Outline of Drug Bio Analytical Specimen Methods
Received: 03-Dec-2022 / Manuscript No. jabt-22-83661 / Editor assigned: 05-Dec-2022 / PreQC No. jabt-22-83661 / Reviewed: 19-Dec-2022 / QC No. jabt-22-83661 / Revised: 21-Dec-2022 / Manuscript No. jabt-22-83661 / Accepted Date: 27-Dec-2022 / Published Date: 28-Dec-2022 QI No. / jabt-22-83661
Abstract
Due to the complexity and individual problems presented by each biological matrix, sample preparation is regarded as the bottleneck phase in bio analysis. An essential stage in every bio analytical technique is competent sample preparation to extract the required analyses and remove superfluous components. In the processing of bio analytical samples, the matrix effect is a significant barrier that has drawn a lot of attention. Over the past ten years, novel sample preparation methods have gained popularity due to their advantages over traditional methods in terms of accuracy, automation, simplicity of sample preparation, storage, and shipment. Our goal is to give a thorough overview of recent advancements in a variety of bioanalytical sample preparation methods for chromatographic and spectroscopic analyses. With the help of preferred instances, it is also indicated how these procedures have attracted a lot of attention in bio analytical research during the past 10 years. The focus is mostly on current developments in sorbent-based micro extraction techniques and other modern trends in bioanalytical sample preparation.
Keywords
Bio analysis; Bioactive matrices; The matrices effect; Microscopic extraction; Sample preparation methods
Introduction
Due to the continual requirement to achieve improved sensitivity, accuracy, and speed of analysis in complex biofluids, the development of bioanalytical sample preparation techniques has grown more difficult over time (e.g., blood, serum, plasma, saliva, feces, and urine). Additionally, samples must frequently be preconcentrated prior to analysis due to the analytes’ low concentration. But frequently, this raises the amounts of components that interfere, including small molecules (like medicines, salts, and metabolites) or large molecules (e.g., nucleic acids, proteins, and peptides). As a result, precise and selective bioanalysis for regulatory reasons requires extremely specific sample clean-up procedures [1]. These investigations subsequently provide regulatory filing support for documents including investigational new drug applications, regular new drug applications, and abbreviated new drug applications [2]. Therefore, before they can be used in actual sample analysis, bioanalytical sample preparation techniques need to be properly validated. The majority of biological samples contain significant levels of salts, lipids, proteins, carbohydrates, and other endogenous substances. They can interfere with the preferred trace analytes through matrix effects, hence getting rid of them is the main goal of sample preparation before analysis. Additionally, numerous bioanalytical studies using solid-phase extraction and liquid-liquid extraction (LLE) have been published (SPE). Due to their advantages in clinical studies, electromembrane extraction (EME) and dispersive liquid-liquid microextraction (DLLME) have recently gained in popularity. Therefore, to speed up bioanalytical research, fresh sample preparation and microfluidics-based procedures must continually be improved. In this article, we examine recent works that discuss sample preparation methods used in bioanalytics. This article’s goal is to discuss the fundamentals, benefits and drawbacks, and practicability of bioanalytical laboratories based on the combined knowledge and experiences of the authors, not to be all-inclusive.
Case Presentation
Biological matrices relevant in bioanalysis
Numerous biological matrices (such as blood, plasma, serum, urine, hair, human breast milk, saliva, perspiration, cerebrospinal fluid (CSF), and tissue) must be examined in bioanalytical research.
Additionally, each matrix has different difficulties. For instance, plasma has higher levels of phospholipids while urine has higher levels of salt [3]. Biofluids, such as blood, serum, plasma, saliva, perspiration, urine, and tissue, are frequently employed in bioanalysis in conventional practise [4]. In recent years, other biological specimens have included hair, human breast milk, and faeces. Hair has a high degree of deterioration in post-mortem investigations [4] and is a stable, robust matrix that is simple to handle and infrequently messed with during collection. Excellent indicators of pharmaceuticals and environmental contaminants can be found in human breast milk [5]. Some herbal medications may be digested by intestinal bacteria and expelled in faeces, which is comparable to excretion in breast milk. Feces can be difficult for analytical systems to process because they are nondigested, nonhomogeneous, complicated, and full of macromolecules and particles. Feces metabolic monitoring globally is difficult from a biochemical and analytical perspective [6]. Below is a brief overview of biological samples [7, 8].
Blood, plasma, and serum
Different types of blood cells suspended in plasma make up blood. In humans, plasma makes up around 55% of the blood fluid and contains substances including glucose, proteins, hormones, minerals, and blood cells. Blood’s liquid and soluble component, serum, is free of fibrinogens. It has a wide range of metabolites that can be employed in the diagnosis of numerous serious illnesses as well as a wide range of clinical problems [9].
Feces
Fecal matter, which is the waste produced by the human body, typically consists of inedible food particles, inorganic materials (such as calcium and iron phosphate), and a small amount of dead bacteria. The intestinal microflora may metabolise some medications, which are then eliminated in the stool. The examination of herbal medicines metabolised by the intestinal microbiota uses the faecal sample as an excellent specimen. The person is typically expected to fast before sampling the faeces. Following collection, the faecal samples are stored in normal saline for later processing. Fecal analysis is largely used in clinical settings to diagnose illnesses of the pancreas, liver, and digestive system.
Urine
Ninety-five percent of urine is made up of water, along with inorganic ions including sodium, phosphate, and ammonia, urea, creatinine, proteins, and coloured blood breakdown products (e.g., urochrome). Prior diagnostic and prognostic biomarkers of conditions such urinary tract infections and chronic kidney illnesses are anticipated to be monitored using urine metabolomics methods [10].
Tissue
A collection of cells with comparable roles and physical characteristics make form tissues. They can be divided into three groups: soft tissues, hard tissues, and tissues with a harsh exterior. Soft tissues are easy to handle, such as the lung, liver, kidney, brain, and spleen. The proper techniques must be used on hard tissues, such as the placenta, artery, heart, and stomach. Due to possible low concentrations, small sample sizes, and the hard makeup of skin, quantifying medicines in the skin can be difficult. Hard tissues, such as cartilage, skeletal muscle, nails, bones, and hair, go through a conventional, well-defined collecting process. Therefore, accurate sample preparation is necessary for all tissues before analysis can begin. For clinical diagnostic purposes, such as the detection of tumours and cancer, tissues are crucial.
CSF
The choroid plexus, which is found in the spinal cord, subarachnoid space, and ventricles of the brain, produces around 80% of the central nervous system’s (CNS) secretory fluid, or CSF. The CSF metabolome can provide numerous clinically significant insights into serious CNS diseases, such as Parkinson’s disease, multiple sclerosis, brain damage, and Guillain-Barre syndrome.
Techniques For Biological Studies For Medical Diagnostics
In order to obtain these advantages, recent studies have concentrated on the development of sample preparation methods. Robotics-based sample preparation techniques such as SPE, LPME, and LLE have been cybernated, creating new and beautiful perspectives on bioanalysis. Solid-phase microextraction (SPME) was first proposed by Arthur and Pawliszyn in 1990. The SPME approach, which combines sampling, preconcentration, and extraction in one step, is not thorough. This method’s advantages include quick and easy operation, excellent accuracy, improved sample cleanup, and reduced solvent usage. The simultaneous preconcentration and separation of volatile and nonvolatile materials is offered by SPME.
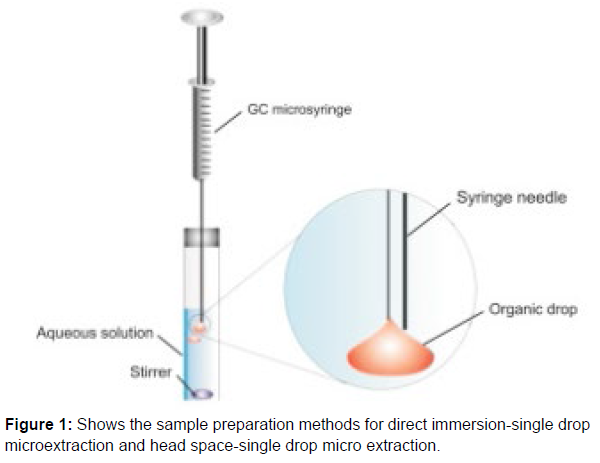
Single drop micro extraction (SDME)
In order to obtain these advantages, recent studies have concentrated on the development of sample preparation methods. Robotics-based sample preparation techniques such as SPE, LPME, and LLE have been cybernated, creating new and beautiful perspectives on bioanalysis.
Solid-phase microextraction (SPME) was first proposed by Arthur and Pawliszyn in 1990. The SPME approach, which combines sampling, preconcentration, and extraction in one step, is not thorough. This method’s advantages (Figure 1) include quick and easy operation, excellent accuracy, improved sample cleanup, and reduced solvent usage. The simultaneous preconcentration and separation of volatile and nonvolatile materials is offered by SPME. Single droplet extraction (SDME) was used as the extraction medium. Typically, the microdrop contains an organic solvent in the amount of 1 to 10 L. There is no carryover in a straightforward, affordable, and environmentally friendly microextraction method. Small solvent volume usage makes SDME an environmentally favourable analytical procedure that generates little to no waste.
Stir bar sorptive micro extraction (SBSME)
Baltussen first introduced SBSME, a sorptive extraction-based system, in 1999. The deposition position is different from that in the SPME, and that is the sole difference. In SBSME, extraction and desorption are essential procedures. During the analysis, a stir bar with a polydimethylsiloxane coating was submerged deep in the sample solution. A little more than 125 L of sorbent is used in commercial SBSME, which is more than SPME uses. SBSME cannot be employed for the analysis of very hydrophilic substances due to the covering material’s hydrophobicity. Researchers have suggested using dualphase stir bars, improved coating materials, molecularly imprinted polymers (MIPs), and monolithic materials to get around this problem. For coating purposes in SBSME, recently developed materials such monoliths, metal-organic frameworks (MOFs), carbon nanotubes, graphene, graphene oxide, and porous organic polymers are helpful.
Conclusion
This technique is used very early in the drug development process to provide support to drug discovery programs on the metabolic fate and pharmacokinetics of chemicals in living cells and in animals.
References
- Burlikowska K, Stryjak I, Bogusiewicz J (2020) Comparison of metabolomic profiles of organs in mice of different strains based on SPME-LC-HRMS. Metabolites 10:1-10.
- Beutler E, Waalen J (2006) The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 107: 1747-1750.
- US Department of Health and Human Services. (n.d.). Blood tests - blood tests. National Heart Lung and Blood Institute.
- Sundermann FW (1956) Status of clinical hemoglobinometry in the United States. Am J Clin Pathol 43: 9-15.
- Wolf HU, Lang W, Zander R (1984) Alkaline haematin D-575, a new tool for the determination of haemoglobin as an alternative to the cyanhaemiglobin method. II. Standardisation of the method using pure chlorohaemin. Clin Chim Acta 136: 95-104.
- Shah VB, Shah BS, Puranik GV (2011) Evaluation of non cyanide methods for hemoglobin estimation. Indian J Pathol Micr 54: 764-768.
- Karakochuk CD, Hess SY, Moorthy D, Namaste S, Parker ME, et al. (2019) Measurement and interpretation of hemoglobin concentration in clinical and field settings: a narrative review. Ann NY Acad Sci 1450: 126-146.
- Kang SH, Kim HK, Ham CK, Lee DS, Cho HI (2008) Comparison of four hematology analyzers, CELL-DYN Sapphire, ADVIA 120, Coulter LH 750, and Sysmex XE-2100, in terms of clinical usefulness. Int J Lab Hem 30: 480-486.
- Whitehead Jr RD, Zhang M, Sternberg MR, Schleicher RL, Drammeh B, et al. (2017) Effects of preanalytical factors on hemoglobin measurement: A comparison of two HemoCue point-of-care analyzers. Clin Biochem 50: 513-520.
- Ingram CF, Lewis SM (2000) Clinical use of WHO haemoglobin colour scale: validation and critique. J Clin Pathol 53: 933-937.
Indexed in, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Ingle RG (2022) Outline of Drug Bio Analytical Specimen Methods. J Anal Bioanal Tech 13: 488.
Copyright: © 2022 Ingle RG. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 1189
- [From(publication date): 0-2022 - Apr 11, 2025]
- Breakdown by view type
- HTML page views: 872
- PDF downloads: 317