P. [V27i; E114g] Compound Heterozygous State in Gjb2 Gene Could Be an Indicator of the Severity of Congenital Hearing Loss
Received: 09-Aug-2015 / Accepted Date: 20-Aug-2015 / Published Date: 27-Aug-2015 DOI: 10.4172/2161-119X.1000209
Abstract
Hearing loss (HL) is the most common sensory disorder, affecting all age groups, ethnicities, and genders. Several genes responsible for hearing loss are related to ion recycling and homeostasis in the inner ear. Mutations in GJB2 gene, the gene encoding gap junction protein connexin26 (Cx26), are most common detected in patients with congenital, recessively inherited, nonsyndromic HL in humans. In order to investigate the molecular etiology of patients with congenital, recessively inherited, and nonsyndromic HL and healthy individuals as control in a family, they were included in this study. Thus, exons of GJB2 gene were amplified by polymerase chain reaction (PCR) and sequenced. In this family, V27I missense mutation and V27I + E114G compound heterozygosity were detected in the results of sequence analysis. The V27I mutation was found in patient with severe HL and healthy individuals. The V27I + E114G compound heterozygosity was detected only in deaf patients. Based on our data, V27I mutations could be considered as a polymorphism not leading to HL. Since V27I + E114G compound heterozygosity was found only in deaf patients, it could be considered as a contributor of HL severity.
Keywords: GJB2 gene Hearing loss Sequencing, Missense mutation
250010Introduction
Hearing loss is the most common congenital sensory impairment worldwide [1], which affects approximately 3% of the population [2]. In most of these cases, the inheritance pattern is autosomal recessive (75-80%), although autosomal dominant (20%), X-linked (2-5%) and mitochondrial (<1%) inheritance also occur [3]. 30% of cases of prelingual hearing loss disorders are classified as syndromic, in cases where additional physical findings lead to HL; the remainders are nonsyndromic [4].
Hearing loss affects approximately 70 million people worldwide. Environmental causes, account for 50-60% of cases, can range from neonatal insults, such as prematurity, jaundice, or prenatal infection to iatrogenic causes. The genetic etiologies, account for 40-50% of cases, can be inherited as either syndromic or non-syndromic forms, or have a spectrum of inheritance patterns [2,5]. Non-syndromic deafness accounts for 60-70% of inherited hearing impairment and involves more than 100 different genes with autosomal dominant (DFNA), autosomal recessive (DNFB), X-linked (DFN), and maternal inheritance. The most common cause of non-syndromic autosomal recessive hearing loss is mutations in Cx26, a gap junction protein encoded by the GJB2gene which is located at chromosome 13 q11-12 [6]. Expression of GJB2 has been well documented in a variety of cells and tissues. In the cochlea, it is believed that GJB2 plays a critical role in the recycling of K+ by ferrying K+ away from the hair cells during auditory transduction [7].
Autosomal recessive nonsyndromic sensorineural hearing impairment (ARNSHI) comprises 80% of familial HL cases. Mutations in the GJB2, the gene encoding gap junction protein Cx26, are the largest genetic etiologic contributor to ARNSHI [7]. To date, more than 100 mutations either leading or contributing to HL have been reported in literature. The most common mutation in Europe, North America, and the Mediterranean is a deletion of 6 guanine nucleotides referred to as 35delG [2]. The V37I and V27I are other GJB2 variants, which are much discussed in literature and usually considered as a genetic risk-indicator of HL and a determinant of severity of disease [8].
It has been well documented that GJB2 mutations diverge largely among different ethnic groups. 35delG is commonly seen in Caucasians [4], although 167delT has high prevalence in the Ashkenazi Jewish population [9]. This present study was performed to determine GJB2 gene variants of a Turkish family with autosomal recessive nonsyndromic congenital HL.
Materials and Methods
Subjects and molecular genetic analysis
A familial HL patients with autosomal recessive nonsyndromic congenital HL and their healthy family members were included in this study if the following criteria were met
(1) There exist hearing-impaired siblings.
(2) There exist healthy control sibling at least one.
(3) There is no evidence of any obvious syndrome. The family with hearing loss consists of 4 deaf patients and 2 healthy persons.
Genomic DNA was extracted from peripheral blood using Qiagen DNA extraction kit (Qiagen, Hilden, Germany) and coding sequences of GJB2 gene were amplified by polymerase chain reaction (PCR) using GML Sequence Primers followed by Big-Dye Terminator 3.1 Cycle Sequencing kit (Applied Biosystems, Inc., Foster City, CA). The sequence results were analyzed using Sanger sequencing machine.
Results
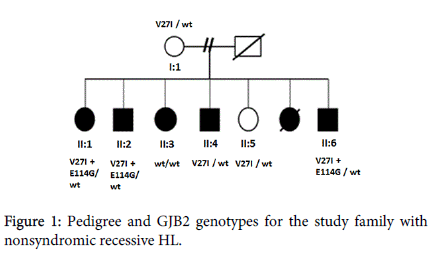
A familial HL case showing autosomal recessive non-syndromic sensorineural hearing impairment was included in present study as a case report shown in (Figure 1). Their hearing losses were nonprogressive and were either congenital or prelingual in onset. Cases’ disease severities are indicated in (Table 1).
| Patient and Control Numbers | Genotype | Disease Severity |
|---|---|---|
| II:1 | V27I+E114G/ wt | Deaf |
| II:2 | V27I+E114G/ wt | Deaf |
| II:3 | wt / wt | Severe |
| II:4 | V27I/ wt | Severe |
| II:5 | V27I/ wt | Healthy |
| II:6 | V27I+E114G/ wt | Deaf |
| I:1 | V27I/ wt | Healthy |
Table 1: Genotype and disease severity in patients and controls.
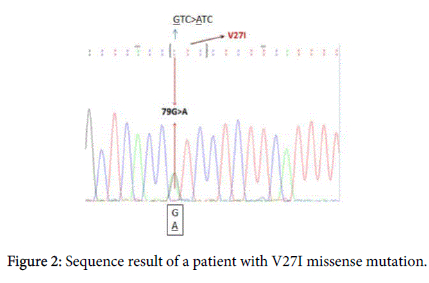
Results of GJB2 sequence analysis in the present study showed that V27I mutation shown in Figure 2 was found in both healthy individuals and patients with severe HL. Previous studies have indicated that V27I mutation is usually considered as a noncausative mutation when it is seen alone [10,11]. The V27I mutation was also detected in a compound heterozygous state with E114G mutation, the Glu to Gly at amino acid position 114 shown in (Figure 2), in only completely deaf patients.
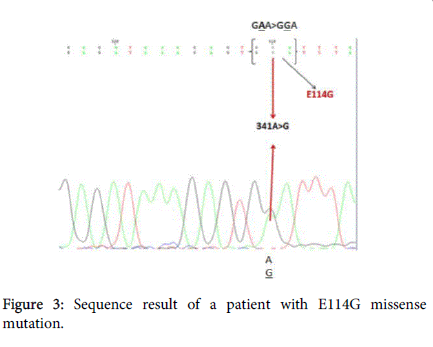
In autosomal recessive single gene disorders, the allelic configurations of detected variants play a key role both in determining correct molecular diagnosis and in disease treatment [12]. V27I +E114G heterozygous state in cis markedly devastated the function of the gap junctions that have crucial role in hearing process and it has been shown that E114G mutation was always detected on the same chromosome that carries the V27I variant [13]. So the heterozygous state found in present study could be considered as contributor to disease severity (Figure 3).
Discussion
To date, over 20 different genes associated with recessive deafness have been identified. GJB2 gene, which is largely expressed in the cochlea, is involved in both syndromic and nonsyndromic HL with a dominant or recessive inheritance pattern. Mutation of the GJB2 gene is the major contributor to autosomal recessive HL and, to a small percentage, autosomal dominant HL. The molecular genetic analyses of the GJB2 gene in a family that consist of both HL patients and healthy individuals showed various variants depending on the severity of the disease. As can be seen in (Figure 1) showing the molecular genetic results of the family, V27I missense mutation in both patients with mild to severe HL and healthy members was identified, although V27I+E114G compound heterozygote was detected only in deaf patients.
The V27I mutation is very often found in patients with HL and it has been considered as a polymorphism not leading to HL [10,11]. Since V27I, the sequence change of the Val to Ile at amino acid position 27, was also detected in healthy individuals in the present study, it cannot be regarded as causative mutation of HL. It might be thought as a contributor because of being located in transmembrane protein domain. The V27I mutation was also detected in a compound heterozygous state with E114G mutation, the Glu to Gly change at amino acid position 114. In autosomal recessive single gene disorders, the allelic configurations of detected variants play a key role both in determining correct molecular diagnosis and in disease treatment. Depending on whether these changes are on the same (in cis) or on opposite (in trans) chromosomes, they can be considered as a causative or noncausative [12].
Previous results of in vitro functional studies on the Xenopus oocyte system demonstrated that [13]. It has been found that the V27I and E114G variants are appeared very often in both deaf probands and hearing controls and the E114G variant is always in cis with the V27I variant. Although in vitro experiments suggest a pathogenic role for the complex allele, the equal distribution of p.[V27I; E114G] in deaf probands and hearing controls makes it a less likely cause of profound congenital deafness [14] Pandya et al. suggested that when these two variants are shown together in cis-configuration, homozygous V27I +E114G or compound heterozygote with another mutation could cause hearing loss [13]. Choi et al. used in-vitro assay and population study to examine the pathogenesis of V27I and E114G variants and indicated that E114G variant is deleterious but V27I+E114G represent a nonpathogenic polymorphism and suggesting that only E114G homozygote or compound heterozygote carrying E114G type with other mutations may cause HL [15]. Also, V27I+E114G/wt genotype was found as the cause of hearing loss in a family involved in a previous study [16]. Two polymorphisms (V27I and E114G) detected in GJB2 gene were not pathogenic variants causing inherited deafness which had been described in a previous study [17]. Moreover, while p.V27I mutation is not leading to HL, V27I + R75W compound heterozygous state was reported as a disease causative mutation [18].
Based on our data, V27I missense mutation and V27I + E114G compound heterozygote detected in GJB2 gene involved in a familial HL case are nonpathogenic variants. Our study indicates that these mutations are not the main causative mutations associated with HL in this family. However, since V27I + E114G compound heterozygous state in our study was found in only deaf patients, it could be thought that these variants impair the function of gap junctions and play a role in increasing the severity of HL. This information will be valuable in considering HL cases or early diagnosis.
References
- Mehl AL, Thomson V (2002) Thecolorado newborn hearing screening project, 1992–1999: On the threshold of effective population-based universal newborn hearing screening. Pediatrics 109: e7.
- Zhong LX (2013) Non-syndromic hearing loss and high-throughput strategies to decipher its genetic heterogeneity. Journal of Otology 8: 6-24.
- Smith RJ, Bale JF Jr, White KR (2005) Sensorineural hearing loss in children. Lancet 365: 879-890.
- Denoyelle F, Marlin S, Weil D, Moatti L, Chauvin P, et al. (1999) Clinical features of the prevalent form of childhood deafness, DFNB, due to a connexin-26 gene defect: implications for genetic counselling. Lancet 353: 1298-1303.
- Morton NE1 (1991) Genetic epidemiology of hearing impairment. Ann N Y AcadSci 630: 16-31.
- Bitner-Glindzicz M1 (2002) Hereditary deafness and phenotyping in humans. Br Med Bull 63: 73-94.
- Kikuchi T, Kimura RS, Paul DL, Adams JC (1995) Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. AnatEmbryol (Berl) 191: 101-118.
- Gallant E (2013) Homozygosity for the V37I GJB2 mutation in fifteen probands with mild to moderate sensorineural hearing impairment: Further confirmation of pathogenicity and haplotype analysis in Asian populations. American Journal of Medical 161: 2148-2157.
- Ben-Yosef T, Friedman TB (2003) The genetic bases for syndromic and nonsyndromic deafness among Jews. Trends Mol Med 9: 496-502.
- Kelley PM, Harris DJ, Comer BC, Askew JW, Fowler T, et al. (1998) Novel mutations in the connexin 26 gene (GJB2) that cause autosomal recessive (DFNB1) hearing loss. Am J Hum Genet 62: 792-799.
- Prasad S, Cucci RA, Green GE, Smith RJ (2000) Genetic testing for hereditary hearing loss: connexin 26 (GJB2) allele variants and two novel deafness-causing mutations (R32C and 645-648delTAGA). Hum Mutat 16: 502-508.
- Chen N, Schrijver I (2011) Allelic discrimination of cis-trans relationships by digital polymerase chain reaction: GJB2 (p.V27I/p.E114G) and CFTR (p.R117H/5T). Genet Med 13: 1025-1031.
- Pandya A, Arnos KS, Xia XJ, Welch KO, Blanton SH, et al. (2003) Frequency and distribution of GJB2 (connexin 26) and GJB6 (connexin 30) mutations in a large North American repository of deaf probands. Genet Med 5: 295-303.
- Tekin M, Xia XJ, Erdenetungalag R, Cengiz FB, White TW, et al. (2010) GJB2 mutations in Mongolia: complex alleles, low frequency, and reduced fitness of the deaf. Ann Hum Genet 74: 155-164.
- Choi SY, Lee KY, Kim HJ, Kim HK, Chang Q, et al. (2011) Functional evaluation of GJB2 variants in nonsyndromic hearing loss. Mol Med 17: 550-556.
- Davarnia B, Babanejad M, Fattahi Z, Nikzat N, Bazazzadegan N, et al. (2012) Spectrum of GJB2 (Cx26) gene mutations in Iranian Azeri patients with nonsyndromic autosomal recessive hearing loss. Int J PediatrOtorhinolaryngol 76: 268-271.
- Han SH, Park HJ, Kang EJ, Ryu JS, Lee A, et al. (2008) Carrier frequency of GJB2 (connexin-26) mutations causing inherited deafness in the Korean population. J Hum Genet 53: 1022-1028.
- Dalamón V, Béhèran A, Diamante F, Pallares N, Diamante V, et al. (2005) Prevalence of GJB2 mutations and the del(GJB6-D13S1830) in Argentinean non-syndromic deaf patients. Hear Res 207: 43-49.
Citation: Tufan T, Erkoc MA, Yilmaz MB, Comertpay G, Alptekin D (2015) P. [V27i; E114g] Compound Heterozygous State in Gjb2 Gene Could Be an Indicator of the Severity of Congenital Hearing Loss. Otolaryngol (Sunnyvale) 5:209. DOI: 10.4172/2161-119X.1000209
Copyright: © 2015 Tufan T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14861
- [From(publication date): 9-2015 - Apr 06, 2025]
- Breakdown by view type
- HTML page views: 10285
- PDF downloads: 4576