Oxidative Stress in Homocysteinemia: The Prevention of the Therapy-Induced Peripheral Neuropathy
Received: 23-Jan-2017 / Accepted Date: 13-Mar-2017 / Published Date: 22-Mar-2017
Abstract
Oxidative stress (OS) is an unhealthy condition that results from the imbalance between the production of reactive oxygen species (ROS) and the rate of their elimination through the anti-oxidant defence mechanisms. This condition is a part of the pathogenesis of many disease conditions. In this paper, the development of OS in homocysteinemia is highlighted, where some light is shed on the mechanism of the increased ROS production and the potential protective strategy in the clinical practice.
Keywords: Oxidative stress; Vitamin supplementation; Homocysteinemia
5724Introduction
Oxidative stress (OS) is a condition that is characterized by the lack of balance between the reactive oxygen species (ROS) and the anti-oxidant cellular defense mechanisms [1]. OS is one of the most harmful situations in the human body, which is incorporated in many diseases, such as DNA damage, cancer, heart diseases, and stroke [2].
The Molecular Hazards Of ROS Could Be Summarized In The Following:
Lipid peroxidation
Peroxidation of the lipids of the cellular membranes can result in the loss of membrane fluidity and elasticity, which destroys the membrane functions and may lead to cellular death. The direct products of lipid peroxidation, such as malondialdehyde (MDA), isoprostanes, and 4- hydroxynonenal play an important role in the development of various diseases.
Malondialdehyde is a reactive carbonyl compound known to be a mutagenic and carcinogenic compound [3]. Isoprostanes are prostaglandin-like substances produced by free radical-induced peroxidation of arachidonic acid. The increase in F2-isoprostane is incorporated in the development of asthma [4], Alzheimer’s disease [5], scleroderma [6] and hepatic cirrhosis [7].
Protein damage
Protein oxidation consequences can include protein fragmentation and the formation of protein-protein cross-linkages, as well as the alteration of the physiological conformation, which results in the alteration of the functions. Oxidation of protein side chains produces high levels of protein carbonyl (CO) groups that may participate in the pathogenesis of rheumatoid arthritis, Alzheimer’s disease, diabetes, sepsis and chronic renal failure [8].
DNA damage
Oxidative DNA injury can result in base alteration and or gene expression changes [9].
Homocysteinemia
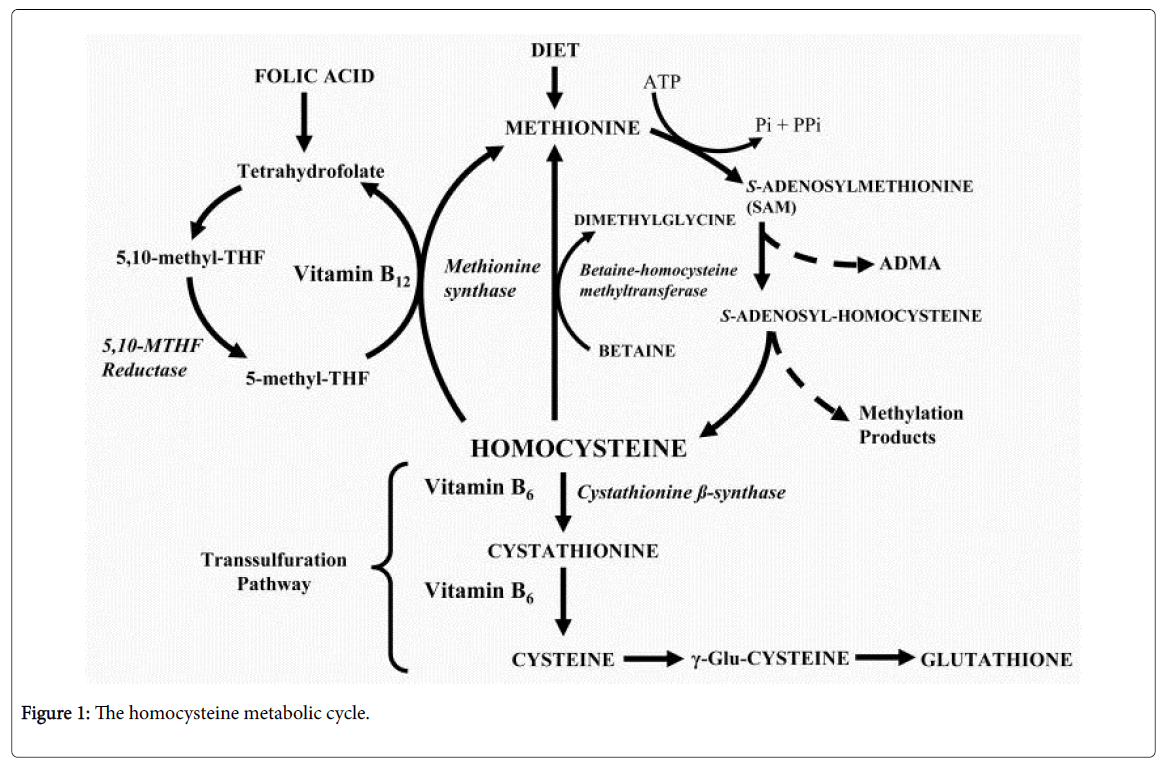
Homocysteine (Hcy) is a synthetic amino acid not present in the diet, but formed by the conversion of methionine to cysteine. Hyperhomocysteinemia causes endothelial dysfunction, which renders it an independent risk factor for the development of cardiovascular diseases. Hcy may increase the production of thrombin by the endothelial cells, which activates protease-activated receptors (PARs) that belong to the G protein-coupled receptor family and include PAR-1, PAR-2, PAR-3, and PAR-4. The PARs are expressed in the endothelial cells, where their activation increases the production of ROS and NADPH oxidase, while decreasing the production of thioredoxin. Thus, hyperhomocysteinemia can lead to OS and the accumulation of asymmetric dimethylarginine (ADMA), which is an endogenous NOS inhibitor, opposing the activity of endothelial NOS (eNOS), and impairing NO signaling [10].
Role Of Folic Acid And The Vitamins B6 And B12
Folic acid is important for the treatment of homocysteinemia because it is important for the cellular production of tetrahydrofolate (THF), which is a precursor of 5-methyl-THF that is essential for the normal methionine synthase enzyme activity. Vitamin B12 is an essential cofactor that is also required for the normal methionine synthase activity. Vitamin B6 is required for the conversion of homocysteine to cystathionine by cystathionine β-synthase (CBS). Supplementation with vitamin B6 decreases the thromboembolic events associated with hyperhomocysteinemia (Figure 1) [11]. Recently, an additional advantage of vitamin B6 during hyperhomocysteinemia has been revealed, where the vitamin plays a significant role in the management of hyperhomocysteinemia-induced OS. The provisional mechanism seems to be related to the distribution of glutathione to vital organs, such as the liver [12].
A Clinical Remark
While the vitamin therapy in hyperhomocysteinemia is inevitable, the vitamin supplementation should be carefully controlled, so that, the levels of the vitamins are kept within the guideline levels, and the toxicity is avoided. In the case of homocysteinemia, the patient is at risk of the development of peripheral neuropathy due to many factors. At first, the elevated plasma level of homocysteine has the ability to induce peripheral neuropathy, where the sensory manifestations predominate [13]. Secondly, any associated deficiency of folic acid, vitamin B12 and or vitamin B6 would act synergistically to worsen the condition. Thirdly, as the vitamin supplementation is an integral part of the therapy plan, the patients are usually advised to utilize one tablet that contains a mixture of the vitamins, in order to improve the patient's compliance to therapy. This carries the risk of keeping certain vitamins within the normal levels, while increasing others to higher levels. The therapy with a tablet, containing a mixture of vitamin B6, B12 and folic acid, may be associated with normal levels of folic acid and vitamin B12, but increased level of vitamin B6, which, similar to its deficiency, can lead to peripheral neuropathy [14]. Accordingly, the use of separated supplementation of the vitamins, together with the frequent measurement of their blood levels, should be recommended, in order to provide the controlled requirements that keep the vitamins within the normal and or therapeutic guideline levels.
Conflicts of Interest
The intellectual properties included in this manuscript belong solely to the author. Reproduction or use of any of them requires the author's written permission. No funding was provided for the development of this work.
References
- Kala C, Salman AS, Abid M, Rajpoot S, Khan AN (2015) Protection Against FCA Induced Oxidative Stress Induced DNA Damage as a Model of Arthritis and In vitro Anti-arthritic Potential of Costus speciosus Rhizome Extract. Inter J Pharma Phyto Res 7: 383-389.
- Joseph N, Zhang-James Y, Perl A, Faraone SV (2015) Oxidative Stress and ADHD: A Meta-Analysis. J Atten Disord 19: 915-924.
- Marnett LJ (1999) Lipid peroxidation–DNA damage by malondialdehyde. Mutat Res-Fund Mol M 424: 83-95.
- Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov S, et al. (1999) Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med 160: 216-220.
- Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbsery WR, et al. (2002) Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic Biol Med 33: 620-626.
- Cracowski JL, Marpeau C, Carpentier PH, Imbert B, Hunt M, et al. (2001) Enhanced in vivo lipid peroxidation in scleroderma spectrum disorders. Arthritis Rheum 44: 1143-1148
- Pratico D, Iuliano L, Basili S, Ferro D, Camastra C, et al. (1998) Enhanced lipid peroxidation in hepatic cirrhosis. J Invest Med 46: 51-57
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329: 23-38.
- Tsuboi H, Kouda K, Takeuchi H, Takigawa M, Masamoto Y, et al. (1998) 8-Hydroxydeoxyguanosine in Urine As an Index of Oxidative Damage to DNA in the Evaluation of Atopic Dermatitis. Br J Dermatol 138: 1033-1035.
- Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, et al. (2005) Mechanisms of homocysteine-induced oxidative stress. American Journal of Physiology-Heart and Circulatory Physiology 289: 2649-2656.
- Maron BA, Loscalzo J (2009) The Treatment of Hyperhomocysteinemia. Annu Rev Med 60:Â 39-54.
- Hsu CC, Cheng CH, Hsu CL, Lee WJ, Huang SC, et al. (2015) Role of vitamin b6 status on antioxidant defenses, glutathione, and related enzyme activities in mice with homocysteine-induced oxidative stress. Food & Nutrition Research 59: 25702.
- Shandal V, Luo JJ (2016) Clinical Manifestations of Isolated Elevated Homocysteine-Induced Peripheral Neuropathy in Adults. J Clin Neuromuscul Dis 17: 106-109.
- Scott K, Zeris S, Kothari MJ (2008) Elevated B6 levels and peripheral neuropathies. Electromyogr Clin Neurophysiol 48: 219-223.
Citation: Shehata M (2017) Oxidative Stress in Homocysteinemia: The Prevention of the Therapy-Induced Peripheral Neuropathy. J Clin Exp Neuroimmunol 2: 111.
Copyright: © 2017 Shehata M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4554
- [From(publication date): 0-2017 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 3674
- PDF downloads: 880