Case Report Open Access
Oxidative Stress and FGF-1 Release from Astrocytes
Jin-ichi Ito*, Jiansheng Gong and Makoto Michikawa
Biochemistry, Graduate School of Medical Sciences, Nagoya City University, Kawasumi 1, Mizuho-cho, Mizuhoku, Nagoya, Japan
- Corresponding Author:
- Jin-ichi Ito
Biochemistry, Graduate School of Medical Sciences
Nagoya City University, Kawasumi 1
Mizuho-cho, Mizuho-ku, Nagoya, Japan
E-mail: jitoh@med.nagoya-cu.ac.jp
Received date: July 22, 2013; Accepted date: November 15, 2013; Published date: November 22, 2013
Citation: Ito J, Gong J, Michikawa M (2013) Oxidative Stress and FGF-1 Release from Astrocytes. J Alzheimers Dis Parkinsonism 3:133. doi:10.4172/2161-0460.1000133
Copyright: © 2013 Ito J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
The brain is an organ to consume highly oxygen and to produce Reactive Oxygen Species (ROS) at high level. ROS facilitate to produce many kinds of peroxide lipids in the lipid-rich brain. Accordingly, it is considered that the brain is an organ to undergo easily oxidative stress. Severe diseases of brain such as Parkinsonism and Alzheimer’s disease are seemingly accompanied with oxidative stress. The construction of protection system against oxidative stress is very important to maintain normal neuron function in the brain. We are studying the production and function of Fibroblast Growth Factor 1 (FGF-1) in astrocytes undergoing oxidative stress. Astrocytes increase FGF-1 release under stressful condition such as oxidative stress and long-term cultured stress. In vitro treatment of rat astrocytes with 100 μM Hydrogen Peroxide (H2O2) for 10 min enhances the release of FGF-1 along with cytosolic proteins such as HSP90, HSP70, PK-Cδ without inducing apoptosis. The treatment with H2O2 enhanced the flow into the cells of exogenous compounds such as trypsin and Rhodamin-phalloidin also, suggesting increase in permeability of plasma membrane in astrocytes treated with H2O2. Oxidative stress suppressed transiently cholesterol synthesis and enhanced cholesterol release from the cell surface, resulted in impairment of the cholesterol metabolism. The change of cholesterol metabolism may participate in the increase in membrane permeability in astrocytes undergone oxidative stress. FGF-1 released from rat astrocytes enhances apoE-containing HDL-like lipoproteins (apoE/HDL) generation of astrocytes in the manner of autocrine action. In this review, we describe physiological significance of FGF-1 released from astrocytes stimulated by oxidative stress to relate with generation of apoE/HDL of astrocytes in the brain.
Keywords
Astrocytes; FGF1; ApoE; HDL; Oxidative stress; Cholesterol
Introduction
Glias are known to occupy approximately 10-fold higher population than neurons, and astrocytes are most abundant glial cells in the brain. The population of astrocytes is approximately 1.4-fold as many as that of neurons in the cerebrum. It has been thought that astrocytes function to support structure and distribution of neurons in the brain, regulate molecular transport between the inside and outside of brain through the Blood-Brain Barier (BBB) along with brain capillary endothelial cells, control energy methabolism of neurons, and keep homeostasis of extracellular emvironment of neural cells. Astrocytes has been considered to play subordinate roles from a viewpoint of the brain function to organize neuronal signal networks in the Central Nervous System (CNS). Recentrly, it was found that astrocytes have receptors of various neurotransmitters and release cytokines and transmittors such as glutamate and D-serine as glio-transmitters, sugesting that astrocytes actively function to regulate various information in neuronal signal networks in the CNS [1,2].
There have been increasing reports that astrocytes function to protect the brain against stresses. Oxidative stress reportedly activates calcium/calmodulin-dependent protein kinase II (CaMKII), followed by activation of Apoptosis Signal-regulating Kinase 1 (ASK1) to induce apoptosis in astrocytes [3]. However, it has been found that protein phosphatase 5 (PP5) localizes widely in the brain and inhibits ASK1 activity, resulted in the protection of neurons against oxidative stress [4]. Astrocytes produce and release Glutathione (GSH) and metallothionein to protect neurons against oxidative stress [5-7]. Furthermore, astrocytes secrete ATP and activate a specific ATP receptor, P2Y1, in the autocrine manner, and then enhance the expression and release of Thioredoxin Reductase (TrxR) in/from astrocytes for protection of the brain against oxidative stress [8]. We found recently that astrocytes increase the FGF- 1 secretion under oxidative stress which, in turn, stimulated generation of apoE/HDL in an autocrine manner for protection of brain against oxidative stress [9-12]. Thus, astrocytes own many kinds of means for protection of neural cells from oxidative stress.
Production and Release of FGF-1 from Astrocytes Induced by Oxidative Stress
FGF-1 (acidic FGF) is classified into the FGF family and stimulates the proliferation and differentiation of mesodermal and neuroectodermal cells such as fibroblasts and astrocytes, similarly to FGF-2 (basic FGF). FGF-1 was shown to localize in all motor neurons and primary sensory neurons in the mesencephalon and in a small number of glial cells in the white matter [13]. It have been reported that in Alzheimer’s disease, some astrocytes prodece FGF-1 in both the gray and white matters [14]. FGF-1 has already been known to contribute to neuron survival, neurite outgrowth, and angiogenesis in vitro and in vivo [9,15]. Because FGF-1 and FGF-2 have no amino-terminal signal sequence, these factors are considered to be synthesized and to localize in the cytosol and nucleus of FGF-producing cells [16,17]. Accordingly, their release to the extracellular space is unlikely mediated by the classical intracellular transport system through the Endoplasmic Reticulum (ER)/Golgi pathway after their biosynthesis.
The mechanism underlying FGF-1 release is not so much clear at present despite of many previous investigations [18-20]. FGF-1 seems to be released in response to sublethal cell injuries such as oxidative stress, heat shock, hypoxia, and serum starvation [21-24]. Previous reports have shown that FGF-1 is expressed in reactive astrocytes in the brains of patients with Alzheimer’s disease, which is known to be associated with chronic inflammation and oxidative stress [25]. We previously observed that the production and release of FGF-1 are enhanced in rat astrocytes under the long-term cultured stress [10]. These lines of evidence suggest that FGF-1 release is enhanced under stressful conditions such as oxidative stress.
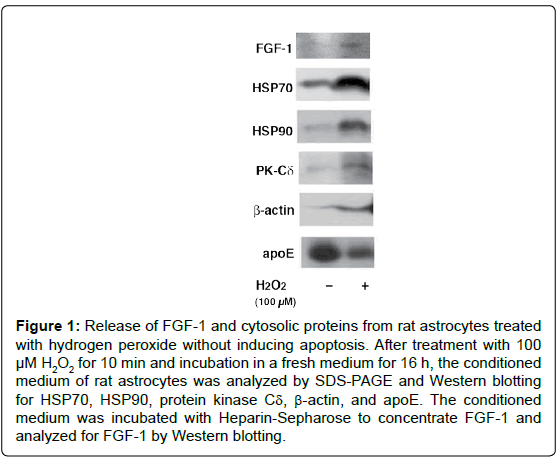
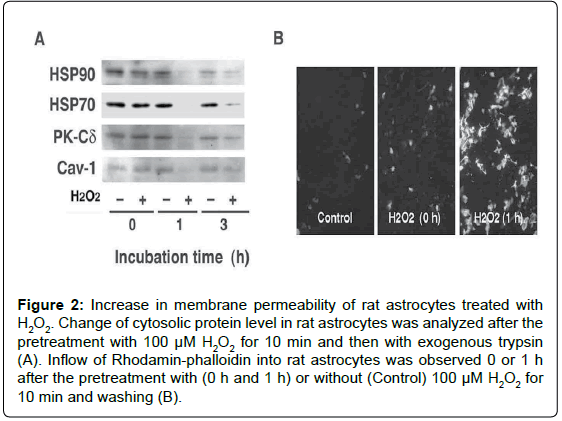
We examined in vitro whether the release of FGF-1 from the brainderived astrocytes is actually triggered by oxidative stress [12]. The treatment of rat astrocytes with 100 μM hydrogen peroxide (H2O2) for 10 min enhanced FGF-1 release without inducing apoptosis. After the treatment with H2O2, the conditioned medium of rat astrocytes cultured in a fresh medium had the FGF-1-like activities, which enhanced cholesterol synthesis, signalings to phosphorylate Akt and ERK, and apoE secretion. The oxidative stress induced by H2O2 enhanced the release of cytosolic proteins such as HSP70 and HSP90 in addition to FGF-1, suggesting the release of FGF-1 takes place along with that of cytosolic proteins from astrocytes undergoing oxidative stress (Figure 1). Antioxidants such as ascorbic acid and ebselen suppresses the release of cytosolic proteins induced by H2O2 treatment. We examined whether exogeneously added trypsin goes through membrane into cytosol in astrocytes treated with H2O2. The treatment with exogenous trypsin decreased the cellular levels of cytosolic proteins such as HSP90, HSP70, PK-Cδ, and Cav-1 1 h or 3 h after the incubation of the cells with H2O2, whereas they remained unchanged in the cells without H2O2 (Figure 2A). Rhodamin-phalloidin also flowed into the cells 1 h after H2O2 treatment and fixation with paraform aldehyde (Figure 2B). These findings suggest that H2O2 treatment enhanced permeability of trypsin and Rhodamin-phalloidin, which enhanced proteolysis of cytoplasmic proteins and staining of cellular actin filaments, respectively. These findings suggest that oxidative stress is one of the candidates which triggers FGF-1 release from astrocytes in the brain.
Mechanism Underlying FGF-1 Release from Astrocytes Induced by Oxidative Stress
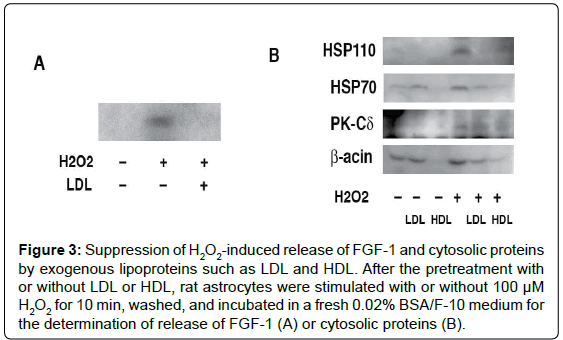
The addition of lipoproteins such as Low Density Lipoproteins (LDL) canceles H2O2-induced release of FGF-1 and cytosolic proteins (Figure 3) [12]. Furthermore, the pretreatment with LDL suppressed exogenous trypsin-induced proteolysis of cytosolic proteins in the H2O2-treated rat astrocytes, suggesting that the H2O2-induced increase in permeability of exogenous compounds is prevented by the uptake of lipids from exogenous lipoproteins. These findings imply that the increase of membrane permeability induced by H2O2 correlates strongly with lipid metabolism.
Figure 1: Release of FGF-1 and cytosolic proteins from rat astrocytes treated with hydrogen peroxide without inducing apoptosis. After treatment with 100 μM H2O2 for 10 min and incubation in a fresh medium for 16 h, the conditioned medium of rat astrocytes was analyzed by SDS-PAGE and Western blotting for HSP70, HSP90, protein kinase Cδ, β-actin, and apoE. The conditioned medium was incubated with Heparin-Sepharose to concentrate FGF-1 and analyzed for FGF-1 by Western blotting.
Interestingly, the treatment with H2O2 strongly suppressed apoE secretion from astrocytes, although it did not suppress FGF-1 release, suggesting that oxidative stress may suppress the classical intracellular transport through the ER/Golgi pathway as shown in apoE secretion from astrocytes. This indicates that FGF-1 is released from astrocytes through a process different from the classical secretion pathway. As [35S]-labeled cytosolic proteins of a wide range of molecular weights were released from rat astrocytes treated with 100 μM H2O2, it is suggested that the enhancement of FGF-1 release by oxidative stress depends on the mechanism nonspecific to the FGF-1 molecule in astrocytes.
It is interesting also that the release of cytosolic proteins such as HSP90 and HSP70 from astrocytes was enhanced by H2O2 at low concentrations, although in other cell types, the release was markedly attenuated in Balb3T3 cells, remained unchanged in bovine endothelium cells, or diminished in HepG2 cells [12]. These findings suggest that astrocytes are more sensitive to H2O2 in terms of the release of cytosolic proteins.
Figure 2: Increase in membrane permeability of rat astrocytes treated with H2O2. Change of cytosolic protein level in rat astrocytes was analyzed after the pretreatment with 100 μM H2O2 for 10 min and then with exogenous trypsin (A). Inflow of Rhodamin-phalloidin into rat astrocytes was observed 0 or 1 h after the pretreatment with (0 h and 1 h) or without (Control) 100 μM H2O2 for 10 min and washing (B).
Figure 3: Suppression of H2O2-induced release of FGF-1 and cytosolic proteins by exogenous lipoproteins such as LDL and HDL. After the pretreatment with or without LDL or HDL, rat astrocytes were stimulated with or without 100 μM H2O2 for 10 min, washed, and incubated in a fresh 0.02% BSA/F-10 medium for the determination of release of FGF-1 (A) or cytosolic proteins (B).
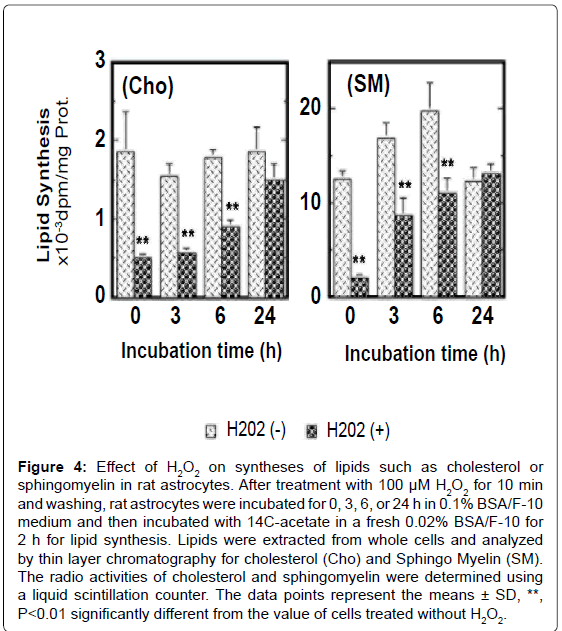
The treatment with 100 μM H2O2 for 10 min inhibited transiently syntheses of cholesterol and sphingomyelin in rat astrocytes and these syntheses were restored approximately 24 h after withdraw of H2O2 (Figure 4). The transport and incorporation of metabolically synthesized cholesterol to/in mambrane fraction, especially the membrane lipid rafts, were significantly suppressed by the treatment with H2O2 in rat astrocytes. The level of caveolin-1 in membrane lipid rafts was reduced by oxidative stress also. The transport of sphingomyelin to lipid rafts was suppressed as well. These findings suggest that oxidative stress without apoptosis significantly suppresses lipid synthesis and disturbs construction of membrane lipid raft microdomain. Oxidative stress, furthermore, enhances non-specific release of lipids from the cell surface of rat astrocytes (unpublished data), suggesting oxidative stress lowers the lipid level of plasma membrane to make the plasma membrane structure unstable in astrocytes. This may enhance the permeability of cytosolic proteins through the unstable plasma membrane.
Malecki et al. observed that FGF-1 is able to across vesicular membranes from endosomes into the cytosol during G1-phase in the manner dependent on proton pumps [26,27]. FGF-1 appears to be localized in the neural tissue as a high-molecular-weight complex. The complex contains FGF-1 and p40 extravasecular domain of synaptotagmin (Syn)-1, and the brain-derived FGF-1/p40 Syn-1 complex is found to be associated with the calcium-binding protein S100A13 [28,29]. FGF-1 is released as a latent homodimer with the p40 extravasecular domain of Syn-1, as induced by heat-shock stress [30]. These findings indicate that FGF-1 is released as complex forms under stressful conditions. The role of the complex formation of FGF-1 in the release of FGF-1 to extracellular space under stressful condition is as yet unknown [31]. Graziani et al. suggested that the nonclassical pathway of release of FGF-1 and p40 Syn-1 involves the destabilization of the membrane containing acidic phospholipids [32]. We also observed in this study that oxidative stress suppresses syntheses of lipids such as cholesterol and sphingomyelin and incorporation of de novo synthesized lipids to the lipid raft/caveolae domain in the membrane fraction. This may impair the plasma membrane functions and alter lipid raft structure. The sensitive response to oxidative stress to enhance the release of FGF-1 and cytosolic proteins may be a functional feature of astrocytes for protection of the brain from oxidative stress through the released FGF-1.
Figure 4:Effect of H2O2 on syntheses of lipids such as cholesterol or sphingomyelin in rat astrocytes. After treatment with 100 μM H2O2 for 10 min and washing, rat astrocytes were incubated for 0, 3, 6, or 24 h in 0.1% BSA/F-10 medium and then incubated with 14C-acetate in a fresh 0.02% BSA/F-10 for 2 h for lipid synthesis. Lipids were extracted from whole cells and analyzed by thin layer chromatography for cholesterol (Cho) and Sphingo Myelin (SM). The radio activities of cholesterol and sphingomyelin were determined using a liquid scintillation counter. The data points represent the means ± SD, **, P<0.01 significantly different from the value of cells treated without H2O2.
FGF-1 Enhances apoE/HDL Generation of Astrocytes in a Manner of Autocrine or Paracrine Action
Human apoE is a glycoprotein composed of 299 amino acids with a molecular weight of 34 kDa. ApoE is produced by various types of cells such as macrophages and steroidogenic cells, and the plasma apoE is mainly secreted from the liver. In the brain, the most abundant apolipoprotein is an apoE produced predominantly by astroctes and partly by microglias, and it is secreted for intercellular transport of cholesterol as apoE/HDL with diameters of 10-17 nm in the CNS. As the supply of cholesterol to the CNS is segregated by the BBB from the lipoprotein system in the circulation, apoE/HDL in the brain may be mainly supplied by astrocytes in the brain [33,34].
Endogenous apoE is seemingly transported intracellularly via membrane lipid rafts in astrocytes and generates apoE/HDL through the interaction with ATP-Binding Cassette A1 (ABCA1) [35]. We found that FGF-1 enhances apoE/HDL generation of astrocytes accompanied by the up-regulation of syntheses of apoE and cholesterol, very likely in an autocrine manner [9,10]. It was also observed that the productions of FGF-1 and apoE are increased in the astrocytes around a cryoinjuryinduced lesion in the mouse brain [9]. The production of FGF-1 was increased prior to the apoE production in astrocytes after brain injury. Wound healing was substantially delayed in apoE-deficient mice, although the production of FGF-1 was increased in the injured brain also. These findings support the hypothesis that injury and stress induce astrocytes to produce and secrete FGF-1 and up-regulate apoE/HDL generation. It is, thus, possible that astrocytes protect neurons from stress and injury through the FGF-1/apoE/HDL system.
Interestingly, FGF-1 enhances apoE/HDL generation in healthy astrocytes under the stress-less condition but not in unhealthy cells under the stressful condition such as oxidative stress and long-term cultured stress. FGF-1 released from unhealthy astrocytes under some stresses may act on healthy astrocytes to induce apoE/HDL generation through the paracrine action for the protection of neural cells in the brain. In apoE-deficient mouse astrocytes, FGF-1 stimulates cholesterol biosynthesis but not enhancing its release, indicating the cholesterol release is enhanced by FGF-1 dependently on intrtacellular apoE in astrocytes. Suppression of PI3-kinase activity by LY294002 inhibited apoE/HDL secretion and suppression of MAP kinase cascade by U0126, an inhibitor of MEK and ERK, inhibited cholesterol synthesis without suppression of apoE/HDL generation [9]. However, these compounds failed to suppress FGF-1-induced increase of apoE mRNA expression. FGF-1 increases apoE mRNA expression through the increase of mRNA expression of Liver X Receptor α (LXRα) [9]. These findings suggest that FGF-1 upregulates apoE/HDL generation through at least three independent signaling pathways.
Construction of FGF-1/apoE/HDL System of Astrocytes in Alzuheimar’s Disease
Tooyama et al. reported that FGF-1 expression increases in the brain of patients with Alzheimer’s disease [14]. There, furthermore, is a report that FGF-1 concentration increases in the serum and cerebrospinal fluid of patients with Alzheimer’s disease [36]. FGF-1 immunoreactivity shows significantly high level in neurons of Alzheimer’s disease brains except for neurons in entorhinal cortex [37]. These findings suggest the relationship between the progress of Alzheimer’s disease and FGF- 1 production in the brain. It is very interesting that FGF-1 expression level is increased in reactive astrocytes surrounding senile plaques [38]. These findings indicate that not only neurons but also astrocytes express FGF-1 in the brain. As production of FGF-1 is enhanced in the brain by the injury and oxidative stress, neuroregenerative diseases such as Alzheimer’s disease may enhance FGF-1 expression in an affected part of brain. If so, what is a physiological relevance of FGF-1 production in the brain of Alzheimer’s disease?
Alzheimer’s disease involves chronic inflammatory reactions, oxidative stress, proteasome inhibition, and high cholesterol level in addition to β-amyloid accumulation. Chronic inflammatory reaction is one of important factors to induce Alzheimer’s disease to induce neurodegenerative hypothesis of AD inflammatory cytokines such as IFN-γ, TNF-α, and interleukin-1α [39]. These factors may activate and induce reactive astrocytes. Perhaps, FGF-1 production is incresed in the brain during this process as reported by Tooyama et al. [14]. There are many reports regarding the interaction of β-amyloid with apoE but not with FGF-1 [40,41]. We demonstrated that FGF-1 enhances apoE/HDL generation through the increase in production of apoE and cholesterol by three different signal pathways in astrocytes [9]. We, furthermore, reported that the production of FGF-1 is ahead of that of apoE in the mouse brain with cryo-injury. These observations suggest a possibility that FGF-1 enhances apoE/HDL generation in astrocytes in response to increasing production of β-amyloid to protect the brain from the attack of β-amyloid.
ApoE appears to promote the proteolytic degradation of β-amyloid [42]. ApoE is actively able to associate with soluble nonaggregated β-amyloid peptides as scavengers. β-amyloid-associated apoE/HDL binds to apoE receptors and then is internalized in glial and neuronal cells. Internalyzed β-amyloid is degradated via the endosome/lysosomal pathway. Jiang et al. reported that apoE dramatically enhances the endolytic degradation of β-amyloid peptides by neprilysin within microglia [43]. The capacity of apoE to promote β-amyloid degradation is dependent upon the apoE isoform and its lipidation status. ABCA1 is a key protein to influence apoE lipidation [35]. Inactivation of the ABCA1 gene in APP transgenic mice resulted in reduced levels of apoE, so that these mice exhibit a paradoxical elevation of brain β-amyloid peptide levels [44]. FGF-1 enhances not only apoE production but also cholesterol synthesis to upregulate apoE/HDL generation in astrocytes. Accordingly, FGF-1 is considered to have an indirectly important role to enhance clearance of β-amyloid from the brain through enhancing the level of apoE/HDL, which binds β-amyloid peptide. The clinical regulation of FGF-1 expression in the brain, therefore, is expected as a target for β–amyloid therapy.
Conclusion
It was described physiological significance and mechanism of FGF-1 release from astrocytes undergone oxidative stress. The release of cytosolic proteins such as HSP90 and HSP70 along with FGF-1 from astrocytes was greatly induced by the treatment with very low density of H2O2 as compared with other cell strains. In this sense, astrocytes have a higher performance of sensor to oxidative stress for cytosolic protein release. The change of cholesterol metabolism induced by oxidative stress contributes to release of cytosolic proteins in astrocytes. The release of cytosolic proteins from astrocytes is accompanied by FGF-1 release and it may be used for protection of neural cells against oxidative stress in the brain. It is possible that the FGF-1 released from stress-loaded astrocytes stimulates healthy astrocytes for the generation of apoE/HDL. The apoE/HDL is thought to function to protect neural cells against oxidative stress and β-amyloid. Thus, astrocytes have roles to remove or reduce β-amyloid peptides through a FGF-1/apoE/HDL system regulated by astrocytes in an autocrine manner.
References
- Van Horn MR, Sild M, Ruthazer ES (2013) D-serine as a gliotransmitter and its roles in brain development and disease. Front Cell Neurosci 7: 39.
- Butt AM (2011) ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin Cell Dev Biol 22: 205-213.
- Liu G, Zhao J, Chang Z, Guo G (2013) CaMKII activates ASK1 to induce apoptosis of spinal astrocytes under oxygen-glucose deprivation. Cell Mol Neurobiol 33: 543-549.
- Sanchez-Ortiz E, Hahm BK, Armstrong DL, Rossie S (2009) Protein phosphatase 5 protects neurons against amyloid-beta toxicity. J Neurochem 111: 391-402.
- Wang XF, Cynader MS (2000) Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem 74: 1434-1442.
- Dringen R, Pfeiffer B, Hamprecht B (1999) Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19: 562-569.
- Chung RS, Adlard PA, Dittmann J, Vickers JC, Chuah MI, et al. (2004) Neuron-glia communication: metallothionein expression is specifically up-regulated by astrocytes in response to neuronal injury. J Neurochem 88: 454-461.
- Eftekharpour E, Holmgren A, Juurlink BH (2000) Thioredoxin reductase and glutathione synthesis is upregulated by t-butylhydroquinone in cortical astrocytes but not in cortical neurons. Glia 31: 241-248.
- Lu R, Ito J, Iwamoto N, Nishimaki-Mogami T, Yokoyama S (2009) FGF-1 induces expression of LXRalpha and production of 25-hydroxycholesterol to upregulate the apoE gene in rat astrocytes. J Lipid Res 50: 1156-1164.
- Ueno S, Ito J-i, Nagayasu Y, Furukawa T, Yokoyama S (2002) An acidic fibroblast growth factor-like factor secreted into the brain cell culture medium upregulates apoE synthesis, HDL secretion and cholesterol metabolism in rat astrocytes. Biochim Biophys Acta 1589: 261-272.
- Nagayasu Y, Ito J, Nishida T, Yokoyama S (2008) Reactivity of astrocytes to fibroblast growth factor-1 for biogenesis of apolipoprotein E-high density lipoprotein is down-regulated by long-time secondary culture. J Biochem 143: 611-616.
- Ito J, Nagayasu Y, Hoshikawa M, Kato KH, Miura Y, et al. (2013) Enhancement of FGF-1 release along with cytosolic proteins from rat astrocytes by hydrogen peroxide. Brain Res 1522: 12-21.
- Eckenstein FP, Andersson C, Kuzis K, Woodward WR (1994) Distribution of acidic and basic fibroblast growth factors in the mature, injured and developing rat nervous system. Prog Brain Res 103: 55-64.
- Tooyama I, Akiyama H, McGeer PL, Hara Y, Yasuhara O, et al. (1991) Acidic fibroblast growth factor-like immunoreactivity in brain of Alzheimer patients. Neurosci Lett 121: 155-158.
- Mohiuddin L, Fernyhough P, Tomlinson DR (1996) Acidic fibroblast growth factor enhances neurite outgrowth and stimulates expression of GAP-43 and T alpha 1 alpha-tubulin in cultured neurones from adult rat dorsal root ganglia. Neurosci Lett 215: 111-114.
- Mason IJ (1994) The ins and outs of fibroblast growth factors. Cell 78: 547-552.
- Wiedlocha A, Nilsen T, Wesche J, Sorensen V, Malecki J, et al. (2005) Phosphorylation-regulated nucleocytoplasmic trafficking of internalized fibroblast growth factor-1. Mol Biol Cell 16: 794-810.
- Carreira CM, LaVallee TM, Tarantini F, Jackson A, Lathrop JT, et al. (1998). S100A13 is involved in the regulation of fibroblast growth factor-1 and P40 synaptotagmin-1 release in vitro. J Biol Chem 273: 22224-22231.
- Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, et al. (2003) The non-classical export routes: FGF1 and IL-1alpha point the way. J Cell Sci 116: 4871-4881.
- Mohan SK, Rani SG, Yu C (2010) The heterohexameric complex structure, a component in the non-classical pathway for fibroblast growth factor 1 (FGF1) secretion. J Biol Chem 285: 15464-15475.
- Jackson A, Friedman S, Zhan X, Engleka KA, Forough R, et al. (1992) Heat shock induces the release of fibroblast growth factor 1 from NIH3T3 cells. Proc Natl Acad Sci U S A 89: 10691-10695.
- Shin JT, Opalenik SR, Wehby JN, Mahesh VK, Jackson A, et al. (1996) Serum-starvation induces the extracellular appearance of FGF-1. Biochim Biophys Acta 1312: 27-38.
- Opalenik SR, Ding Q, Mallery SR, Thompson JA (1998) Glutathione depletion associated with the HIV-1 TAT protein mediates the extracellular appearance of acidic fibroblast growth factor. Arch Biochem Biophys 351: 17-26.
- Mouta Carreira C, Landriscina M, Bellum S, Prudovsky I, Maciag T (2001) The comparative release of FGF1 by hypoxia and temperature stress. Growth Factors 18: 277-285.
- Takami K, Matsuo A, Terai K, Walker DG, McGeer EG, et al. (1998) Fibroblast growth factor receptor-1 expression in the cortex and hippocampus in Alzheimer's disease. Brain Res 802: 89-97.
- Malecki J, Wesche J, Skjerpen CS, Wiedlocha A, Olsnes S (2004) Translocation of FGF-1 and FGF-2 across vesicular membranes occurs during G1-phase by a common mechanism. Mol Biol Cell 15: 801-814.
- Malecki J, Wiedlocha A, Wesche J, Olsnes S (2002) Vesicle transmembrane potential is required for translocation to the cytosol of externally added FGF-1. EMBO J 21: 4480-4490.
- LaVallee TM, Tarantini F, Gamble S, Carreira CM, Jackson A, et al. (1998) Synaptotagmin-1 is required for fibroblast growth factor-1 release. J Biol Chem 273: 22217-22223.
- Landriscina M, Bagala C, Mandinova A, Soldi R, Micussi I, et al. (2001) Copper induces the assembly of a multiprotein aggregate implicated in the release of fibroblast growth factor 1 in response to stress. J Biol Chem 276: 25549-25557.
- Tarantini F, LaVallee T, Jackson A, Gamble S, Mouta Carreira C, et al. (1998) The extravesicular domain of synaptotagmin-1 is released with the latent fibroblast growth factor-1 homodimer in response to heat shock. J Biol Chem 273: 22209-22216.
- Chapman ER, Davis AF (1998) Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J Biol Chem 273: 13995-14001.
- Graziani I, Bagalá C, Duarte M, Soldi R, Kolev V, et al. (2006) Release of FGF1 and p40 synaptotagmin 1 correlates with their membrane destabilizing ability. Biochem Biophys Res Commun 349: 192-199.
- Dietschy JM, Turley SD (2001) Cholesterol metabolism in the brain. Curr Opin Lipidol 12: 105-112.
- Linton MF, Gish R, Hubl ST, Bütler E, Esquivel C, et al. (1991) Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest 88: 270-281.
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, et al. (2004) ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem 279: 40987-40993.
- Mashayekhi F, Hadavi M, Vaziri HR, Naji M (2010) Increased acidic fibroblast growth factor concentrations in the serum and cerebrospinal fluid of patients with Alzheimer's disease. J Clin Neurosci 17: 357-359.
- Thorns V, Masliah E (1999) Evidence for neuroprotective effects of acidic fibroblast growth factor in Alzheimer disease. J Neuropathol Exp Neurol 58: 296-306.
- Kimura H, Tooyama I, McGeer PL (1994) Acidic FGF expression in the surroundings of senile plaques. Tohoku J Exp Med 174: 279-293.
- Niranjan R (2013) Molecular basis of etiological implications in Alzheimer's disease: focus on neuroinflammation. Mol Neurobiol 48: 412-428.
- Leduc V, Domenger D, De Beaumont L, Lalonde D, Bélanger-Jasmin S, et al. (2011) Function and comorbidities of apolipoprotein e in Alzheimer's disease. Int J Alzheimers Dis 2011: 974361.
- Fagan AM, Holtzman DM (2000) Astrocyte lipoproteins, effects of apoE on neuronal function, and role of apoE in amyloid-beta deposition in vivo. Microsc Res Tech 50: 297-304.
- Tokuda T, Matsubara C, Vidal R, Kumar A, Permanne B, et al. (2000) Lipidation of apolipoprotein E influences its isoform-specif interaction with Alzheimer's amyloid beta peptides. Biochem J 348: 359-365.
- Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, et al. (2008) ApoE promotes the proteolytic degradation of Abeta. Neuron 58: 681-693.
- Hirsch-Reinshagen V, Maia LF, Burgess BL, Blain JF, Naus KE, et al. (2005) The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem 280: 43243-43256.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 13656
- [From(publication date):
February-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9150
- PDF downloads : 4506