Paediatric Aphakic Glaucoma: A Diagnostic and Management Challenge
Received: 26-Feb-2016 / Accepted Date: 13-May-2016 / Published Date: 20-May-2016 DOI: 10.4172/2476-2075.1000115
6545Introduction
Pediatric aphakic glaucoma is a potentially blinding condition, which poses significant management challenges - the patient is young and treatment must aim to preserve vision for perhaps 80 years or more of life expectancy. The purpose of this study is to highlight that early detection is important to improve the prognosis for these patients and their families. Misdiagnosis of this condition may adversely affect vision preservation. However, diagnosis can be difficult, especially when children may demonstrate vague symptoms such as irritability, photophobia and epiphora which themselves may further impair clinical co-operation. Despite considerable advances in techniques employed in paediatric cataract surgery, aphakic glaucoma continues to occur and remains notoriously challenging to manage [1]. We present a case of glaucoma after cataract surgery that masqueraded as corneal infection which was challenging to diagnose and treat. Prompt recognition of pediatric aphakic glaucoma and intraocular pressure (IOP) control has resulted in a favorable clinical outcome, thus far.
Statement of Ethics
In accordance with the declaration of Helsinki, the patient provided informed consent prior to participation. We certify that all applicable institutional and governmental regulations concerning the use of human volunteers were followed during this research.
Case Report
Medical and ophthalmic patient history
A 16-month old male infant of African-Polish origin, presented to our Ophthalmology department with irritability, bilateral sore eyes, yellow purulent discharge and hazy cornea, following a recent contact lens (CL) change. The patient had a history of congenital cataracts, for which no identifiable cause was found. At the age of 2 months, the patient underwent right lensectomy, followed by 3 weeks later by left lensectomy. Both cataract surgeries included planned posterior capsulotomy and anterior vitrectomy. The patient was subsequently fitted with 30D CL bilaterally. His birth was at full term by normal vaginal delivery. He did not have any developmental issues, other ocular or systemic disorders. He lived at home with his parents and older brother. Neither parent had any ocular complaints nor was there a family history of glaucoma.
Clinical investigations and management
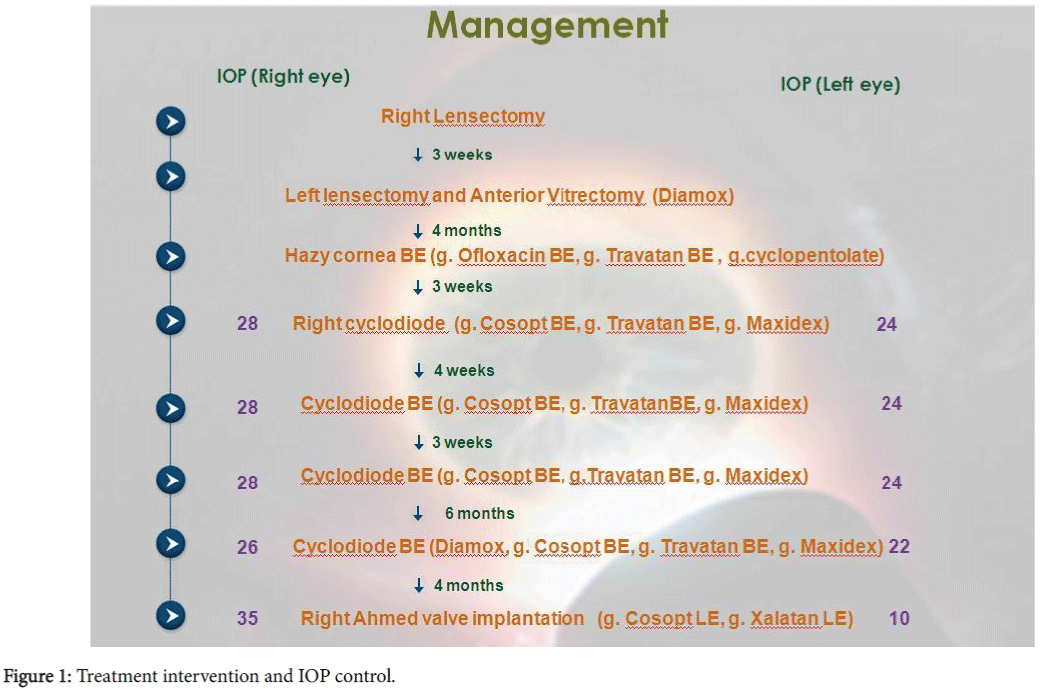
Approximately, 5 months following his initial right cataract surgery, the infant presented with bilateral hazy cornea. He had poor fixation to light in both eyes. The working diagnosis was CL keratitis, with paediatric aphakic glaucoma as a possible differential diagnosis. The patient was promptly commenced on topical ofloxacin 0.3%, in addition to travoprost (Travatan) 0.004% and cyclopentolate 0.5% eye drops and closely followed-up. An urgent examination under anaesthesia (EUA) was organised when the corneal oedema failed to resolve completely. The IOPs measured by Perkins tonometry were 28 mmHg (right) and 24 mmHg (left eye). The horizontal corneal diameters were 12.5 mm (right) and 12.0 mm (left). His axial length (AL) was 24.9 mm (right) and 21.7 mm (left). Refraction was + 8.50/1.00 × 180 (right) and + 14.50 (left). The optic nerve cup-to-disc (CDR) ratios measured 0.5 right and 0.3 left. The anterior chamber depth in both eyes was normal. Direct gonioscopy with Koeppe lens revealed open angles.
To provide IOP control, in addition to topical glaucoma medication: timolol- dorzolamide combination (Cosopt), travoprost (Travatan) and a weight appropriate dose of oral acetazolamide (Diamox). The patient underwent several repeated cyclodiode treatments to some success before ultimately requiring an Ahmed valve (FP7 model, New World Medical) implantation. The sequelae of events are summarised as Figure 1.
At his most recent EUA, his IOP remains stable (12 mmHg in the right and 10 mmHg left) with a functioning Ahmed valve and no complications noted. The measured horizontal corneal diameter was 13 mm (right) and 12.5 mm (left). His AL was 25.9 mm (right) and 21.9 mm (left). The CDR measured 0.5 right and 0.2 left eyes. The patient remains on timolol-dorzolamide and travoprost eye drops to his left eye only and wears a +13.5D CL (right) and + 19.5D (left eye).
Discussion
Aphakic glaucoma, the most common long-term complication following congenital cataract surgery, has an incidence between 15 and 45%, depending on the duration of reported post-operative follow-up [2]. We will review what has been published in literature and how this relates to our case. In our paper, we aim to provide guidelines for ophthalmologists treating glaucoma patients with paediatric aphakic glaucoma. We hope to contribute to existing ophthalmological knowledge by sharing our experience and the challenges encountered in managing this case and looking for the best possible treatment strategy.
Cataract surgery
In our patient early, uncomplicated cataract surgery and ethnicity may have been important risk factors. Lensectomy at an early age, especially during the first year of life has been shown to increase the risk of glaucoma, which is associated with a worse visual prognosis [3]. Retrospective studies have shown that the highest incidence of glaucoma occurs when cataract surgery was performed prior to one month or at five to six months of age [4]. The critical period for developing a fixation reflex is between 2 and 4 months of age. It is a fine balance of timing between early surgery for improved visual prognosis and delaying surgery to avoid the risk of glaucoma. Primary intraocular lens (IOL) implantation has not been shown to result in a lower rate of glaucoma, provide a more permanent correction and correcting most of the aphakia [5]. Kirwan et al. reported a glaucoma incidence of 33% in the aphakic group compared to 13% in the pseudophakic group, whereas Asrani and colleagues found an 11.3% incidence of glaucoma in the aphakic group compared to 0.27% in the pseudophakic group [6,7]. It is postulated that the IOL either provides a barrier from the toxic material of the vitreous (chemical theory) or supports the angle, preventing trabecular meshwork (TM) collapse (mechanical theory) [7]. The lower incidence of aphakic glaucoma in pseudophakic eyes may likely be a consequence of many surgeons avoiding inserting IOLs in high risk eyes. In contrast, CL often results in better optical correction and the diopteric power can be adjusted throughout life. A main drawback is its cost, risk of infection and potential loss.
Pathophysiological mechanisms
The pathophysiology of aphakic glaucoma remains to be elucidated. Our patient had early-onset glaucoma with open-angles. Open-angle glaucoma following congenital cataract surgery may be due to the interaction between TM cells and lens epithelial cells [8]. The role of the vitreous in aphakic glaucoma is poorly understood. In our case, it is likely that exposure of the angle to vitreous may have further caused an alteration to the aqueous outflow system.
Diagnostic challenges
Aphakic glaucoma poses a significant diagnostic challenge, as children often lack the classical manifestations and are uncooperative due to their visual symptoms. It can be extremely challenging, as in our case to measure the IOP with the child awake. EUA is usually required, but general anaesthesia in infants and young children, even in good health, carries a small but significant risk and should not be regarded lightly [9].
Clinical investigations
The mean aplanation IOP in children is lower than adults, but by the age of 10, they are equivalent [10]. Accurate IOP measurement in the paediatric population is often difficult, because it is subject to many influences including sedation, mode of airway securement during EUA, tonometer type and central corneal thickness [11]. This uncertainty is why clinical experience in addition to other ancillary examination (e.g. optic nerve, corneal diameter and AL) findingsshould be weighted more heavily when making judgements as to whether the child’s glaucoma is stable or progressing. The AL measurements with the A-scan ultrasound is especially useful to monitor the progression of glaucoma especially in children less than 5 years of age, who have more distensible globes [12]. Stabilisation or decrease in AL often occurs with IOP reduction. Normal black children, tend to have thinner corneas [13]. However, unlike adults, one should not adjust the measured IOP based on the CCT. Instead, children with thin corneas should be monitored more closely on a lowered target IOP.
Most general anaesthetics tend to lower IOP by variable amounts and at variable times after administration. Some ophthalmologists prefer to use ketamine, rather than sevoflurane in children for IOP measurement, as sevoflurane appreciably lowers IOP [14]. Furthermore, the IOP should be measured as soon as possible following induction of the anaesthesia, to get an accurate measurement. Many tonometers have been shown to estimate IOP inaccurately in children [15]. The Icare (Icare Finland Oy; Helsinki, Finland) rebound tonometer although useful in clinical practice without the use of anaesthetic eye drops, tends to overestimate IOP in those with thick corneas when compared to the Perkins (hand-held Goldmann applanation) tonometer [16]. Moreover, the child needs to be upright for IOP measurement in a rebound tonometer, and cannot be used in our child with contact lenses. CCT can further affect IOP measurements and patients with aphakic glaucoma tend to have significantly thickened corneas [17].
Management options
The treatment of paediatric aphakic glaucoma is challenging and communication with parents must be clear and informative to establish the trust for a child’s often prolonged care. Additionally, there are important secondary management issues unique to the paediatric population that need to be addressed including increased IOP which may cause myopic refractive errors, corneal scarring from resolved corneal oedema which can result in induced astigmatism, strabismus from glaucoma surgery or as a result of sensory vision loss, and amblyopia. Medical management is the first line of treatment of aphakic glaucomas with open angles, but surgical intervention may be necessary if inadequate IOP control persists. Prompt peripheral iridectomy with possible synechiolyisis is necessary as management of angle closure glaucoma in an aphakic eye.
Medical therapy
Medical management is often first line or as an adjunctive to maximize IOP lowering in addition to surgery. To date, the most useful topical medications in children are beta blockers and carbonic anhydrase inhibitors. Issues in medical management include side effects of topical treatment and compliance. Acetazolamide, a carbonic anhydrase inhibitor, however, is not ideal for long-term use due to potential for growth suppression, chronic fatigue and liver toxicities. Moreover, acetazolamide in infants is associated with hypercapnea from renal acidosis, dehydration and anorexia [18]. Due to ethical considerations, children are often not included by regulatory agencies in studies for the approval of glaucoma drugs. The adverse effects of medications are depending on the age and weight of the child. Plasma levels in children especially less than 2 years of age, after timolol 0.25% has been greatly exceed than in adults given 0.5%, which may increase the risk of side effects such as bronchospasm and bradycardia [19]. Therefore, punctal occlusion should be used. Travoprost eye drops may result in conjunctival redness, periocular pigmentation and ocular irritation. Some medications can have life-threatening side effects in young children. Brimonidine, a selective alpha-adrenergic receptor, is contraindicated as it is associated with extreme fatigue and somnolence. Some children with aphakic glaucoma also do not respond well to latanoprost (Xalatan), a prostaglandin analogue, presumed secondary to TM immaturity, which does not mature until the age of 8 years of age [20]. Moreover, the ocular hypertensive response with steroids in younger children can be more severe with a more rapid onset [21].
Surgical interventions
When medical treatment fails, surgery is required and options include trabeculectomy, glaucoma drainage devices (GDD) and cyclodestructive procedures. Comparisons of various interventions are difficult because of different success criteria used and follow up duration. Repeat surgery is generally the norm and parents should be counselled in advance to have realistic expectations. The role of trabeculectomy in treating paediatric aphakic glaucoma has largely been superseded by GDD. Trabeculectomy without the use of adjunctive antifibrotic agents (e.g. Mitomycin C) have been largely unsuccessful [22]. Antifibrotics however are associated with increased risk of serious complications such as late-onset- bleb-relatedendophthalmitis, which occurs more frequently in young children and with CL use [23].
Cyclodestructive procedures
Cycloablative procedures include diode laser cyclophotocoagulation and endocyclophotocoagulation. Diode laser is portable, compact, better tolerated, with quicker recovery and safer than Nd:YAG laser or cyclocryotherapy. In high risk cases, cyclodiode is a safer option than surgery. Cyclodiode treatment can be used effectively in eyes with good visual acuity, although response may be temporary with apparently low risk of serious complications [24]. Retreatment frequently results in a more sustained IOP control, but may increase the likelihood of significant sight-threatening complications including retinal detachment and phthisis [25]. A 79% success rate was attained with cyclodiode therapy in those with aphakic glaucoma, with 41% reduction in IOP following one treatment, with its effect lasting for a longer duration those phakic eyes [26]. The effect of cyclodiode on subsequent surgery is unknown with some reporting lower IOP following GDD implants and others reporting extensive conjunctival scarring have made drainage procedures ineffective [2,27].
Endocyclophotocoagulation has been shown to have success rates of 34% following one treatment, increasing to 43% with multiple treatments [28]. The biggest drawback is that it is an invasive procedure and primary results do not seem to support an extended role at this time.
Glaucoma drainage device (GDD)
GDD are an effective option for aphakic paediatric glaucoma. Newer devices are smaller and more biocompatible. GDD are better implanted sooner rather than later in the hope of achieving, early definite IOP control. Tube shunt surgery for children is technically similar to adults, but the GDD plate can be trimmed to a more appropriate size for the paediatric eye. A hypertensive effect usually occurs around one month, due to fibrosis around the valve site. Drainage surgery may be complicated by hypotony when the eye is large and has a thin sclera or from failure to control IOP caused by an aggressive healing response [29].
The Ahmed valve offers the advantage of immediate IOP lowering, which is a potential advantage in a patient with an oedematous cornea from an amblyopia perspective. Pakravan et al. reported success rates with the Ahmed valve in treating aphakic glaucoma as 66.7% [30].
Although GDD offer the most effective long term treatment for IOP control in refractory cases, complications occur frequently, related to the drainage plate or malpositioning of the drainage tube [31]. Children are particularly prone to extrusion and exposure of the implant from changes in the ocular dimensions following successful IOP control, normal growth and development, the elastic nature of the paediatric eye and eye rubbing. Exposure of GDD is the main risk factor for endophthalmitis and necessitates revision of the device. Corneal decompensation is another long-term risk given the potential lifespan of this population.
Conclusions
Despite considerable advances, the diagnosis and management of aphakic glaucoma still poses a significant clinical challenge. Two thirds of aphakic children eventually end up with a mean visual acuity of ≤ 6/120, secondary to blindness from amblyopia, poor IOP control from medication compliance or surgical complications [32]. Lifelong followup is necessary. The physical and emotional effects of treatment including multiple hospital visits on the child and their family must always be borne in mind. A holistic management of patient care involving a multidisciplinary team approach is required to optimise visual prognosis.
Acknowledgements
V.Swetha E. Jeganathan, Finalist Alcon Glaucoma Case Presentation UK in 2013.
References
- Beck AD, Freedman SF, Lynn MJ, Bothun E, Neely DE, et al. (2012) Glaucoma-related adverse events in the Infant Aphakia Treatment Study: 1-year results.Arch Ophthalmol 130: 300-305.
- Kirwan C, O'Keefe M (2006) Paediatric aphakic glaucoma.ActaOphthalmolScand 84: 734-739.
- Chen TC, Bhatia LS, Walton DS (2005) Complications of pediatriclensectomy in 193 eyes.Ophthalmic Surg Lasers Imaging 36: 6-13.
- Lawrence MG, Kramarevsky NY, Christiansen SP, Wright MM, Young TL, et al. (2005) Glaucoma following cataract surgery in children: surgically modifiable risk factors.Trans Am Ophthalmol Soc 103: 46-55.
- Michaelides M, Bunce C, Adams GG (2007) Glaucoma following congenital cataract surgery--the role of early surgery and posterior capsulotomy. BMC Ophthalmol 7:13.
- Kirwan C, Lanigan B, O'Keefe M (2010) Glaucoma in aphakic and pseudophakic eyes following surgery for congenital cataract in the first year of life.ActaOphthalmol 88: 53-59.
- Asrani S, Freedman S, Hasselblad V, Buckley EG, Egbert J, et al. (2000) Does primary intraocular lens implantation prevent "aphakic" glaucoma in children?J AAPOS 4: 33-39.
- Michael I, Shmoish M, Walton DS, Levenberg S (2008) Interactions between trabecular meshwork cells and lens epithelial cells: a possible mechanism in infantile aphakic glaucoma. Invest Ophthalmol Vis Sci 49: 3981-3987.
- Koc F, Kargi S, Biglan AW, Chu CT, Davis JS (2006) The aetiology in paediatric aphakic glaucoma.Eye (Lond) 20: 1360-1365.
- Sihota R, Tuli D, Dada T, Gupta V, Sachdeva MM (2006) Distribution and determinants of intraocular pressure in a normal pediatric population.J Pediatr Ophthalmol Strabismus 43: 14-18.
- Beck AD (2001) Diagnosis and management of pediatric glaucoma.OphthalmolClin North Am 14: 501-512.
- Law SK, Bui D, Caprioli J (2001) Serial axial length measurements in congenital glaucoma.Am J Ophthalmol 132: 926-928.
- Muir KW, Duncan L, Enyedi LB, Freedman SF (2006) Central corneal thickness in children: Racial differences (black vs. white) and correlation with measured intraocular pressure.J Glaucoma 15: 520-523.
- Jones L, Sung V, Lascaratos G, Nagi H, Holder R (2010) Intraocular pressures after ketamine and sevoflurane in children with glaucoma undergoing examination under anaesthesia. Br J Ophthalmol 94: 33-35.
- Lasseck J, Jehle T, Feltgen N, Lagrèze WA (2008) Comparison of intraocular tonometry using three different non-invasive tonometers in children.Graefes Arch Clin Exp Ophthalmol 246: 1463-1466.
- Li Y, Tang L, Xiao M, Jia S, Liu P, et al. (2013) Comparison of the Icare tonometer and the hand-held goldmannapplanation tonometer in pediatricaphakia. J Glaucoma 22: 550-554.
- Lopes JE, Wilson RR, Alvim HS, Shields CL, Shields JA, et al. (2007) Central corneal thickness in pediatric glaucoma. J PediatrOphthalmol Strabismus 44: 112-117.
- Matthews YY (2008) Drugs used in childhood idiopathic or benign intracranial hypertension.Arch Dis Child EducPract Ed 93: 19-25.
- Mandal AK, Netland PA (2004) Glaucoma in aphakia and pseudophakia after congenital cataract surgery. Indian J Ophthalmol 52: 185-198.
- Black AC, Jones S, Yanovitch TL, Enyedi LB, Stinnett SS, et al. (2009) Latanoprost in pediatric glaucoma--pediatric exposure over a decade.J AAPOS 13: 558-562.
- Brookes JL, Khaw PT (2005) Steroid response in children.Clin Experiment Ophthalmol 33: 229-230.
- Bellows AR, Johnstone MA (1983) Surgical management of chronic glaucoma in aphakia.Ophthalmology 90: 807-813.
- Yoon PS, Singh K (2004) Update on antifibrotic use in glaucoma surgery, including use in trabeculectomy and glaucoma drainage implants and combined cataract and glaucoma surgery. CurrOpinOphthalmol 15: 141-146.
- Kirwan JF, Shah P, Khaw PT (2002) Diode laser cyclophotocoagulation: role in the management of refractory pediatricglaucomas. Ophthalmology 109: 316-323.
- Bloom PA, Tsai JC, Sharma K, Miller MH, Rice NS, et al. (1997) "Cyclodiode". Trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology 104: 1508-1519.
- Autrata R, Rehurek J (2003) Long-term results of transscleralcyclophotocoagulation in refractory pediatric glaucoma patients.Ophthalmologica 217: 393-400.
- Kirwan C, O'Keefe M, Lanigan B, Mahmood U (2005) Ahmed valve drainage implant surgery in the management of paediatric aphakic glaucoma.Br J Ophthalmol 89: 855-858.
- Neely DE, Plager DA (2001) Endocyclophotocoagulation for management of difficult pediatricglaucomas. J AAPOS 5: 221-229.
- Huang MC, Netland PA, Coleman AL, Siegner SW, Moster MR, et al. (1999) Intermediate-term clinical experience with the Ahmed Glaucoma Valve implant.Am J Ophthalmol 127: 27-33.
- Pakravan M, Homayoon N, Shahin Y, Ali Reza BR (2007) Trabeculectomy with mitomycin C versus Ahmed glaucoma implant with mitomycin C for treatment of pediatricaphakic glaucoma.J Glaucoma 16: 631-636.
- Ou Y, Caprioli J (2012) Surgical management of pediatric glaucoma.Dev Ophthalmol 50: 157-172.
- Allen RJ, Speedwell L, Russell-Eggitt I (2010) Long-term visual outcome after extraction of unilateral congenital cataracts.Eye (Lond) 24: 1263-1267.
Citation: Jeganathan VSE, Mulvihill A, Montgomery D (2016) Paediatric Aphakic Glaucoma: A Diagnostic and Management Challenge. Optom Open Access 1: 115. DOI: 10.4172/2476-2075.1000115
Copyright: © 2016 Jeganathan VSE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.