Overexpression of Pepper Capsaicinoid Pathway Genes in Tomato
Received: 08-Nov-2023 / Manuscript No. jpgb-23-119545 / Editor assigned: 10-Nov-2023 / PreQC No. jpgb-23-119545 (PQ) / Reviewed: 18-Nov-2023 / QC No. jpgb-23-119545 / Revised: 23-Nov-2023 / Manuscript No. jpgb-23-119545 (R) / Published Date: 30-Nov-2023 DOI: 10.4172/jpgb.1000176
Abstract
In this study we attempted to overexpress three pepper genes in tomato to produce spicy fruits. The three genes, BCAT (branched-chain amino acid aminotransferase), Kas (ketoacyl-ACP synthase) and CS/AT (Capsaicin synthase/acyltransferase), were separated by P2Am and T2Am sequences in a tricistronic cassette driven by the 35S promoter. The genes were expressed in transgenic tomato although tomato fruits were not spicy based on twoperson tasting evaluations. Further studies may be necessary with tissue-specific promoters.
Keywords
Capsaicinoid pathway genes; Overexpression; Pepper; Tomato
Introduction
The pungency or heat of hot pepper is the result of accumulation of group of alkaloids called capsaicinoids whose major representatives are capsaicin and dihydrocapsaicin [1]. The heat sensation created by these alkaloids is such defining aspect of these crop the genus name Capsicum come from Greek kapto, which means ‘to bite’ [2]. Capsaicinoids are synthesized in pepper fruit placenta with integration of two different biochemical pathways: the phenylpropanoid pathway uses phenylalanine as substrate to produce vanillylamine, and branched chain fatty acid pathway which produces 8-methyl nonenoic acid using valine as a substrate [3]. The enzyme CS (Capsaicin synthase) combines both compounds to produce capsaicin and other capsaicinoids by condensing the vanillylamine with different fatty acid substrates [2].
Chilli pepper is an indispensable spice used as a basic ingredient in varieties of cuisine all over the world, the nutritional value of Capsicum is high and is considered as an excellent source of vitamins C, A, B-complex and E along with minerals like molybdenum, manganese, folate, potassium, and thiamine [4]. Therapeutic properties of Capsicum are the result of capsaicinoids. As a medicine it is mainly used as counter irritant in lumbago, neuralgia, rheumatic disorder, and nonallergic rhinitis [5]. The plants have also been used as folk remedies for dropsy, colic, diarrhea, asthma, arthritis, muscle cramps and toothache [6]. All these effects suggest regular intake of capsaicinoid is beneficial for health and thus hot peppers should be considered as functional food [7].
Unlike other domesticated members of Solanaceae family such as tomato, potato, and tobacco, Capsicum species are notoriously labor intensive and difficult to cultivate. Pungent varieties are often cultivated in open fields and are vulnerable to environmental conditions that are detrimental to fruit yield [3]. Environmental factors such as high temperature, high CO2 level, and excess rain can all reduce the plant growth/yield and increase disease incidence in the plants [8]. Seed quality and germination rate are highly dependent on maturity of fruits, species, cultivar, and post-harvesting handling [9]. Even if all the agronomic hurdles are resolved, the highly variable capsaicinoid biosynthesis heavily controlled by environment represents further problem in consistent level of pungency production in pepper fruits [10].
Classical breeding and modern genetic manipulation can both be used for the improvements and consistent pungency levels in pepper. Breeding has contributed significantly to the creation of elite Capsicum varieties [11]. Agrobacterium mediated genetic transformation are notoriously hard for Capsicum due to its recalcitrant nature. Current available Capsicum transformation protocols have low efficiency, poor reproducibility, and high genotype-dependence [12,13]. Thus, genetic manipulation is not suitable option for pepper improvements [3]. Engineering alternate model plant to produce capsaicinoid could be a conceptually promising approach for production of these secondary metabolites [14].
Economically, tomato (Solanum lycopersicum) is the most important horticultural crop, and its yield is second only to potato across the world [15]. Despite their clade split at least 19 million years ago [16], genome of Capsicum and tomato are well preserved, with basic chromosome number of x=12 in both species and major conserved syntenic segments between them [17]. Tomato being wellestablished model with highly amenable biotechnological manipulation methods and its high productivity with short cropping cycle [18] can be used as capsaicinoid biofactory model plant [19]. Phylogenetic analysis of gene families involved in capsainoid biosynthesis in pepper and their orthologs in tomato, potato and Arabidopsis identified 51 gene families and of these 13 gene families had independent pepper specific duplication (such as ACLd, AT3, b-CT, C3H, CAD, CCR, Kas I and PAL genes) [17]. Comparative transcriptome analysis revealed several genes in capsaicin biosynthesis pathway having different expression in pepper and tomato fruits. Fruit specific expression of CS (encoding acyltransferase) primarily occurred during pepper placenta development along with other genes necessary for the capsaicinoid biosynthesis, contrary to this the orthologous genes in tomato pathway (BCAT, Kas and CS) are rarely expressed in fruiting stage [2]. Comparative expression studies in non -pungent peppers vs pungent pepper also showed large deletions in CS cause no or very low expression in non-pungent pepper; similar results were observed for other capsicionoid pathway genes as well. These results may indicate that changes in the gene expression of BCAT, Kas and CS/AT enabled capsaicinoid synthesis in hot pepper fruits [20,21]. Mutagenic studies in hot pepper also revealed loss of function in different genes affect the pungency differently. Pungency in pepper, therefore, appears to be under transcriptional control and is directly linked with higher expression levels of capsaicinoid biosynthesis genes in the placental septum of pungent cultivars [21,22]. Compared to chilli peppers, in tomato some genes have lower level of expression (PAL, C4H, ACL, AMT), others have lower level of expression with temporally restricted expression (COMT, FaTA) and few are not expressed at all (Kas, BCAT and CS) [2].
Based on the genetic information on both pepper and tomato, it is theoretically possible to activate capsaicinoid pathway in tomato. Three genome engineering strategies could be used for such endeavor. One is to use of transcriptional activators like effectors (TALEs) or CRISPR/Cas9 for multiplex activation of genes in pepper [23-25]. Second strategy is the use of targeted promoter replacement through genome engineering for activation of inactive genes in tomato [26,27]. And third would be insertion and overexpression of the pepper genes (Kas, BCAT and CS) in tomato through Agrobacterium mediated transformation of tomato.
For our study we employed Agrobacterium mediated tomato transformation to insert and overexpress the three pepper genes involved in capsaicin biosynthesis that were not expressed by tomato, namely BCAT, Kas and CS/AT. Two different vectors were prepared one with 35s promoter and one with fruit specific E8 promoter. All three genes were included in single tricistronic vector for the transformation (more in method). All three genes are involved in capsaicin biosynthesis through branched chain fatty acid pathway, BCAT (branched-chain amino acid aminotransferase) condenses valine to alpha ketoisovalerate, Kas (ketoacyl-ACP synthase) along with ACL (acyl carrier protein) is involved in fatty acid synthesis through isobutyryl-CoA to 8-methyl-6-nonenoic acid and CS/AT/PUN1 (Capsaicin synthase) condenses vanillylamine from phenylpropanoid pathway with 8-methyl-6-noneoyl-CoA from branched chain fatty acid pathway to synthesize capsaicin [2].
Material and Method
Preparation of overexpression gene construct
pCAMBIA1301 vector was used in preparation of overexpression gene construct. The tricistronic cassette with three pepper genes was arranged as shown in Figure 1A. The three genes were separated by P2Ap and T2Ap peptides (Figure 1B) from Osborn MJ [28]. The peptides were reverse translated to DNA sequence (P2A and T2A in (Figure 1B) and optimized for plants (Arabidopsis) using JCat [29] to produce the P2Am and T2Am (Figure 1B) used in Figure 1A. The cassette (Figure 1C) was synthesized by BioBasic (Amherst, NY, USA) and ligated into pCAMBIA1301 after digestion with NcoI and BstEII. The construct was used to transform Agrobacterium tumefaciens strain LBA4404 and positive clones were used for tomato transformation.
Figure 1: Tricistronic overexpression cassette. (A) Schematic presentation of the tricistronic cassette. (B) P2Am and T2Am sequences used to facilitate cleavage of the three proteins. (C) DNA sequence of the cassette (P2Am and T2Am are in bold/green). BCAT-branched-chain amino acid aminotransferase; Kas-ketoacyl- ACP synthase; CS/AT/PUN1-Capsaicin synthase/acyltransferase.
Agrobacterium preparation
Competent Agrobacterium tumefaciens strain LBA4404 cells were transformed with the construct described above using electroporation. A single colony from transformed Agrobacterium cells was inoculated into 10 ml LB broth with 50 mg l-1 kanamycin and grown for 24 hours at 28°C. An aliquot of the cultured cells was subsequently inoculated into 50 ml LB with 50 mg l-1 kanamycin and grown for another 24 hours. This culture was harvested and used for transformation described below.
Transformation of tomato
Tomato seeds (Solanum lycopersicum) cv Micro-Tom and Micro- Tina from Tomato Growers Supply Company, Fort Meyers, FL were surface sterilized in 40 ml of 25% bleach with 2 drops of tween 20 for 15 minutes and rinsed 5-7 times with distilled water before plating in seed germination medium (MS salt 4.3 g l-1, Nitsch vitamin 1 ml l-1, sucrose 30 g l-1 and agar 6 g l-1, pH 5.8). Hypocotyls and cotyledon leaves of 7-10 days old seedlings were used for transformation. Three days before transformation, Agrobacterium culture was started in 20 ml LB medium supplemented with 50 mg l-1 kanamycin. A day before transformation hypocotyl and cotyledon leaves were cut from seedling at the petioles and at the tip using sterile razor on the cocultivation media (MS salt 4.3 g l-1, thiamine-HCL 0.4 mg l-1, myo-inositol 100 mg l-1, sucrose 30 g l-1, 2,4-D 0.2 mg l-1, agar 6 g l-1 and kinetin 0.1 mg l-1) with sterile filter paper laid on the surface. Bacteria from LB broth was harvested by centrifugation and resuspended in cocultivation media (without agar). Leaves from overnight incubation were scrapped and mixed with bacteria and incubated at room temperature for 30 minutes with occasional mixing. Bacterial suspension was then drained, leaves were dried on sterile paper towels and returned to cocultivation media with filter paper on the surface. These plates were sealed with micropore tapes and incubated in dark at room temperature for 3 days. Following 3 days of cocultivation leaves were placed on regeneration medium (MS salts 4.3 g l-1, Nitsch vitamin ml l-1, sucrose 30 g l-1, zeatin 1.5 mg l-1, IAA 0.2 mg l-1, carbenicillin 400 mg l-1, hygromycin 30 mg l-1, agar 6 g l-1 and pH 5.8) and incubated under natural day/light cycle for 3-8 weeks with media change every 3 weeks. Shoots will regenerate during this period. Regenerated shoots were transferred to rooting medium (MS salts 4.3 g l-1, Nitsch vitamin 1ml l-1, sucrose 30 g l-1, IBA 0.5 mg l-1, carbenicillin 400 mg l-1, hygromycin 30 mg l-1, agar 6 g l-1 and pH 5.8) for further growth and rooting then transferred to soil after acclimatization for few days.
Transformation confirmation
Transformation and insertion of transgene was confirmed by plant regeneration on hygromycin containing regeneration media then by PCR using hygromycin primers (forward: GATGTTGGCGACCTCGTATT and reverse: GATGTAGGAGGGCGTGGATA) on DNA from transgenic plants.
RNA extraction
From T0 transgenic plants, RNA was extracted for RT-PCR. Leaf samples from young transgenic plants were bulked to isolate RNA. Fresh 50-100 mg of leaf tissue was frozen in liquid N2 and grinded to powder using mortar and pestle. One ml of TRIzol® reagent was used to homogenize 50-100 mg of tissue. After 5 minutes of incubation, 0.2 ml of chloroform was added to the sample and further incubated for 3 minutes. The mixture was centrifuged at 4°C for 15 minutes. The supernatant was transferred to a new tube and mixed with 0.5 ml of isopropanol and incubated for 10 minutes. After centrifuging at 4°C for 5 minutes, supernatant was discarded and RNA pellet was mixed with 1 ml of 75% ethanol and centrifuged for 5 minutes at 4°C, supernatant was discarded, and pellet were dried by inverting tubes on clean filter paper for 10 minutes. Around 25-30 μl of DNase/RNase free water was used to dissolve the RNA. Aliquot of this RNA was treated with DNase I at 37°C for 10 minutes. This followed phenol: chloroform extraction with isopropanol precipitation and two 75% ethanol wash. The purity of RNA was treated with DNase I and quantified using Nanodrop. Samples with 260/280 values between 1.9-2.1 were used for further analysis.
RT-PCR Semiquantitative RT-PCR was done to confirm the transgene expression of using qScript® XLT One-Step RT-PCR kit from QuantaBio in Applied Biosynthesis 2720 Thermocycler. Manufacturer’s guidelines were followed for reaction set up and thermocycler procedures. In short, RT-PCR was done for RNA of both transgenic and control plants in 10 μl volume. All the reagents, RNA and primers were thawed in ice for 15 minutes and the reaction was set up on ice. For each 10 μl reaction, 5 μl one-step ToughMix (20X), 0.2 μl each of forward and reverse primers, 0.4 μl of qScript® XLT One-Step reverse transcriptase (25X), 2.2 μl of nuclease free water and 2 μl of RNA were added and mixed. PCR plate was then spun briefly to remove any bubble and collect the content at the bottom of the well. In thermocycler, RT-PCR was programmed as follows: cDNA synthesis at 48°C for 20 minutes, initial denaturation 94°C for 3 minutes, 35 cycles of denaturation 94°C for 20 seconds, annealing 56°C for 30 seconds and extension at 72°C for 1 minute. This was followed by 72°C for 5 minutes and 4°C for forever. PCR product was run on 1% agarose gel along with loading dye to visualize the presence of transgene in RNA. Phenotypic observations Transgenic tomatoes were regularly checked for any visual phenotypic changes in their fruit shape, surface texture on early fruit stages to late mature stages. Ripened tomato fruits were tasted for spiciness by two people in lab. Results Transformation of tomato Two tomato lines (Micro-Tina and Micro-Tom) were used for the transformation studies. Construct with 35S promoter was used to transform Micro-Tina and construct with E8 promoter was used to transform Micro-Tom. Although the number of infected leaves was not counted, regeneration of callus from leaves was variable for both Micro-Tina and Micro-Tom line. Micro-Tom showed early advantage with better germination, fast growth compared to Micro-Tina (data not collected). During callus regeneration care was taken to place the leaves adaxial side up to increase the chance of regeneration. Care was taken not to overcrowd the plates and explants were subcultured every 2 weeks. Once calli started to produce the shoot, they were transferred to Magenta box to give enough nutrients and room for plantlets to grow faster. In total 30 transgenic plants from 35S vector and 35 transgenic lines from E8 vector were generated.
Confirmation of transgene insertion
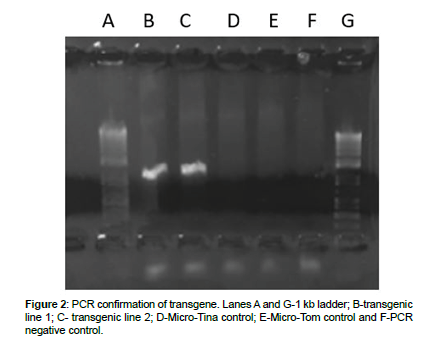
Genomic DNA was extracted from the leaves of transgenic and control tomato plants to perform PCR. Hygromycin primers were used to confirm the presence of transgene in T0 generation of transgenic plants (Figure 2). Hygromycin bands were observed in both Micro- Tina and Micro-Tom transgenic lines but were absent from control plants confirming the insertion of transgene in tomato genome. Gene expression
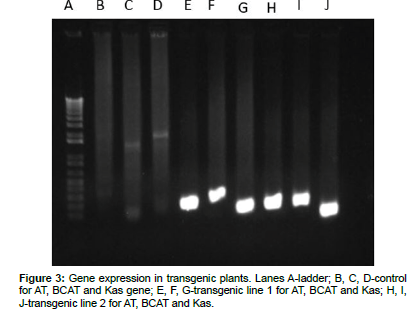
Expression of transgene was confirmed by semiquantitative RTPCR using pepper gene specific primers. For each primer set, once the primers were designed, they were blasted against tomato sequence to confirm their absence from tomato. For each of three gene one specific primer set was designed. RT-PCR was done using these primers on RNA derived from control as well as transgenic plants. Gel electrophoresis of the RT-PCR product showed the gene bands for all three target genes (AT, BCAT and Kas) in both transgenic lines tested while the control plant did not have any transgene expression (Figure 3). This shows all three pepper genes were integrated in tomato genome and were being expressed.
Phenotypic changes
Morphological changes: Transgenic plants were observed for any phenotypic structural changes in plant as well in fruits. There were no overwhelming changes that were consistent throughout all transgenic lines, some transgenic tomato plants, however, did produce fruits with pepper like rough skin.
Spiciness: Ripened transgenic tomato fruits were tasted for hint of hotness. Two people tasted the juice from the same fruit to determine whether there is any hotness in it. There was consensus, none of transgenic tomato fruits carried any spiciness on them. Although our results from RT-PCR suggested the expression of all three gene in transgenic plants, lack of hotness in fruit suggest either there is no detectable increase in capsaicin production, or the biosynthetic pathway requires more than 3 genes to be overexpressed.
Discussion
Tomato as a biofactory for production of secondary metabolites is not a new concept, it has been demonstrated tomato can be used in such purpose [30] were able to use tomato to produce betalain (food color). Other studies have used tomatoes to increase the production of various flavonoids [31], phenylpropanoid compounds like resveratrol and genistin [32]. Tomato fruits are rich in metabolites such as sucrose, hexoses, citrate, malate and ascorbic acids as well as secondary metabolites such as carotenoids, phenylpropoids and terpenoids is some extent [33]. The presence of such compounds suggests the presence of basic biosynthetic pathway for each in tomato. The presence of these biosynthetic pathways has led many to believe tomato can be used as a chassis to produce various economically as well as pharmacologically important metabolites such as retinol (Vitamin A) through B-carotene biosynthesis [34] and dioscin through steroidal glycoalkaloids production [35].
The presence of defunct capsaicinoid biosynthesis pathway in tomatoes offers an opportunity to produce capsaicin in tomatoes. This will alleviate the problems associated with pepper farming such as varying levels of pungency, environmental distress, slow seed germination and long-life cycle as well as high levels of soil borne diseases and nematode infection [3]. Both pepper and tomato being the member of same nightshade family (Solanaceae) have major conserved synteny with basic chromosome number of x=12 in both species and share many traits between them [18]. Comparative genomics revealed presence of all necessary capsaicinoid genes in tomato with varying expression [2]. Overexpression of those less expressed genes in tomatoes should in theory make tomato fruit spicy.
By inserting three capsaicinoid genes (BCAT, Kas and AT) we intended to activate the capsacinoid pathway in tomato. Agrobacterium mediated transformation method was successful in delivery of all three genes as evidenced by regeneration of hygromycin resistant transgenic plants and expression of three pepper genes as seen in RT-PCR. This, however, did not result in any increase in capsaicin synthesis or accumulation in tomato fruits. The generation of transgenic tomato plants expressing pepper gene, however, is a significant step toward the generation of spicy tomato. There could be many reasons why our plants were not producing spicy fruits. It could be that too little capsaicin to be detected by tasting, or the three genes might not be enough to activate whole capsaicin biosynthesis pathway as it comprises more than 51 gene families [18]. Gene duplication during capsicum evolution meant biosynthesis pathway has up to 13 pepper specific duplication compared to tomato [18], also tissue specific and developmental expression of genes involved in capsaicinoid biosynthesis could play role in making tomato spicy.
With phenotypic analysis we did not find any significant changes between transgenic and control tomatoes. Transgenic tomatoes in some cases, however, did have long stingers in early stages of fruit development and produced rough skin unlike smooth skin of control plants. These characters were not prevalent. We did not necessarily expect transgenic tomatoes to have altered shape or size, but these were some interesting observations.
Capsaicin is an important secondary metabolite that has been a central element in culinary and pharmacological activities in many countries and cultures. The demand for such a valuable product will likely increase in coming years. It is ideal we have alternate way of getting capsaicin without necessarily depending on unreliable and labor-intensive pepper farming. Tomatoes being close relative of pepper and having defunct capsaicin biosynthetic pathway could be answer as it has recently been attracting interest as a biofactory model for secondary metabolites.
Acknowledgements
CSP thanks the Graduate Student Organization (GSO) for their support. This project was supported in part by the University of Louisiana at Lafayette. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contribution
CSP performed all the experiments and wrote the manuscript; YHW conceived the project and finalized the manuscript. All authors have read and approved the manuscript for submission.
Competing Interest Statement
None declared.
Declaration of Generative AI in Scientific Writing
None declared.
References
- Aza-González C, Nunez-Palenius HG, Ochoa-Alejo N (2011) Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Rep 30: 695-706.
- Kim S, Park M, Yeom SI, Kim YM, Lee JM, et al. (2014) Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat Genet 46: 270-278.
- Naves ER, Silva L De A, Sulpice R, Araujo WL, Nunes-Nesi A, et al. (2019) Capsaicinoids: Pungency beyond Capsicum. Trends Plant Sci 24: 109-120.
- Simonne AH, Simonne EH, Eitenmiller RR, Mill HA, Green NR, et al. (1997) Ascorbic acid and pro-vitamin A contents in unusually colored bell peppers (Capsicum annuum L.). J Food Comp Analy 10: 299-311.
- Kothari SL, Joshi A, Kachhwaha S, Ochoa-Alejo N (2010) Chilli pepper- A review on tissue culture and transgenesis. Biotechnol Adv 28: 35-48.
- Ravishankar GA, Suresh B, Giridhar P, Rao SR, Johnson TS, et al. (2003) Biotechnological studies on Capsicum metabolite production and plant improvements. Capsicum: the genus Capsicum. London: CRC Press.
- Hardy G (2000) Nutraceuticals and functional foods: introduction and meaning. Nutrition 16: 688-689.
- Lee SG, Kim SK, Lee HJ, Lee HS, Lee JH, et al. (2018) Impact of moderate and extreme climate change scenarios of growth, morphological features, photosynthesis, and fruit production of hot pepper. Ecol Evol 8: 197-206.
- Demir I, Ellis RH (1992) Development of pepper (Capsicum annuum) seed quality. Ann App Biol 121: 385-399.
- Zewdie Y, Bosland PW (2000) Evaluation of genotype, environment, and the genotype-by-environment interaction for capsaicinoids in Capsicum annuum L. Euphytica 111: 185-190.
- Onus AN, Pickersgill B (2004) Unilateral incompatibility in Capsicum (Solanaceae): occurrence and taxonomic distribution. Ann Bot 94: 289-295.
- Heidmann I, Boutilier K (2015) Pepper, sweet (Capsicum annuum). Methods Mol Biol 1223: 321-334.
- Min J, Shin SH, Jeon EM, Park JM, Hyun JY, et al. (2015) Pepper, chilli (Capsicum annuum). Method Mol Biol 1223: 311-320
- Li Y, Wang H, Zhang Y, Martin C (2018) Can the world’s favorite fruit, tomato, provide an effective biosynthetic chassis for high-value metabolites?. Plant Cell Rep 37: 1443-1450.
- Peixoto JV, Neto Cm, Campos LF, Dourado WS, Nogueira AP, et al. (2017) Industrial tomato lines: morphological properties and productivity. Genet Mol Res 16: 1-15.
- Sarkinen T, Bohs L, Olmstead RG, Knapp S (2013) A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): a dated 1000-tip tree. BMC Evol Biol 13: 214.
- Qin C, Yu C, Shen Y, Fang X, Chen L, et al. (2014) Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc Natl Acad Sci USA 111: 5135-5140.
- Daniel JG (2013) Food and Agriculture Organization of the United Nations 2015. Indian J Med Res 138: 398-410.
- Jenkins T, Bovi A, Edwards R (2011) Plants: biofactories for a sustainable future? Philos Trans A Math Phys Eng Sci 369: 1826-1839.
- Stewart C, Kang BC, Liu K, Mazourek M, Moore SL, et al. (2005) Pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant J 42: 675-688.
- Kim M, Kim S, Kim S, Kim BD (2001) Isolation of cDNA clones differentially accumulated in the placenta of pungent pepper by suppression subtractive hybridization. Mole Cells 11: 213-219.
- Tanaka Y, Nakashima F, Kirii E, Goto T, Yoshida Y, et al. (2017) Difference in capsaicinoid biosynthesis gene expression in the pericarp reveals elevation of capsaicinoid contents in chilli peppers (Capsicum chinense). Plant Cell Rep 36: 267-279.
- Sanjana NE, Cong L, Zhou Y, Cunniff M, Feng G, et al. (2012) A transcriptional activator-like effector toolbox for genome engineering. Nat Protoc 7: 171-192.
- Loeder LG, Zhou J, Zhang Y, Malzahn A, Zhong Z, et al. (2018) Robust transcriptional activation in plants using multiplex CRISPR-Act2.0 and mTALE-Act systems. Mol Plant 11: 245-256.
- Songstad DD, Petolino JF, Voytas DF, Reichert NA (2017) Genome editing in plants. Crit Rev Plant Sci 36: 1-23.
- Hou H, Atlihan N, Lu ZX (2014) New biotechnology enhances the application of cisgenesis in plant breeding. Front Plant Sci 5: 389.
- Schouten HJ, Jacobsen E (2008) Cisgenesis and intragenesis, sisters in innovative plant breeding. Trends Plant Sci 13: 260-261.
- Osborn MJ, Panoskaltsis-Mortari A, McElmurry RT, Bell SK, Vignali DA, et al. (2008) A picornaviral 2A-like sequence-based tricistronic vector allowing for high-level therapeutic gene expression coupled to a dual-reporter system. Mol Ther 12: 569-74.
- Grote A, Hiller K, Scheer M, Münch R, Nörtemann B, et al. (2005) JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res 33: W526-31.
- Butelli E, Titta L, Giorgio M, Mock HP, Matros A, et al. (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26: 1301-1308.
- Tzin V, Rogachev I, Meir S, Moyal Ben Zvi M, Masci T, et al. (2013) Tomato fruits expressing bacterial feedback insensitive 3-deoxy-d-arabino-heptuloconate 7-phosphate synthase of shikimate pathway possess enhanced levels of multiple specialized metabolites and upgraded aroma. J Exp Bot 64: 4441-4452.
- Zhang Y, Butelli E, Alseekh S, Tohge T, Rallapalli G, et al. (2015) Multi-level engineering facilitates the production of phenylpropanoid compounds in tomato. Nat Commun 6: 8635-8655.
- Siddiqui MW, Ayalazavala JF, Dhua RS (2015) Genotypic variation in tomatoes affecting processing and antioxidant attributes. Crit Rev Food Sci Nutr 55: 1819-1835.
- Ru M, Wang K, Bai Z, Peng L, He S, et al. (2016) Molecular cloning and characterization of two enzymes involved in the rosmarinic acid biosynthesis pathway of Prunella vulgaris L. Plant Cell Tiss Organ Cult 128: 381-390.
- Sonawane PD, Pollier J, Panda S, Szymanski J, Massalha H, et al. (2016) Plant cholesterol biosynthetic pathways overlaps with phytosterol metabolism. Nat Plants 3: 16205-16218.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Prakash CS, Wang YH (2023) Overexpression of Pepper CapsaicinoidPathway Genes in Tomato. J Plant Genet Breed 7: 176. DOI: 10.4172/jpgb.1000176
Copyright: © 2023 Prakash CS, et al. This is an open-access article distributedunder the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided theoriginal author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1096
- [From(publication date): 0-2023 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 900
- PDF downloads: 196