Editorial Open Access
p53 Mutation: Critical Mediator of Therapy Resistance against Tumor Microenvironment
Ali S Arbab, Meenu Jain and B R Achyut*
Tumor Angiogenesis Lab, Department of Biochemistry and Molecular Biology, Georgia Cancer Center, Augusta University, Augusta, GA 30912, USA
- *Corresponding Author:
- B R Achyut, Ph.D.
Assistant Professor, Biochemistry and Molecular Biology
Member- Tumor Signaling and Angiogenesis, Georgia Cancer Center
Augusta University, 1410 Laney Walker Blvd
Augusta, GA, 30912
Tel: 706-721-9344
Fax: 706-721-0101
E-mail: bachyut@augusta.edu
Received date: October 21, 2016; Accepted date: October 22, 2016; Published date: October 28, 2016
Citation: Arbab AS, Jain M, Achyut BR (2016) p53 Mutation: Critical Mediator of Therapy Resistance against Tumor Microenvironment. Biochem Physiol 5:e153. doi:10.4172/2168-9652.1000e153
Copyright: © 2016 Arbab AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biochemistry & Physiology: Open Access
Editorial
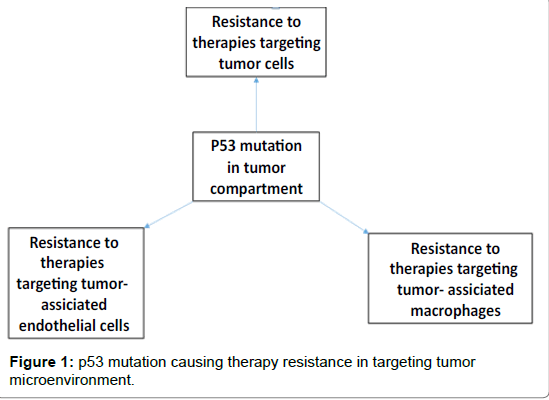
There has been a trend of use of conventional or non-conventional therapies against cancer such as targeting “king pin” tumor cells itself, targeting tumor vasculature lined by endothelial cells, targeting tumor associated macrophages and recently, targeting inhibitory signals on T cells through immunotherapies in tumor microenvironment (TME). However, most of the cases have witnessed therapy resistance flowing a short-term or transient benefit. Despite of several failures in clinical trials targeting tumors and their microenvironments, our understanding is improving every day. It is evident that mutations in tumor cell compartment play a critical role in cancer development as well as therapy resistance. Here, we have discussed studies representing therapy resistance, through p53 as a model mutation and glioblastoma (GBM) as a model tumor.
Targeting tumor cells in glioblastoma
GBM, a grade IV glioma classified by World Health Organization, is considered highly malignant, vascular and invasive subtype [1]. Hypoxia and neovascularization are signature histopathologic features of GBM [2], which is most lethal during first year after initial diagnosis despite surgical resection and other standard therapies [1,3]. Temozolomide chemotherapy and radiotherapy against GBM tumor cells have led to a significant improvement in tumor growth and patient survival in newly diagnosed and recurrent GBM [4,5]. The survival advantage conferred by temozolomide chemotherapy is associated with methylation of the promoter region of the gene encoding O6-methylguanine DNAmethyltransferase (MGMT) [6]. Both tumor protein p53 (TP53) and MGMT are involved in DNA repair after chemotherapy or radiotherapy, which may contribute to drug resistance. In addition, tumor cells acquiring several mutations during tumor progression could contribute to therapy resistance in GBM.
p53 mutations in GBM causing therapy resistance
Many different types of cancer including GBM show a high incidence of TP53 mutations, leading to the stabilization and overexpression of mutant p53 proteins [7,8]. Mutant p53 have both lost wild-type p53 tumor suppressor activity and gained functions that help to contribute to tumor progression [9]. Mutations in p53 gene is reported in 30- 50% of GBMs [10] and strongly associated with a poor prognosis for overall survival in patients with GBM. In addition to role of p53 mutations in promoting tumor growth, p53 mutation drive resistance to antiangiogenic therapy (AAT) targeting GBM vasculature [11]. Also, p53 mutation may decrease the chemo-sensitivity of GBM to temozolomide by increasing MGMT expression [9]. Classical mechanisms of tumor cell–intrinsic resistance to targeted agents have been well-defined in literature, including aberrant drug metabolism and transport, drug target mutation, and activation survival pathways [7].
Targeting tumor microenvironment in GBM
Therapies targeted against TME represent a promising approach for anti-cancer therapy. Targeting TME may have decreased likelihood of acquired resistance through mutations in target TME cells, as is frequently observed with tumor cell–targeted therapies. TME-targeted agents such as targeting VEGF-VEGFR pathways in endothelial cells mediated vasculature and targeting CSF1R positive macrophages that constitute immune suppressive niche in TME, has been in routine use in preclinical studies and clinical trials. It still remains unclear whether resistance to TME-directed therapies follows similar principles as tumor cells. Therefore, it is becoming critical to mechanistically define how resistance may evolve in response to TME-targeted therapies in order to provide long-term disease management.
Targeting endothelial cell related angiogenesis in GBM
Since endothelial cell associated vasculature is important for providing nourishment to the growing tumor, AAT was applied in GBM targeting vascular endothelial growth factor (VEGF)–VEGF receptor axis with small molecular receptor tyrosine kinase inhibitors (RTKIs) and anti-VEGF antibody. AAT did not produce expected results in both clinical and preclinical studies [12-16] (Figure 1). Regrettably, benefits of AAT are at best transitory, and this period of clinical benefit (measured in weeks or months) is followed by restoration of tumor growth and progression [17,18]. Evidence of relapse to progressive tumor growth following treatment reflects development of resistance to AATs [19]. Preclinical studies indicated the development of resistance to the AATs in animal models of GBM [15,16,20]. One possible mechanism for resistance to AAT might be the activation of alternative angiogenesis signaling pathways [21-24]. Hypoxia with increased production of bFGF, angiopoietin1/2, granulocyte colony stimulating factor (G-CSF), monocyte chemotactic protein-1 (MCP-1) and SDF-1α were seen following AAT [16]. A second potential mechanism of AAT resistance might be due to recruitment of BMDCs in the TME. Hypoxia creates conditions permissive for the recruitment of a heterogeneous population of macrophages that promote immune suppression, neovascularization, and tumor growth [16,20,25]. Subsequent analysis showed critical myeloid and endothelial cell signatures in the tumors following AAT [20]. Therefore, targeting of BMDCs acquiring protumor myeloid phenotypes may block the activation of alternative mechanisms drive AAT resistance in GBM.
Targeting tumor associated macrophages in GBM
Macrophages and microglia are of the most abundant noncancerous cell types in GBM, in some cases accounting for up to 30% of the total tumor composition [26,27]. Recent studies have shown that myeloid populations of BMDCs are critical in tumor development [20,28]. Myeloid derived suppressor cells (MDSCs) are immunosuppressive cells that are abundant in TME and inhibit T-cell-mediated anti-tumor immunity [29-31]. Macrophages in the TME are skewed towards a M2 polarized state. This M2 polarized state is closely related to the tumor associated macrophage (TAMs) profile. Several chemokines, such as macrophage colony-stimulating factor-1 (MCSF/CSF1) and monocyte chemotactic protein-1 (MCP1/CCL2) are known to contribute in the recruitment of TAMs and myeloid cells to tumors due to the presence of CSF1R [30,32,33]. CSF1R expression has been reported on immunosuppressive myeloid cells, TAMs and dendritic cells (DCs) [34-36]. CSF1-CSF1R signaling regulates survival, differentiation, and proliferation of monocytes and macrophages [37,38] and has a critical role in angiogenesis and tumor progression [39,40]. Recently, GW2580 was identified as an inhibitor of the CSF1R pathway by acting as a competitive inhibitor of ATP binding to the CSF1R kinase [41,42]. CSF1R blockade through other class of inhibitor has been shown to reverse macrophage polarization, inhibited GBM progression [43] and improved efficacy of radiotherapy [35]. Recently we found that GW2580 was able to reduce glioma growth by limiting CXCL7 production in tumor-recruited bone marrow-derived myeloid cells [20] in a novel chimeric mouse model [25]. However, combining AAT with anti-CSF1R did not improve the results [20,25].
Resistance to anti-CSF1R therapy targeting TAMs in GBM
Studies indicated that TME accumulated TAMs can be targeted through anti-CSF1R with short-term treatment protocol to inhibit GBM progression in animal models [20,25,43,44]. However, studies are rare to investigate how resistance emerges in response to continuous long-term CSF-1R blockade in GBM. Recent study published in journal “Science”, identified that although overall survival is significantly prolonged in response to long-term CSF-1R inhibition, subset of tumors recur eventually in >50% of mice [45]. Transplantation of recurrent tumor cells into naïve animals, GBM reestablish sensitivity to CSF-1R inhibition, indicating that resistance is microenvironment driven [45]. Consequently, combining IGF-1R or PI3K blockade with continuous CSF-1R inhibition in recurrent tumors significantly prolonged overall survival [45]. However, study did not answer whether resistance to anti- CSF1R targeting TAMs in TME is depended on mutational status of the tumor cells.
These findings support the view that although cells in microenvironment are less susceptible to genetic mutation than cancer cells, a tumor can however acquire a resistant phenotype by exploiting its microenvironment. Idea that understanding the functions of tumor cell associated p53 status in terms of tumor growth differences, recruited or residual TAMs, their polarization and functions in TME is important. Therefore, development of new therapeutic approaches that may be advantageous for several cancers are required to explore [7]. Importantly, targeting gain-of -function p53 mutation in combination with anti-TME could be the key for future preclinical and clinical trials [7].
Funding
Supported by in part grant# IRG-14-193-01 by American Cancer Society to B R Achyut and National Institutes of Health grants R01CA160216 and R01CA172048 to Ali S Arbab.
References
- Olar A, Aldape KD (2014) Using the molecular classification of glioblastoma to inform personalized treatment. J Pathol 232: 165-177.
- Brat DJ, Van Meir EG (2004) Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis and accelerated growth in glioblastoma. Lab Invest 84: 397-405.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987-996.
- Linz U (2010) Commentary on Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5year analysis of the EORTC-NCIC trial. Cancer 116: 1844-1846.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, et al. (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459-466.
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, et al. (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352: 997-1003.
- Muller PA, Vousden KH (2014) Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell 25: 304-317.
- Van Oijen MG, Slootweg PJ (2000) Gain-of-function mutations in the tumor suppressor gene p53. Clin Cancer Res 6: 2138-2145.
- Wang X, Chen JX, Liu JP, You C, Liu YH, et al. (2014) Gain of function of mutant TP53 in glioblastoma: Prognosis and response to temozolomide. Ann SurgOncol 21: 1337-1344.
- Louis DN, Von Deimling A, Chung RY, Rubio MP, Whaley JM, et al. (1993) Comparative study of p53 gene and protein alterations in human astrocytic tumors. J NeuropatholExpNeurol 52: 31-38.
- Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS (2002) Effect of p53 status on tumor response to anti-angiogenic therapy. Science 295: 1526-1528.
- Gilbert MR (2016) Antiangiogenictherapy for glioblastoma: Complex biology and complicated results. J ClinOncol 34: 1567-1569.
- Chinot OL, Reardon DA (2014) The future of anti-angiogenic treatment in glioblastoma. CurrOpinNeurol 27: 675-682.
- BatchelorTT, Reardon DA, de Groot JF, Wick W, Weller M (2014) Anti-angiogenic therapy for glioblastoma: Current status and future prospects. Clin Cancer Res 20: 5612-5619
- Ali MM, Janic B, Babajani-Feremi A, Varma NR, Iskander AS, et al. (2010) Changes in vascular permeability and expression of different angiogenic factors following anti-angiogenic treatment in rat glioma. PLoS One 5: e8727.
- Ali MM, Kumar S, Shankar A, Varma NR, Iskander AS, et al. (2013) Effects of tyrosine kinase inhibitors and CXCR4 antagonist on tumor growth and angiogenesis in rat glioma model: MRI and protein analysis study. TranslOncol 6: 660-669.
- Miller KD, Sweeney CJ, Sledge GW Jr (2005) Can tumor angiogenesis be inhibited without resistance? EXS: 95-112.
- Achyut BR, Bader DA, Robles AI, Wangsa D, Harris CC, et al. (2013) Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-ÃÂ?² signaling. PLoS Genet 9: e1003251.
- Bergers G, Hanahan D (2008) Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8: 592-603.
- Achyut BR, Shankar A, Iskander AS, Ara R, Angara K, et al. (2015) Bone marrow derived myeloid cells orchestrate anti-angiogenic resistance in glioblastoma through coordinated molecular networks. Cancer Lett 369: 416-426.
- Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358: 2039-2049.
- Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, et al. (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11: 83-95
- Norden AD, Drappatz J, Wen PY (2008) Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol 7: 1152-1160.
- Arbab AS (2012) Activation of alternative pathways of angiogenesis and involvement of stem cells following anti-angiogenesis treatment in glioma. HistolHistopathol 27: 549-557.
- Achyut BR, Arbab AS (2016) Myeloid cell signatures in tumor microenvironment predicts therapeutic response in cancer. Onco Targets Ther 9: 1047-1055.
- Morantz RA, Wood GW, Foster M, Clark M, Gollahon K (1979) Macrophages in experimental and human brain tumors. Part 2: Studies of the macrophage content of human brain tumors. J Neurosurg 50: 305-311.
- Kostianovsky AM, Maier LM, Anderson RC, Bruce JN, Anderson DE (2008) Astrocytic regulation of human monocytic/microglial activation. J Immunol 181: 5425-5432.
- Shojaei F, Zhong C, Wu X, Yu L, Ferrara N (2008) Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol 18: 372-378.
- Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, et al. (2010) Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res 70: 6139-6149.
- Achyut BR, Arbab AS (2014) Myeloid Derived Suppressor Cells: Fuel the Fire. BiochemPhysiol 3: e123
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V (2012) Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12: 253-268.
- Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, et al. (2008) Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111: 5457-5466.
- Lin EY, Nguyen AV, Russell RG, Pollard JW (2001) Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193: 727-740.
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, et al. (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood111:4233-4244.
- Xu J, Escamilla J, Mok S, David J, Priceman S, et al. (2013) CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res 73: 2782-2794.
- MacDonald KP, Rowe V, Bofinger HM, Thomas R, Sasmono T, et al. (2005) The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J Immunol 175: 1399-1405.
- Hamilton JA (2008) Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8: 533-544.
- Hume DA, MacDonald KP (2012) Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119: 1810-1820.
- Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, et al. (2010) Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: Combating tumor evasion of anti-angiogenic therapy. Blood 115: 1461-1471.
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, et al. (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74: 5057-5069.
- Conway JG, Pink H, Bergquist ML, Han BJ, Depee S, et al. (2008) Effects of the cFMS kinase inhibitor 5-(3-methoxy-4-((4-methoxybenzyl) oxy)benzyl)pyrimidine-2,4-diamine (GW2580) in normal and arthritic rats. Journal of Pharmacology and Experimental Therapeutics326:41-50.
- Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, et al. (2005) Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. ProcNatlAcadSci USA 102: 16078-16083.
- Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, et al. (2013) CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 19: 1264-1272.
- Achyut BR, Shankar A,Iskander AS, Ara R, Knight RA, et al. (2016)Chimericmouse model to track the migration of bone marrow derived cells in glioblastoma following anti-angiogenic treatments. Cancer BiolTher 17: 280-290.
- Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, et al. (2016) The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352: aad3018.
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 11326
- [From(publication date):
October-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10477
- PDF downloads : 849