Outbreak of SARS-CoV-2 Associated with Omicron Variant Infection in Healthcare Workers in Japan
Received: 04-Jun-2022 / Manuscript No. JIDT-22-65895 / Editor assigned: 07-Jun-2022 / PreQC No. JIDT-22-65895 (PQ) / Reviewed: 21-Jun-2022 / QC No. JIDT-22-65895 / Revised: 28-Jun-2022 / Manuscript No. JIDT-22-65895 (R) / Published Date: 05-Jul-2022 DOI: 10.4172/2332-0877.1000503
Abstract
In Japan, the SARS-CoV-2 Omicron (B.1.1.529) variant has been spreading rapidly since the first case was detected in November 2021. Although this variant has been reported to have immune escape potential and SARS-CoV-2 mRNA vaccines have been reported to be less effective against this variant, the protective effect can be increased by a third dose of vaccine. In National Hospital Organization Hakodate National Hospital, an outbreak of the Omicron variant involving more than 30 Healthcare Workers (HCWs) and 90 hospital inpatients occurred in February 2022, despite most hospital staff having received three doses of vaccine. 95% confidence intervals for the ratio of the median antibody titers were 0.71 to 1.86 and there was no significant difference in antibody titers after the third vaccination between infected and non-infected HCWs. Although all cases of SARS-CoV-2 infection in HCWs were asymptomatic or mild, a third dose of mRNA vaccine may be insufficient to prevent infection with the Omicron variant or vaccine-induced antibody titers may not reflect the preventive effect against Omicron.
Keywords: SARS-CoV-2 Omicron variant; Outbreak; mRNA vaccine; Booster dose; Healthcare workers
About The Study
Since the SARS-CoV-2 B.1.1.529 (Omicron) variant was first reported in South Africa in November 2021, it has been designated as a variant of concern by the World Health Organization and has been spreading rapidly worldwide [1]. In Japan, as in other countries, it is believed that the Omicron variant has taken over from the Delta (B.1.617.2) variant as the dominant SARS-CoV-2 strain. In this situation, accelerated delivery of a third (booster) dose of vaccine is expected to play an important role in preventing infection by the Omicron variant [2,3]. Although the Omicron variant has higher infectivity and is less susceptible to neutralizing antibodies induced by vaccination [4,5], administering a booster dose of mRNA vaccine has maintained its effectiveness to protect against infection by the Omicron variant by induction of neutralizing antibody [4-7].
In the National Hospital Organization (NHO) Hakodate National Hospital, the first confirmed cases in Healthcare Workers (HCWs) were diagnosed on January 31, 2022. As of March 1, 93 hospital inpatients and 37 HCWs had tested positive for SARS-CoV-2 and the hospital was considered to have an outbreak. Although most HCWs working in the hospital had received a booster dose of BNT162b2 (Comirnaty®; Pfizer- BioNTech) vaccine, 32 HCWs vaccinated with three doses experienced breakthrough infections, presumed to be due to the Omicron variant.
The effectiveness of three doses of mRNA vaccine on prevention of infection by the Omicron variant in HCWs and the relationship between antibody titer and susceptibility to infection are still unclear. In this study, we documented breakthrough SARS-CoV-2 infections in HCWs during the hospital outbreak, and evaluated the association between the serum anti-SARS-CoV-2 spike antibody response after vaccination and prevention of SARS-CoV-2 infection.
In NHO Hakodate National Hospital, 91.5% (421/460) of HCWs received a booster dose of BNT162b2 vaccine from December 1 to January 6, approximately 8-9 months after their second vaccination. The serum antibody titers were measured 2 weeks after the third vaccination for those who have completed vaccination by December 8, 2021 using Elecsys® Anti-SARS-CoV-2 S on the Cobas 8000 e601 Analyzer (Roche Diagnostics Rotkreuz, Switzerland). This assay is an electrochemiluminescence immunoassay that detects anti-SARS- CoV-2 spike antibody (predominantly IgG). The measurement range of the assay is 0.4-100000 U/mL (in 400-fold diluted samples). Data on the characteristics of study participants and the symptoms of those with infection were collected from medical records and by telephone interviews. The severity of the symptoms was evaluated according to COVID-19 Treatment Guidelines presented by National Institute of Health [8].
This study was approved by the NHO Hakodate National Hospital Ethics Committee on Clinical Research (#R3-0728001), and was conducted in compliance with the Declaration of Helsinki and all applicable national regulations. All study participants provided oral consent to participate in this study.
The difference in the median of the common logarithm for antibody titer between groups was assessed, and its 95% Confidence Intervals was estimated by bootstrap percentile method with 1,000,000 repetition. All statistical analyses were conducted using SAS®Ver. 9.4 (SAS Institute Inc., Cary, NC, USA).
Immediately after the first SARS-CoV-2 infection in a HCW was confirmed, HCWs who had potential direct or indirect exposure to infected patients or infectious materials were screened for SARS-CoV-2 infection using a Polymerase Chain Reaction (PCR) test. Potential for exposure was defined as having been within 2.0 m of an infected person for 15 minutes or longer in a 24-hour period or an overlap in work assignment in the same unit or ward as an infected person during the 7-days prior to their testing positive (based on close contact or an epidemiological link).
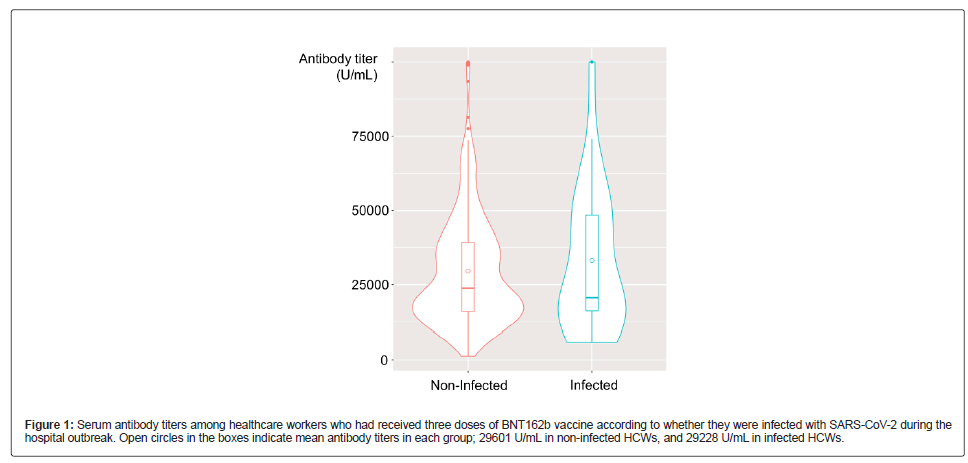
By March 1, 2022, 444 HCWs composed of 131 males and 313 females, including 239 nurses, 40 nurse assistants and 35 physicians were tested for SARS-CoV-2 since January 31. Among them, 37 (8.3%) had a positive PCR result considered to be related to this outbreak. The infected HCWs comprised 8 males and 29 females, including 31 nurses, 2 nurse assistants, 2 physicians, 1 radiological technologist, and 1 physical therapist. The characteristics of the infected HCWs are provided in Table 1. The symptoms were generally mild and none of the infected HCWs required medication and hospital admission. The median antibody titer was 20,570 U/mL (range: 5,941, >100,000; IQR:13,392, 51,353) in infected HCWs, and 23,836 U/mL (range: 1,237, >100,000; IQR:15,935, 39,208) in non-infected HCWs (Figure 1). The difference in the median of the common logarithm for antibody titer 2 weeks after a booster dose was -0.07 (95% CI: -0.15, 0.27), that is, the ratio of the median antibody titer of positive-persons to negative- persons was around 0.71 to 1.86 (Table 1 and Figure 1).
| No | Age | Sex | Occupation1 | Total number of doses | Days from 3rd Dose to Diagnosis | Antibody titer (U/mL)2 | Symptoms | Severity | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Sore throat | Cough | ||||||||
| 1 | 32 | F | RN | 2 | (-) | (-) | + | + | + | Mild |
| 2 | 53 | F | RN | 3 | 59 | 5941 | - | - | - | Asymptomatic |
| 3 | 27 | F | RN | 3 | 59 | 7088 | + | - | + | Mild |
| 4 | 35 | F | RN | 3 | 54 | 13392 | + | + | + | Mild |
| 5 | 22 | F | RN | 3 | 38 | (-) | - | - | - | Asymptomatic |
| 6 | 30 | F | RN | 3 | 60 | 45599 | + | + | + | Mild |
| 7 | 26 | M | RN | 3 | 60 | 39562 | + | + | - | Mild |
| 8 | 28 | F | RN | 3 | 46 | (-) | - | - | - | Asymptomatic |
| 9 | 27 | M | PT | 3 | 42 | (-) | - | + | - | Mild |
| 10 | 38 | M | RN | 2 | (-) | (-) | + | + | + | Mild |
| 11 | 37 | F | RN | 3 | 59 | 20479 | + | + | - | Mild |
| 12 | 40 | F | MD | 3 | 61 | 18901 | - | - | - | Asymptomatic |
| 13 | 31 | M | RN | 3 | 62 | 12236 | - | - | - | Asymptomatic |
| 14 | 43 | F | RN | 3 | 55 | 51353 | - | - | - | Asymptomatic |
| 15 | 44 | M | RN | 3 | 40 | (-) | - | - | - | Asymptomatic |
| 16 | 37 | F | RN | 3 | 62 | 10547 | - | - | - | Asymptomatic |
| 17 | 25 | F | RN | 3 | 55 | 40075 | - | - | - | Asymptomatic |
| 18 | 28 | F | RN | 3 | 61 | 23925 | - | + | - | Mild |
| 19 | 37 | F | RN | 3 | 22 | (-) | - | - | - | Asymptomatic |
| 20 | 25 | F | RN | 3 | 63 | 56889 | - | - | - | Asymptomatic |
| 21 | 45 | F | RN | 3 | 63 | 7742 | - | - | - | Asymptomatic |
| 22 | 55 | F | NA | 3 | 0 | (-) | - | - | - | Asymptomatic |
| 23 | 55 | F | NA | 0 | (-) | (-) | + | + | + | Mild |
| 24 | 57 | F | RN | 3 | 60 | 42752 | - | - | - | Asymptomatic |
| 25 | 23 | F | RN | 3 | 30 | (-) | - | + | - | Mild |
| 26 | 34 | F | RN | 3 | 66 | 19681 | - | - | - | Asymptomatic |
| 27 | 26 | M | RN | 3 | 64 | 74003 | - | - | - | Asymptomatic |
| 28 | 49 | M | MD | 3 | 65 | 19462 | - | + | - | Mild |
| 29 | 24 | F | RN | 3 | 62 | 57918 | - | + | - | Mild |
| 30 | 24 | F | RN | 3 | 62 | 20570 | + | + | + | Mild |
| 31 | 48 | F | RN | 3 | 65 | 19702 | - | + | - | Mild |
| 32 | 39 | M | RT | 2 | (-) | (-) | - | + | + | Mild |
| 33 | 37 | F | RN | 3 | 50 | (-) | - | + | - | Mild |
| 34 | 31 | F | RN | 3 | 56 | (-) | + | + | - | Mild |
| 35 | 62 | F | RN | 3 | 74 | 10000 | - | + | - | Mild |
| 36 | 49 | F | RN | 3 | 74 | 54432 | - | + | - | Mild |
| 37 | 44 | F | RN | 0 | (-) | (-) | + | - | - | Mild |
Note: 1 RN, Registered Nurse; MD. Medical Doctor; NA, Nurse Assistant; PT, Physical Therapist; RT, Radiologic Technologist; 2There is no data for antibody titer for those who have not received a booster dose or vaccinated after December 8, 2021.
Table 1: Characteristics of infected healthcare workers
Figure 1: Serum antibody titers among healthcare workers who had received three doses of BNT162b vaccine according to whether they were infected with SARS-CoV-2 during the hospital outbreak. Open circles in the boxes indicate mean antibody titers in each group; 29601 U/mL in non-infected HCWs, and 29228 U/mL in infected HCWs.
In NHO Hakodate Hospital, the HCWs showed relatively high titer of the anti-spike antibody 5 months after two doses of BNT162b2 mRNA vaccination and no SARS-CoV-2 infections were diagnosed in HCWs before the Omicron variant emerged [9].
However, despite a marked increase in antibody titers following booster vaccination and appropriate use of personal protective equipment, isolation, and facility zoning, vaccinated HCWs were still susceptible to SARS-CoV-2 infection while in a high-risk hospital environment. The hospital is located in a cold region of Japan and the outbreak occurred during the winter, so poor ventilation combined with a severe climate might have contributed to the outbreak [10].
Among the HCWs infected with SARS-CoV-2, only two were tested to determine the variant type and they were both confirmed to have the variant other than Delta. As the Omicron variant was the dominant SARS-CoV-2 strain in Japan at the time of the outbreak [11], Omicron was presumed to be associated with the outbreak.
Since the Omicron variant rapidly spread worldwide, an increased number of cases of breakthrough infection have been reported among individuals who have received three doses of mRNA vaccine [12,13]. In this study, among the HCWs tested, 32 of 406 (7.9%) who had finished three-dose vaccine, 3 of 27 (11%) who had received only two doses and 2 of 11 (18%) who were unvaccinated became infected with SARS-CoV-2. In this outbreak, we found that there was no significant difference in antibody titers between the infected and non-infected HCWs. The Omicron variant is much more transmissible than other variants, and even though booster vaccination increases serum antibody titers [6], this may be insufficient to prevent infection [4].
Nevertheless, the booster vaccination has been shown to protect against severe illness caused by Omicron [14,15]. Though all cases of infection were asymptomatic or mild, it was unclear from our study whether booster vaccination contributed to reducing the severity of the disease. It should be mentioned that the infected HCWs in this outbreak were relatively healthy and young. The mean age was 36.9 years (range 22-51) and had a low risk of developing severe illness. In addition, 38% of infected HCWs had no data for antibody titers, and it was also a limitation of this study.
Conclusion
To our knowledge, this is the first report of a hospital outbreak of breakthrough infection with the Omicron variant among HCWs in Japan who have received a booster dose of mRNA vaccine. These findings suggest three doses of the BNT162b2 vaccine may be insufficient to prevent infection with Omicron or vaccine-induced antibody titers may not reflect the preventive effect against Omicron in HCWs, although no severe symptoms were noted. Further studies of the effectiveness of a booster dose against Omicron are underway.
Acknowledgment
We thank Yoko Kuriyama, Makoto Takahashi, Michio Sato, Takashi Fujita, Yuya Sato, Fuko Imai, and all the participants of this study as well as the staff at the National Hospital Organization Hakodate National Hospital supporting the COVID-19 vaccination program.
Conflict of Interest
IY reports grants from KAKENHI, AMED, and Health, Labour and Welfare Policy Research Grants, research fund by Nihon Medi-Physics, and speaker fees from Chugai Pharmaceutical Co, AstraZeneca PLC, Japan Tobacco Pharmaceutic Division, and Nippon Shinyaku Co., outside the submitted work. All other authors declare no competing interests.
References
- World Health Organization (2022) Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. 26 November 2021.
- Spitzer A, Angel Y, Marudi O, Zeltser D, Saiag E, et al. (2022) Association of a third dose of BNT162b2 vaccine with incidence of SARS-CoV-2 infection among health care workers in Israel. JAMA 327:341-349.
[Crossref] [Google Scholar] [PubMed]
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, et al. (2021) Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med 385:1393-1400.
[Crossref] [Google Scholar] [PubMed]
- Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, et al. (2022) The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 185:447-456.
[Crossref] [Google Scholar] [PubMed]
- Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, et al. (2022) mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 185:457-466.
[Crossref] [Google Scholar] [PubMed]
- Gruell H, Vanshylla K, Tober-Lau P, Hillus D, Schommers P, et al. (2022) mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med 185:457-466.
[Crossref] [Google Scholar] [PubMed]
- Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, et al. (2022) Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. N Engl J Med 386:492-494.
[Crossref] [Google Scholar] [PubMed]
- National Institute of Health. (2021) Coronavirus Disease 2019 (COVID-19) Treament Guidelines. 19 October 2021.
- Otsuka S, Hiraoka K, Suzuoki M, Ujiie H, Kato T, et al. (2022) Antibody responses induced by the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in a single community hospital in Japan. J Infect Chemother 28:539-542.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. (2021) Coronavirus disease (COVID-19): Ventilation and air conditioning. 23 December 2021.
- National Institute of Infectious Diseases Japan. (2022) Current situation of infection, February 9, 2022.
- Kuhlmann C, Mayer CK, Claassen M, Maponga T, Burgers WA, et al. (2022) Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet 399:625-626.
[Crossref] [Google Scholar] [PubMed]
- Loconsole D, Bisceglia L, Centrone F, Sallustio A, Accogli M, et al. (2022) Autochthonous outbreak of SARS-CoV-2 Omicron variant in booster-vaccinated (3 doses) healthcare workers in Southern Italy: Just the tip of the iceberg? Vaccines 10:283.
[Crossref] [Google Scholar] [PubMed]
- Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, et al. (2022) Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA 327:639-651.
[Crossref] [Google Scholar] [PubMed]
- Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, et al. (2021) Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 398:2093-2100.
[Crossref] [Google Scholar] [PubMed]
Citation: Otsuka S, Hiraoka K, Suzuoki M, Ujiie H, Kato T, et al. (2022) Outbreak of SARS-CoV-2 Associated with Omicron Variant Infection in Healthcare Workers in Japan. J Infect Dis Ther 10: 503. DOI: 10.4172/2332-0877.1000503
Copyright: © 2022 Otsuka S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2265
- [From(publication date): 0-2022 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1877
- PDF downloads: 388