8 were selected to be treated with nasopancreatic tube drainage in the early stage of acute pancreatitis, and pathological pancreatic juice in naso-pancreatic tube was collected within 24 hours after operation. The collected pancreatic juice was centrifuged for supernatant collection and filtered for sterilization. The protein concentration of the treated pancreatic juice was detected by BCA protein quantitative kit, and stored at -80℃ for later use, the desired concentration of pancreatic juice was obtained by dilution with complete medium.
Cell culture
Human pancreatic duct epithelial cells line was purchased from Shanghai Cell Bank-ATCC cell Bank (Shanghai, China), and cultured in a 5% CO2 cell incubator at 37°C with 90% DMEM and 10% fetal bovine serum as medium (Procell).
Methods
The logarithmic growth phase cells were inoculated into 6-well plate (104 cells/well), and cultured in the incubator for one day. On the second day, which was treated with different concentrations of the pathological pancreatic juice for 24 hours, and the morphology of HPNE cells was observed under the microscope. Proliferation of HPNE cells was detected by CCK-8 and EDU. Western blotting was used to detect the expression of autophagy and apoptosis-related proteins, and flow cytometry was used to detect apoptosis. In addition, the autophagosome formation was observed under fluorescence microscope, after autophagy was intervened, detected the expression of apoptosis-related proteins and apoptosis of HPNE cells by flow cytometry. p38 MAPK inhibitor was used to interfere with p38 MAPK signaling pathway and detected the expression of autophagy and apoptosis-related proteins, so as to determine the mechanism of HPNE cells apoptosis in pancreatic juice pathological microenvironment.
CCK8 assay
The suspension of HPNE cells was planted in 96-well plate (1000 cells/well), with three wells for each group, and cultured overnight. HPNE cells were treated with different concentrations of the pathological pancreatic juice on the second day. After three days, CCK8 solution (10% CCK8+90% medium, AbMole Bio Science) was added to each well, then the 96-well plate were placed in a 5% CO2 incubator and incubated at 37 for 2 hours. The absorbance value of each well at 450nm was measured with a microplate reader.
EDU assay
HPNE cells at the logarithmic growth stage were inoculated into 24-well plate (2000 cells/well). Before inoculation, round glass sheets were tightly pasted in 24-well plate and cultured until normal growth stage. HPNE cells were treated with different concentrations of the pathological pancreatic juice on the second day and EDU (Beyotime) assay was performed on the third day. EDU working solution 2X was configured, and 2X EDU working solution (20μM) preheated at 37°C was added to the 24-well plate in equal volume with the culture medium. The final concentration of EDU in the 24-well plate was changed to 1X, and the cells were incubated for 2 hours. After that, the culture medium was removed and 4% paraformaldehyde was added, and fixed at room temperature for 15 minutes. Removed fixative solution, added 200 μl washing solution to each well, washed the cells 3 times, 3-5 minutes every time. Removed the washing solution, incubated with 200μl of permeable solution in each well for 10-15 minutes at room temperature, removed the permeable solution, added 200μl of washing solution in each well to wash the cells 2 times, 3-5minutes each time. In order to inactivate the endogenous peroxidase, the slices were incubated with endogenous peroxidase blocking solution at room temperature for 20 minutes under dark conditions. Then washed with washing solution 3 times. Finally, the round glass plate was taken out and placed on a gel drop slide to observe the proliferation of cells under a fluorescence microscope.
Assay of green fluorescent protein-LC3 Puncta
HPNE cells were transfected with mCherry-GFP-LC3 (Beyotime), the cells with stable expression of Green Fluorescent Protein (GFPLC3) in 24-well plate (1000 cells/well). One day later, pathological pancreatic juice was co-cultured with the cells. On the third day, the cells were fixed with 4% paraformaldehyde, and the distribution of GFP-LC3 spots in the cells was observed by fluorescence microscope (Olympus, Japan).
Flow cytometry analysis
Apoptosis was detected by flow cytometry using FACSC ALIBUR cell analyzer (BD Biosciences) and Annexin V-PI (AV-PI) staining (BD 556547 Annexin V-FITC/PI). HPNE cells were digested with trypsin without EDTA, and were washed with cold PBS two times during this procedure, followed by annexin-V-FITC (2μl) and PI (5μl) staining at room temperature for 15 minutes in darkness, followed by flow cytometry to analyze apoptosis of the HPNE cells.
Western blotting
HPNE cells were washed with the cold PBS 3 times, RIPA lysis buffer was added to HPNE cells on ice for 30 minutes, centrifuged at 4°C for 5 minutes, total protein concentration was detected using a BCA kit (Key GEN Bio TECH, China), protein was mixed with sample buffer and boiled for 10 minutes. Protein and sample buffer solution were added to each swimming lane, separated by 10% SDS-PAGE gel, transferred to PVDF membrane, and sealed in 5% skim milk at room temperature for one hour. Cleaved-Caspase-3 (1:1000, Proteintech), c-PARP1 (1:1000, Proteintech), Bax (1:1000, Abcam), Bim (1:1000, Abcam), LC3/Ⅱ(1:1000, Proteintech), P62 (1:1000, Proteintech), pp38 (1:1000, Wanleibio), p38 (1:1000, Wanleibio), Overnight in 4°C refrigerator. After washing, the membrane was incubated with the corresponding secondary antibody GAPDH (1:20000, Abcam) at room temperature for one hour. Finally, the protein bands were displayed by ECL Western blot kit, and the protein expression was analyzed semiquantitatively by ImageJ, and GAPDH was identified as the internal reference protein.
Statistical analysis
GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Experimental data were expressed in the form of mean ± standard deviation. All data were analyzed by oneway ANOVA, and P < 0.05 was considered statistically significant.
Results
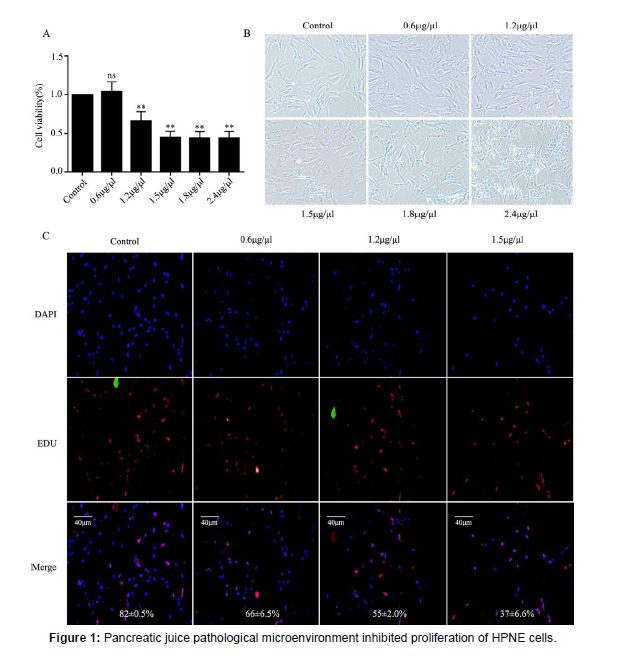
Pancreatic juice pathological microenvironment inhibited proliferation of HPNE cells
HPNE cells were treated with different pancreatic juice concentrations for 72 hours, then CCK-8 assayed the proliferation rate of HPNE cells in different pancreatic juice concentrations.
B: Morphological changes of HPNE cells in different pancreatic juice concentrations for 24 hours under fluorescence microscope, (magnification, 40X). C: EDU assayed the proliferation rate of HPNE cells in different pancreatic juice concentrations for 24 hours. ns: no statistical significance, the data were presented as mean ± standard deviation from three samples.**:P < 0.01, vs the control group. The concentration of pancreatic juice detected by BCA kit was 6 μg/μl.
Pancreatic juice is isotonic fluid rich in digestive enzymes and non-digestive enzymes secreted by acinar cells and HPNE cells [13]. During SAP, pancreatic juice contains various inflammatory factors and activated digestive enzymes, and pancreatic juice inflammatory mediators become an important indicator to measure the local inflammatory environment of the pancreas [3]. In addition, the PH and electrolyte secretion of normal pancreatic microenvironment also changed [14], thus forming the pancreatic juice pathological microenvironment. In order to explore whether pancreatic juice pathological microenvironment could damage HPNE cells, we set up the different concentrations and detected the proliferation of HPNE cells at different concentrations by CCK8 colorimetry. The results showed that the proliferation of HPNE cells was significantly inhibited in the pancreatic juice pathological microenvironment (Figure 1A). The cell morphology showed fibrotic damage and the cytoplasm was lysed (Figure 1B). EDU showed that the number of cell proliferation was reduced under the stimulation of pancreatic juice pathological microenvironment (Figure 1C). In conclusion, pathological pancreatic juice has a toxic effect on HPNE cells and inhibited the proliferation of the cells during SAP progression.
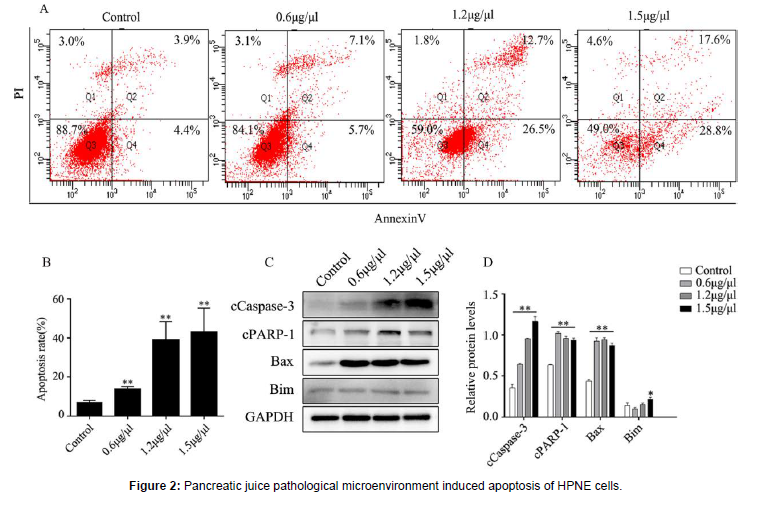
Pancreatic juice pathological microenvironment induced apoptosis of HPNE cells
The HPNE cells were inoculated in 6-well plate, followed by adding different concentrations of pancreatic juice on the second day. One day later, digested with trypsin without EDTA, and staining with apoptosis detection kit, and finally analyzed by flow cytometry. The same steps, the HPNE cells were inoculated in 6-well plate, followed by adding different concentrations of pancreatic juice on the second day. One day later, extracted the total proteins. A-B: apoptosis of cells was assessed by AV-PI staining assay. C-D: Profiling of apoptotic proteins expression. **: P < 0.01, vs the control group; *: P < 0.05, vs the control group.
In the process of SAP progression, pancreatic acinar cells have already been apoptotic, and shown that P53 knockout significantly reduced apoptosis and damage of acinar cells [15], indicating that the P53 pathway may be a new target molecule for the treatment of SAP progression. Whether the HPNE cells exist apoptosis in the pancreatic juice pathological microenvironment. Annexin V-PI (AV-PI) staining was used to detect the apoptosis of HPNE cells in the pancreatic juice pathological microenvironment (Figure 2A-B). In addition, western blotting was used to detect the expression of apoptotic proteins (Figure 2C-D). The results showed that HPNE cells existed apoptosis and the expression of apoptosis-related proteins increased under the stimulation of pancreatic juice pathological microenvironment.
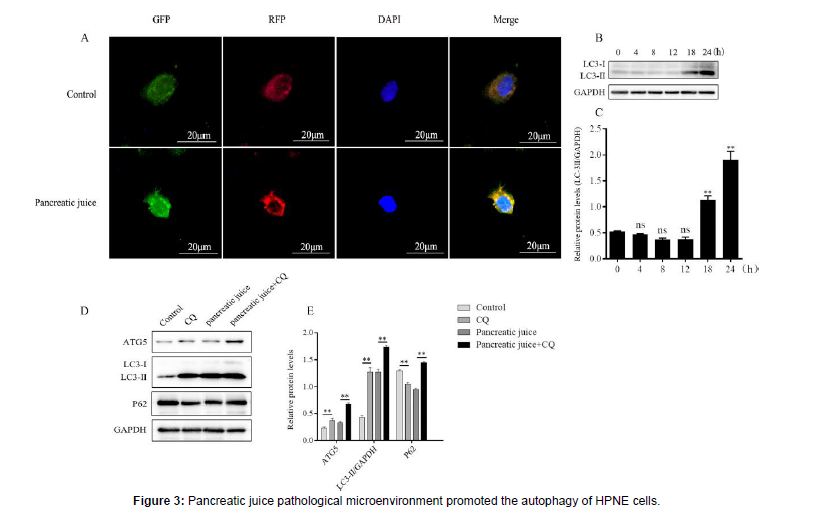
The HPNE cells were inoculated in the 24-well plate after the ground glasses were pressed tightly, on the second day, the HPNE cells were treated with mRFP-GFP-LC3 adenovirus for 48 hours, and on the fourth day the HPNE cells were treated with 1.5 μg/μl pancreatic juice for one day. After one day, all ground glasses were sealed and observed the formation of autophagosomes under fluorescence microscope. Besides, extracted total proteins at different times and analyzed the expression of autophagy marker proteins. The HPNE cells were inoculated in 6-well plate, treated with chloroquine for 4 hours on the second day, washed away with PBS, and treated with 1.5 μg/μl pancreatic juice for 24 hours. On the third day, extracted total proteins and analyzed autophagic protein expression. A: the formation of autophagosome was observed under fluorescence microscope, (magnification, 400X). B-C: expression of autophagy maker proteins at different times. D-E: expression of autophagy proteins after chloroquine treated for 4 hours. ns: no statistical significance, vs the control group. **: P < 0.01.
The disorder of autophagy may promote the inflammatory response of SAP [16]. In order to determine the autophagy of HPNE cells in the pancreatic juice pathological microenvironment, the formation of autophagosome was observed under fluorescence microscope (Figure 3A). Pancreatic juice at certain concentration (1.5 μg/μl) was used to stimulate HPNE cells at different times (Figure 3B-C), after HPNE cells were treated with Chloro Quine (CQ), and detected the expression of autophagy-related proteins by Western blotting (Figure 3D-E). The results showed that the pancreatic juice pathological microenvironment induced autophagy in HPNE cells, and excessive autophagy may aggravate the occurrence and development of acute pancreatitis.
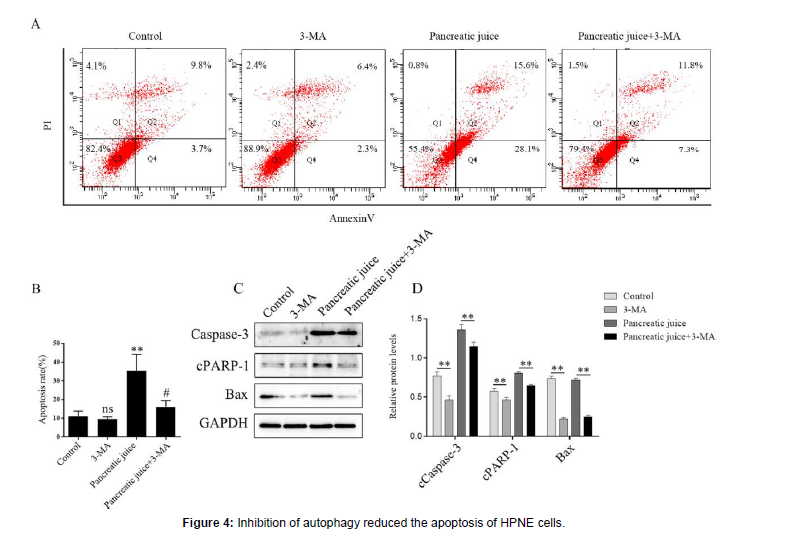
Inhibition of autophagy reduced the apoptosis of pancreatic duct epithelial cells
The HPNE cells were inoculated on 6-well plate, and adding the 3-MA to the 3-MA group and the pancreatic juice+3-MA group for 5 hours on the second day, then washed 3-MA with PBS, adding the medium to the 3-MA group, and adding the 1.5 μg/μl pancreatic juice to the pancreatic juice group and pancreatic juice+3-MA group. one day later, digested with trypsin without EDTA, and staining with apoptosis detection kit, and finally analyzed by flow cytometry. The same steps, extracted the total proteins, and detected the expression the apoptosis proteins. A-B: apoptosis of HPNE cells was assessed by AV-PI staining assay with 3-MA treated for 5 hours and pancreatic juice treated for 24 hours. C-D: expression of apoptosis proteins with 3-MA treated for 5 hours and pancreatic juice treated for 24 hours. ns, vs the control group; **P < 0.05; # < 0.05, vs the pancreatic juice group.
It has been reported that there was a complex interaction between autophagy and apoptosis [17], resulting in different effects on cells. Therefore, we explored whether inhibition of autophagy effectively reduced the apoptosis of HPNE cells. The cells were treated with 3-MA for 5 hours and then stimulated with a certain concentration of pancreatic juice (1.5 μg/μl). The group without pancreatic juice was a negative control. Annexin V-PI (AV-PI) staining showed that inhibition of autophagy reduced the apoptosis of HPNE cells in the pancreatic juice pathological microenvironment (Figure 4A-B), and the expression of apoptosis-related proteins was down-regulated by Western blotting (Figure 4C-D). Therefore, intervention of autophagy may be a potential therapeutic target for preventing HPNE cells apoptosis in the early stage SAP progression.
Inhibition of p38 MAPK signaling pathway reduced the apoptosis of pancreatic duct epithelial cells
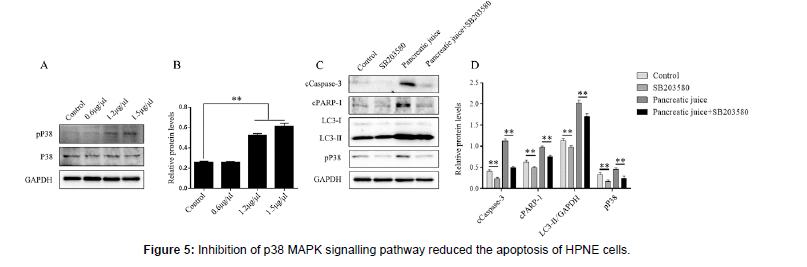
The HPNE cells were inoculated in the medium sized dish of cells, and added different pancreatic juice concentrations on the second day, extracted total proteins on the third day. The HPNE cells were inoculated in six-well plate, added the p38 MAPK signaling pathway inhibitors (SB203580) in SB203580 group and pancreatic juice+ SB203580 group for 4 hours on the second day, washed with PBS, and then added with the 1.5 μg/μl pancreatic juice for 24 hours, extracted total proteins on the third day. A-B: profiling of phosphop38 MAPK protein expression after treated with different pancreatic juice for 24 hours. C-D: expression of apoptosis and autophagy proteins with SB203580 treated for 4 hours and pancreatic juice treated for 24 hours. **P < 0.01, vs the control group.
It has been reported that inhibition of the p38 MAPK signaling pathway reduced the level of inflammatory cytokines, and lowered the risk of developing systemic inflammatory response and multiorgan failure in SAP mouse models [11]. In this work, we found that inhibition of p38 MAPK signaling pathway could reduce the apoptosis of HPNE cells in the pancreatic juice pathological microenvironment. Western blotting detected the expression of pP38 protein (Figure 5AB), after HPNE cells were treated with p38 MAPK inhibitor SB203580 (10μM) for 4 hours. Then the pathological pancreatic juice (1.5 μg/μl) stimulated the HPNE cells, and detected the expression of autophagy and apoptosis-associated proteins (Figure 5C-D). The results showed that inhibition of p38 MAPK signaling pathway alleviated the apoptosis of pancreatic duct epithelial cells in pancreatic juice pathological microenvironment through inhibition of autophagy.
Discussion
SAP is a common clinical acute abdomen, and the mortality rate can reach over 30% [18]. During SAP, the mucosal barrier of the pancreatic duct was impaired, but mechanism of the impaired is still unclear. Therefore, it is particularly important to clarify the mechanism of pancreatic duct mucosal barrier impaired during SAP, find potential therapeutic targets to improve pancreatic duct permeability, and alleviate the development of acute pancreatitis. In this work, we found that proliferation of HPNE cells was inhibited, and apoptosis of HPNE cells occurred in the pancreatic juice pathological microenvironment. In addition, the p38 MAPK signaling pathway is activated in the pancreatic juice pathological microenvironment, Inhibition of p38 MAPK signaling pathway reduced the apoptosis of HPNE cells by regulating autophagy.
Pancreatic juice pathological microenvironment is a stimulus factor with complex components and toxic effects on HPNE cells, and the essence of SAP is inflammation [19]. During SAP progression, pancreatic juice contains various inflammatory factors and activated digestive enzymes [3, 20]. It was found that HPNE cells occurred autophagy and apoptosis in the pancreatic juice pathological microenvironment. In addition, inhibition of autophagy reduced the apoptosis of HPNE cells, and excessive autophagy may be a promoter of HPNE cells apoptosis.
Autophagy is closely related to the occurrence and progression of AP, and even played a key role in determining the severity and progress of AP [4]. Accumulation of autophagosomes in cells caused by impaired autophagy aggravated the pathological process of AP [21]. Therefore, the normal physiological process of autophagy maintains the dynamic balance of cell organelles. Jianhua Wan found that the regulation of autophagy could alleviate the development of AP in mice, because of 3-MA could reduce autophagy flow and inflammation levels [22]. It has been reported that regulation of autophagy could inhibit the progress of SAP [19, 23], and the 3-MA could inhibit autophagy and prevent neuronal apoptosis [24]. The synergistic effect between autophagy and apoptosis aggravated neurovascular degeneration [25]. There is a mutual regulatory relationship between autophagy and apoptosis [26]. We tried to treat HPNE cells with 3-MA to detect the apoptosis of HPNE cells in pancreatic juice pathological microenvironment, the flow cytometric analysis showed that 3-MA reduced the apoptosis of HPNE cells, and the cCaspase-3, cPARP-1 and Bax were downregulated. And these data showed that inhibition of autophagy reduced the occurrence of apoptosis.
p38 MAPK signaling pathway can be strongly activated by environmental and genotoxic stress [27, 28], Which plays an important role in the process of apoptosis [29]. It has been reported that acute Pancreatitis Associated Ascitic Fluid (PAAF) induced hepatocyte apoptosis through activation of p38 MAPK signaling pathway and caspase-3 dependent pro-apoptotic pathway. Inhibition of p38 MAPK signaling pathway by p38 MAPK signaling inhibitors reduced liver damage in rats [30], and RND2 inhibited autophagy and reduced apoptosis by inhibiting p38 MAPK signaling pathway [31]. In conclusion, p38 MAPK is involved in the regulation of autophagy and apoptosis [32]. In the pancreatic juice pathological microenvironment, p38 MAPK signaling pathway was activated, pP38 protein was upregulated, we pretreated HPNE cells with SB203580 (10 μM) for 4 hours, and then co-cultured with HPNE cells in pancreatic juice pathological microenvironment. Western blotting analysis showed that LC3-Ⅱ/GAPDH was down-regulated, cCaspase-3 and cPARP-1 also were down-regulated. All these data suggest that intervention of the p38 MAPK signaling pathway inhibited the autophagy and apoptosis of HPNE cells.
Pancreatic juice pathological microenvironment induced autophagy and apoptosis of HPNE cells by activating p38 MAPK signaling pathway, and inhibition of p38 MAPK signaling pathway reduced autophagy and apoptosis of HPNE cells, which may be a potential therapeutic target for reducing HPNE cells apoptosis, so we provided a theoretical basis for improving the permeability of pancreatic duct mucosal barrier in the progress of acute pancreatitis.
Funding
This work was supported by the Ningxia Department of Science and Technology’s key research and development program (2021BEG03042).
Acknowledgement
We thank Dr. Yong Zhao Zhu for technical guidance.
Declaration of Interest
The authors declare no conflicts of interest and No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- Mederos MA, Reber HA, Girgis MD (2021) Acute Pancreatitis: A Review. JAMA 325: 382-390.
- Konok GP, Thompson AG (1969) Pancreatic ductal mucosa as a protective barrier in the pathogenesis of pancreatitis. Am J Surg 117: 18-23.
- Dalbec KM, Max Schmidt C, Wade TE, Wang S, Swartz-Basile DA, et al. (2010) Adipokines and cytokines in human pancreatic juice: unraveling the local pancreatic inflammatory milieu. Dig Dis Sci 55: 2108-2112.
- Yuan X, Wu J, Guo X, Li W, Luo C, et al. (2021) Autophagy in Acute Pancreatitis: Organelle Interaction and microRNA Regulation. Oxid Med Cell Longev 2021: 8811935.
- Wang H, Li C, Jiang Y, Li H, Zhang D (2020) Effects of Bacterial Translocation and Autophagy on Acute Lung Injury Induced by Severe Acute Pancreatitis. Gastroenterol Res Pract 2020: 8953453.
- Yang H, Ma S, Guo Y, Cui D, Yao J (2019) Bidirectional Effects of Pyrrolidine Dithiocarbamate on Severe Acute Pancreatitis in a Rat Model. Dose Response 17: 1559325819825905.
- Kong L, Deng J, Zhou X, Cai B, Zhang B, et al. (2021) Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis 12: 928.
- Yue J, Lopez JM (2020) Understanding MAPK Signaling Pathways in Apoptosis. Int J Mol Sci 21.
- Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzzi L, et al. (2013) Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol 23: 310-322.
- Cao W, Li J, Yang K, Cao D (2021) An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer 108: 304-322.
- Chen Z, Chen Y, Pan L, Li H, Tu J, et al. (2015) Dachengqi Decoction Attenuates Inflammatory Response via Inhibiting HMGB1 Mediated NF-κB and p38 MAPK Signaling Pathways in Severe Acute Pancreatitis. Cell Physiol Biochem 37: 1379-1389.
- Wei S, Huang Q, Li J, Liu Z, You H, et al. (2012) Taurine attenuates liver injury by downregulating phosphorylated p38 MAPK of Kupffer cells in rats with severe acute pancreatitis. Inflammation 35: 690-701.
- Beaudoin AR, St-Jean P, Grondin G (1989) Pancreatic juice composition: new views about the cellular mechanisms that control the concentration of digestive and nondigestive proteins. Dig Dis 7: 210-220.
- Hegyi P, Petersen OH (2013) The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev Physiol Biochem Pharmacol 165: 1-30.
- Tan JH, Cao RC, Zhou L, Zhou ZT, Chen HJ, J, et al. (2020) ATF6 aggravates acinar cell apoptosis and injury by regulating p53/AIFM2 transcription in Severe Acute Pancreatitis. Theranostics 10: 8298-8314.
- Gukovskaya AS, Gukovsky I, Algul H, Habtezion A (2017) Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology 153: 1212-1226.
- Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N (2013) Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 1833: 3448-3459.
- Munir F, Jamshed MB, Shahid N, Hussain HM, Muhammad SA, et al. (2020) Advances in immunomodulatory therapy for severe acute pancreatitis. Immunol Lett 217: 72-76.
- Tang GX, Yang MS, Xiang KM, Yang BC, Liu ZL, et al. (2021) MiR-20b-5p modulates inflammation, apoptosis and angiogenesis in severe acute pancreatitis through autophagy by targeting AKT3. Autoimmunity 54: 460-470.
- De Oliveira C, Khatua B, Noel P, Kostenko S, Bag A, et al. (2020) Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Invest 130: 1931-1947.
- Barrera K, Stanek A, Okochi K, Niewiadomska Z, Mueller C, et al. (2018) Acinar cell injury induced by inadequate unfolded protein response in acute pancreatitis. World J Gastrointest Pathophysiol 9: 37-46.
- Zhang L, Chen Y, Wang L, Chen XP, Zhang WG, et al. (2013) Chloroquine relieves acute lung injury in rats with acute hemorrhagic necrotizing pancreatitis. J Huazhong Univ Sci Technolog Med Sci 33: 357-360.
- Wang X, Zhou G, Liu C, Wei R, Zhu S, et al. (2016) Acanthopanax versus 3-Methyladenine Ameliorates Sodium Taurocholate-Induced Severe Acute Pancreatitis by Inhibiting the Autophagic Pathway in Rats. Mediators Inflamm 8369704.
- Roux C, Lesueur C, Aligny C, Brasse-Lagnel C, Genty D, et al. (2014) 3-MA inhibits autophagy and favors long-term integration of grafted Gad67-GFP GAB Aergic precursors in the developing neocortex by preventing apoptosis. Cell Transplant 23: 1425-1450.
- Wang XX, Zhang B, Xia R, Jia QY (2020) Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci 24: 9601-9614.
- Maiuri MC, Zalckvar E, Kimchi E, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8: 741-752.
- Han J, Lee JD, Bibbs L, Ulevitch RJ (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265: 808-811.
- Loesch M, Chen G (20081) The p38 MAPK stress pathway as a tumor suppressor or more? Front Biosci 13: 3581-3593.
- Yu X, Fan H, Jiang X, Zheng W, Yang Y, et al. (2020) Apatinib induces apoptosis and autophagy via the PI3K/AKT/mTOR and MAPK/ERK signaling pathways in neuroblastoma. Oncol Lett 20: 52.
- Yang J, Fier A, Carter Y, Liu G, Epling-Burnette PK, Bai F, et al. (2003) Liver injury during acute pancreatitis: the role of Pancreatitis-Associated Ascitic Fluid (PAAF), p38-MAPK, and caspase-3 in inducing hepatocyte apoptosis. J Gastrointest Surg 7: 200-207.
- Xu Y, Sun Q, Yuan F, Dong H, Zhang H, et al. (2020) RND2 attenuates apoptosis and autophagy in glioblastoma cells by targeting the p38 MAPK signalling pathway. J Exp Clin Cancer Res 39: 174.
- Sui X, Kong N, Ye L, Han W, Zhou J, et al. (2014) p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett 344: 174-179.
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Citation: Shi JP, Dong TT, Chen LP, Hao QQ, Yao WJ, et al. (2022) Pancreatic Juice Pathological Microenvironment Induces Apoptosis of Human Pancreatic Duct Epithelial Cells through Autophagy by Activating p38 MAPK Signaling Pathway. Cell Mol Biol, 68: 240. DOI: 10.4172/1165-158X.1000240
Copyright: © 2022 Shi JP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3105
- [From(publication date): 0-2022 - Nov 16, 2025]
- Breakdown by view type
- HTML page views: 2622
- PDF downloads: 483