Osteopontin Isoforms b and c Mediate Prostate Cancer Cell Evasion
Received: 10-Aug-2021 / Accepted Date: 09-Sep-2021 / Published Date: 16-Sep-2021 DOI: 10.4172/1165-158X.1000207
Abstract
Background: Osteopontin (OPN), an extracellular structural protein, along VEGF are attributed to cancer progression and angiogenesis. Therefore, we aimed to evaluate the effect of Curcumin and anti-androgen drug, Flutamide (separately and in combination) on the expression of OPN isoforms and VEGF.
Methods: The human prostate cell lines were treated with different concentrations of Curcumin and prostate cancer conventional drug Flutamide alone and combined to find effective doses and IC50 values. Percentages of apoptotic cells were evaluated by Annexin/PI staining, and mRNA levels of OPN isoforms and VEGF gene expression were investigated by the real-time PCR method.
Results: Our data displayed that Flutamide (20 μM and 12 μM in PC3 and LNCaP cell lines, respectively), Curcumin (15 μM and 10 μM in PC3 and LNCaP cell lines, respectively), and also their combination with same above concentrations significantly increase the percentage of apoptotic cells with upregulation of tumor suppressor P53 gene, and downregulation of anti-apoptotic Bcl-2 gene. Also, it causes overexpression of OPN-b and OPN-c isoforms and VEGF gene in androgen-independent (PC3) and OPN-c isoform and VEGF in androgen-dependent (LNCaP) prostate
Conclusion: We show for the first time that prostate cancer cells probably evade Curcumin and Flutamide antiandrogenic effects via induction of OPN-b and OPN-c isoforms, which are recognized factors with the capacity of promoting cancer progression and angiogenesis. The correlation between androgen-independency and OPN-b isoforms needs further exploration.
Keywords: Prostate cancer; Curcumin; Flutamide; Osteopontin; VEGF; Angiogenesis; Resistance
Abbreviations
ADT: Androgen-Deprivation Therapy; AR: Androgen Receptor; Bcl2: B-Cell Leukemia/Lymphoma 2; IC50: Half Maximal Inhibitory Concentration; DMSO: Dimethyl Sulfoxide; EDTA: Ethylene Diaminetetraacetic Acid; EGFR: Epidermal Growth Factor Receptor; ELISA: Enzyme-Linked Immunosorbent Assay; FBS: Fetal Bovine Serum; MMP: Matrix Metalloproteinase; MTT: Microculture Tetrazolium Test; NF-κB: Nuclear Factor-κB; OPN: Osteopontin; PBS: Phosphate-Buffered Saline; PCa: Prostate Cancer; PCR: Polymerase Chain Reaction; PI: Propidium Iodide; RT-PCR: Real-Time PCR; SD: Standard Deviation; STAT3: Signal Transducer and Activator of Transcription 3; VEGF: Vascular Endothelial Growth Factor
Introduction
Prostate cancer accounts for approximately 30% of all cancers and 9.8% of cancer-related mortality in men as the most common type of malignancy among the male population [1,2]. Radical prostatectomy, radiation therapy, chemotherapy, and anti-androgen therapy are the mainstays of the current prostate cancer management [3,4]; however, these therapeutic measures are suspected with limitations in their survival and quality of life benefits while imposing significant morbidity [5]. Prostate cancer has the potential for extensive metastasis with secondary lesions in the bones, lymph nodes, and other organs.
Metastasis of the tumoral cells relies on the formation of new blood vessels from the existing ones in a process known as angiogenesis [6,7]. This fundamental process is facilitated through several autocrine and paracrine factors. Vascular Endothelial Growth Factor (VEGF), one of the most potent angiogenic factors, has been found in prostate cells of benign and malignant types [8,9]. VEGF plays a pivotal role in microvascular remodeling and angiogenesis-related to prostate cancer progression, metastasis, and osteolysis [10,11].
Osteopontin (OPN), an integrin-binding glycophosphoprotein in the extracellular matrix, can be produced by the neoplastic cells to augment the metastatic ability via regulation of VEGF secretion and angiogenesis [12,13]. However, because of three alternative splicing isoforms (OPN-a, OPN-b, and OPN-c), OPN exerts heterogenous functions regarding cancer progression [14]. OPN-c isoform overexpression modulates angiogenesis, apoptosis, adhesion, invasion, and metastasis in prostate cancer cell lines [14-16].
Targeting these pathways related to prostate cancer angiogenesis may alter the clinical course and metastatic extension of the patients with prostate cancer. Curcumin, a yellow polyphenolic compound derived from the plant turmeric, has recently received great attention as an anti-carcinogenic agent with the potential to abrogate pathological angiogenesis with a safe profile [17,18]. This natural product possesses chemo preventive effects by targeting numerous signaling pathways, such as Nuclear Factor-κB (NF-κB) [19], Activating Protein-1 (AP-1) [20], PI3K/Akt [21], Cyclin D1 [22], Wnt/ß-catenin [23], and Androgen Receptor (AR) [24,25] in both androgen-dependent (LNCaP) and androgen-independent (PC3) prostate cancer cell lines. Curcumin demonstrates anti-angiogenic properties by modulating the OPN/αvβ3 pathway [26]; however, the exact role of OPN isoforms and the effect of Curcumin on these isoforms in prostate cancer progression and angiogenesis remain to be answered.
We aimed to evaluate whether Curcumin isolated and in combination with prostate cancer anti-androgen Flutamide, has the potency to preclude angiogenesis and carcinogenic effects of OPN isoforms and VEGF gene in androgen-dependent (LNCaP) and androgen-independent (PC3) prostate cancer cell lines.
Materials and Methods
Cell lines and cell culture
The human prostate cell linesPC3 (ATCC Number: CRL-1435, NCBI Code: C427) -obtained from androgen resistant metastatic prostate cancer patients and LNCaP (ATCC Number: CRL-10995, NCBI Code: C439) as androgen-dependent lineage were purchased from the National Cell Bank stipulated in conflict of interest section. These cells were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA) supplemented with amino acid mixtures, including four male L-glutamine and 10% heat-inactivated fetal bovine serum (Gibco, Carlsbad, CA), 1000 units/ml Penicillin, and 100 μg/ml streptomycin (Gibco BRL, Grand Island, NY). Cells were incubated in a humid atmosphere at 5% CO2 at 37°C in a Memmert CO2 incubator. The drugs used in this study, Flutamide: 2-Methyl-N-[4-Nitro-3-(trifluoromethyl) Phenyl] Propanamide with CAS Number 13311-84-7 and Curcumin were purchased from the Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO). To prepare a stoke for treat cell lines, Curcumin and Flutamide was dissolved in DMSO (Sigma-Aldrich, St. Louis, MO, USA). RPMI- 1640 medium with 0.1% of DMSO was used as control.
Different methods for determining the survival of prostate cancer cells in response to treatment
Evaluating cell survival in pharmacological and cancer studies is important to determine cell sensitivity and treatment outcome. Determination of cell survival is essential in cell culture-based studies and is used to test various drugs and determine their chemical toxicity. Standard three colorimetric methods of trypan blue, 3- (4, 5- dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide, and finally clonogenic assay are used to determine the survival of cancer cell lines. The trypan blue staining is based on the impermeability of the dye to through cell membrane. Trypan blue is widely used to stain dead cells. In this method, cell survival is determined by counting unstained cells under a microscope. A cell that can reproduce and form a colony is clonogenic, and the loss of this ability leads to reproductive death. This definition applies to cancer cells and cells with a high proliferation rate, and the lack of this ability indicates cell death. Accordingly, after the cell has been exposed to various toxins, the cell may still be healthy and able to produce protein and make new DNA. However, if it loses its ability to multiply, it is considered dead. In this method, the number of colonies is counted as cell survival by microscopy. Exponentially growing prostate cell lines were detached by 1% trypsin-EDTA (sterile-filtered, Bio Reagent, Gibco BRL, Grand Island, NY) in RPMI 1640 medium. A growth inhibition assay and cell viability of the prostate cancer PC3 and LNCaP cell lines were carried out to determine the growth inhibition effect of Flutamide and Curcumin using trypan blue, clonogenic assay, and MTT assay.
Trypan blue dye: The treated prostate cells with Flutamide and Curcumin were centrifuged and re-suspended with 0.1 ml of PBS solution. Then 20 μl of the cell suspension was mixed with 20 μl of trypan blue dye 0.4%, and after 1 or 2 min, 10 μl of the resulting mixture was placed on a Neubauer slide. The cells with the damaged membrane turned blue, and the living cells remained colorless and completely transparent. The cells were counted, and the percentage of cell survival was calculated as (total number of cells - stained cells)/total number of cells) × 100. All experiments were carried out independently three times.
Microculture tetrazolium test (MTT): To measure the inhibitory effect of Flutamide and Curcumin on the metabolic activity of PC3 and LNCaP cells by MTT Assay (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide, Sigma-Aldrich, St. Louis, MO, USA), the following was performed: Both PC3 and LNCaP cell lines were seeded at 5 × 103/mL per well in 96-well plates treated for 24 hr, 48 hr and 72 hr in absence and presence of various concentrations of Flutamide and Curcumin and their combinations at 37°C with 5% CO2 saturation.
At selected times, 100 μl of 0.5 mg/mL MTT solution prepared fresh in RPMI-1640 without FBS each time it was used was added to the cells and incubated for 4 hours at 37°C to metabolize this solution. The formed formazan crystals were dissolved with 100 μl of dimethyl sulfoxide (DMSO), and a purple-colored solution was formed. The optical density was read at an absorbance of 570 nm wavelength in an ELISA microplate reader (MPR4+), Hyperion, Medizintechnik GmbH & Co.KG, Germany). The experiment was accomplished in triplicate (n=3) to determine the anti-proliferative effect of the IC50 (half maximal inhibitory concentration) of Flutamide and Curcumin against the PC3 and LNCaP cell lines. Dose-response curves were plotted, and IC50, an inhibitory concentration of 50% of the control cell’s growth, was calculated by Graph-Pad PRISM software version 8 (San Diego, CA).
In vitro 3D colony formation assay: A colony-forming assay was performed to evaluate the invasiveness of prostate cancer cells in a cell culture medium. For this purpose, 2% and 0.7% sterile agarose were prepared and stored in the laboratory water bath at 45°C. The cells were harvested and poured on 2% agar in a six-well plate (2 × 105 cells/well) with RPMI culture medium, 0.7% agarose, and 10% FBS serum. The plate was incubated at 37°C, and after 14 days, each well was examined for colony formation under an inverted microscope. Cells accumulate 50 or more like a colony, and a set of 3 to 50 cells was obtained as a cluster. All experiments were carried out independently three times.
Measurement of cell apoptosis by flow cytometry
Briefly, one of the available methods for studying apoptosis is the analysis and study of Phosphatidylserine (PS) molecules present on the membrane’s outer surface during the apoptotic process. Annexin-V is a phospholipid-binding protein in the presence of calcium ions. This substance has a high affinity for the PS molecule. Therefore, it is very suitable for detecting cells undergoing apoptosis in a cell population. The Annexin-V-Fluos co-staining assay kit contains both V-FITC (fluorescein isothiocyanate) and PI dye (propidium iodide), making it possible to distinguish and clean apoptotic cells from necrotic cells. Annexin-V-Flous diagnostic kit was used to count apoptotic cells [27]. First, the 1 × 105 cells/ml were cultured in a 6-well plate at 1640 RPMI medium containing 10% serum and incubated for 24 hours at 37°C and 5% CO2. Flutamide and Curcumin were then added to the wells at specific concentrations (for cell death in this test to be controlled and significant, a concentration lower than the lethal concentration of 50% or LD50 was used) in triplicate, and incubation was continued for 48 hours. Cells were collected after treatment and washed twice with PBS. Then, PI and Annexin-v were added to the cell suspension in the binding buffer. The cells were incubated in the dark for 15 minutes at 37°C and analyzed using flow cytometry. Cell discrimination was performed in the form of apoptosis (Annexin+/PI- [early apoptosis] and Annexin+/PI+ [late apoptosis]).
DNA cell cycle analysis
Both Prostate cell lines PC3 and LNCaP (5 × 105 cells/well), permeabilized and treated with specified concentrations of Flutamide and Curcumin for 48h, were washed with PBS, afterward fixed with 70% cold ethanol 24 hr. Cells were washed twice with PBS and incubated at 37°C for 30 mins with RNase I and dye DNA with 500 μL Propidium Iodide (PI) (50 μg/mL in 0.1% Triton X-100/0.1% sodium citrate). Cells were separated by BD flow cytometer set and performed with the Flowjo software (Tree Star Inc., version 9.6.3, USA). According to PI staining, both Prostate cell lines were measured with sub-G0/G1 pattern (before DNA synthesis) as apoptotic cells.
RNA extraction, cDNA synthesis and gene expression analysis by real-time PCR
TriPure Isolation Reagent (Roche Applied Science, Peuzberg, Germany) was used to extract the total RNA of the cells according to the manufacturer’s instructions. Colibri Microvolume Spectrometer (Titertek-Berthold.AZoNetwork UK Ltd., Manchester) was used to measure the quantity of RNA concentration of the samples. The cDNA synthesis kit (Takara Bio Inc., Otsu, Japan) was used for generating cDNA from RNA with Thermocycler Senso Quest Lab cycler, Germany.
Real-time PCR was performed with QIAGEN’s real-time PCR cycler, the Rotor-Gene Q instrument according to the following protocol to adjust the total volume to 20 μl; 2-fold diluted cDNA solution (2 μl), from each forward and reverse primers (10 pmol, 0.5 μl), nuclease-free water; Depc Water (7 μl) and finally SYBR Premix Ex Taq technology (Takara Bio Inc, Otsu, Japan, 10 μl). The specificity of PCR reactions was confirmed by melting curve analysis. To estimate the relative expression levels, we investigated GAPDH mRNA expression levels and the 2−ΔΔCT method used for calculating the relative expression. The PCR temperature program included a pre-denaturation step at 95°C for 30 seconds, followed by 40 cycles including the denaturation phase at 95°C for 5 seconds and a combined annealing/extension phase at 60°C for 20 seconds. To amplify each gene fragment, a specific primer pair was selected for each gene, which included the Forward and Reverse primers. All primers are certified with Primer-BLAST online software (NCBI website, Table 1).
| Gene | Accession Number | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Size (bp) |
|---|---|---|---|---|
| OPN-a | NM_001040058.1 | ATCTCCTAGCCCCACAGAAT | CATCAGACTGGTGAGAATCATC | 208 |
| OPN-b | NM_000582.2 | ATCTCCTAGCCCCAGAGAC | AAAATCAGTGACCAGTTCATCAG | 209 |
| OPN-c | NM_001040060.1 | TGAGGAAAAGCAGAATGCTG | GTCAATGGAGTCCTGGCTGT | 155 |
| VEGF | NM_001316955.1 | CTCACCAAGGCCAGCACATAGG | ATCTGGTTCCGAAAACCCTGAG | 159 |
| Bcl2 | NM_000633 | GGGGAGGATTGTGGCCTTC | CAGGGCGATGTTGTCCACC | 90 |
| P53 | NM-011640 | AGACCTATGGAAACTACTTC | GGACAGCATCAAATCATC | 76 |
| GAPDH | NM-001289746.1 | GTGAACCATGAGAAGTATGACAAC | CATGAGTCCTTCCACGATACC | 123 |
Table 1: Nucleotide sequences of the Osteopontin specific isoforms and VEGF primers used for real-time PCR.
Statistical analysis
All data were presented as means ± Standard Deviation (SD) of triplicate determinants. Analysis of variance (ANOVA) method and T-test were used to evaluate the results. Statistical significance was defined at *P<0.05, **P<0.01, and ***P<0.001 compared to the corresponding control.
Results
Morphological changes
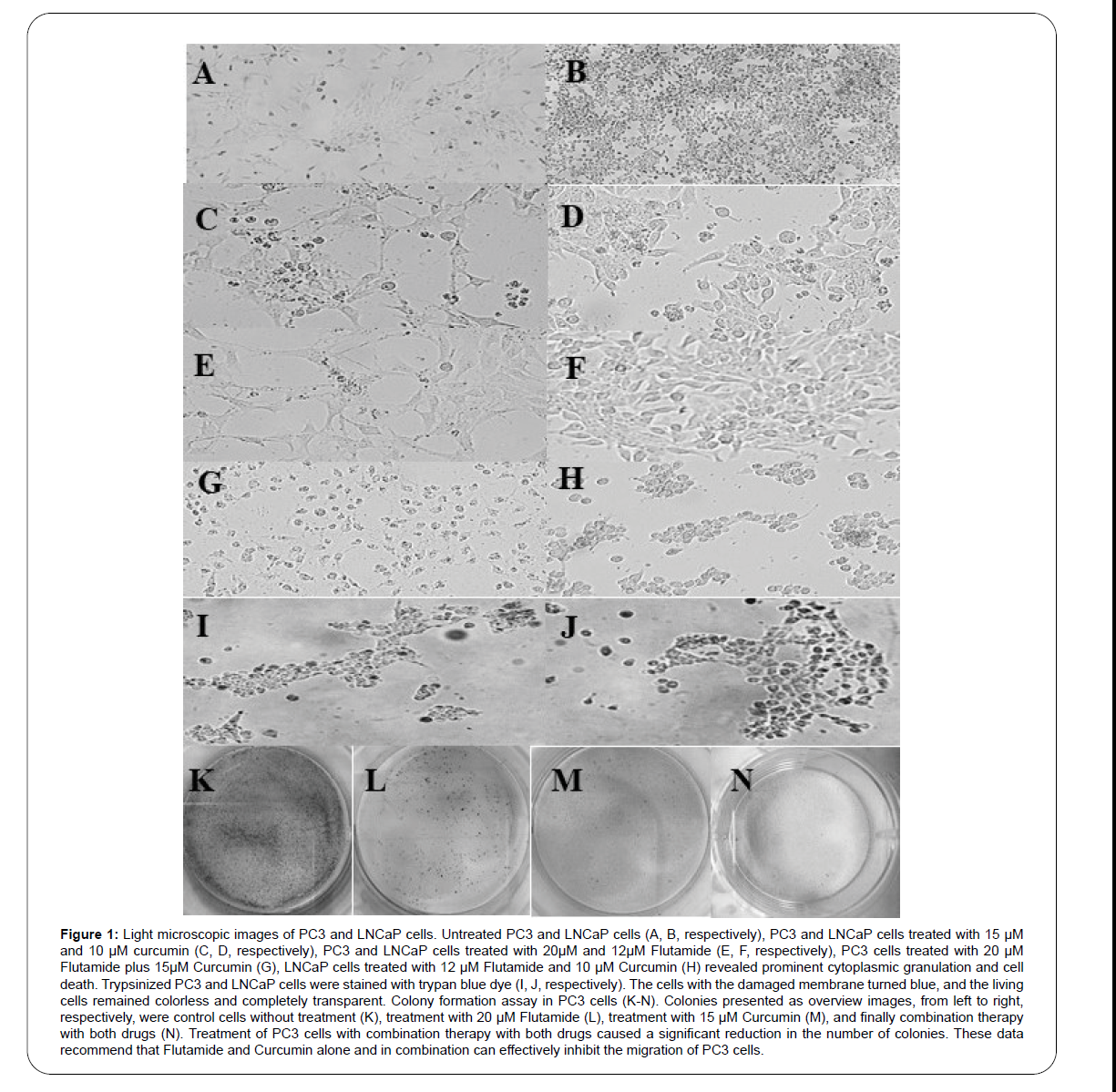
Morphological changes of PC3 and LNCaP cells were examined using an inverted microscope. As shown in Figure 1, the cells changed such as shrinkage, rounding, and membrane protrusion after treatment with Flutamide and Curcumin. These morphological changes suggest that Flutamide and Curcumin may induce apoptosis in PC3 and LNCaP cells.
Figure 1: Light microscopic images of PC3 and LNCaP cells. Untreated PC3 and LNCaP cells (A, B, respectively), PC3 and LNCaP cells treated with 15 μM and 10 μM curcumin (C, D, respectively), PC3 and LNCaP cells treated with 20μM and 12μM Flutamide (E, F, respectively), PC3 cells treated with 20 μM Flutamide plus 15μM Curcumin (G), LNCaP cells treated with 12 μM Flutamide and 10 μM Curcumin (H) revealed prominent cytoplasmic granulation and cell death. Trypsinized PC3 and LNCaP cells were stained with trypan blue dye (I, J, respectively). The cells with the damaged membrane turned blue, and the living cells remained colorless and completely transparent. Colony formation assay in PC3 cells (K-N). Colonies presented as overview images, from left to right, respectively, were control cells without treatment (K), treatment with 20 μM Flutamide (L), treatment with 15 μM Curcumin (M), and finally combination therapy with both drugs (N). Treatment of PC3 cells with combination therapy with both drugs caused a significant reduction in the number of colonies. These data recommend that Flutamide and Curcumin alone and in combination can effectively inhibit the migration of PC3 cells.
Evaluation of cytotoxicity
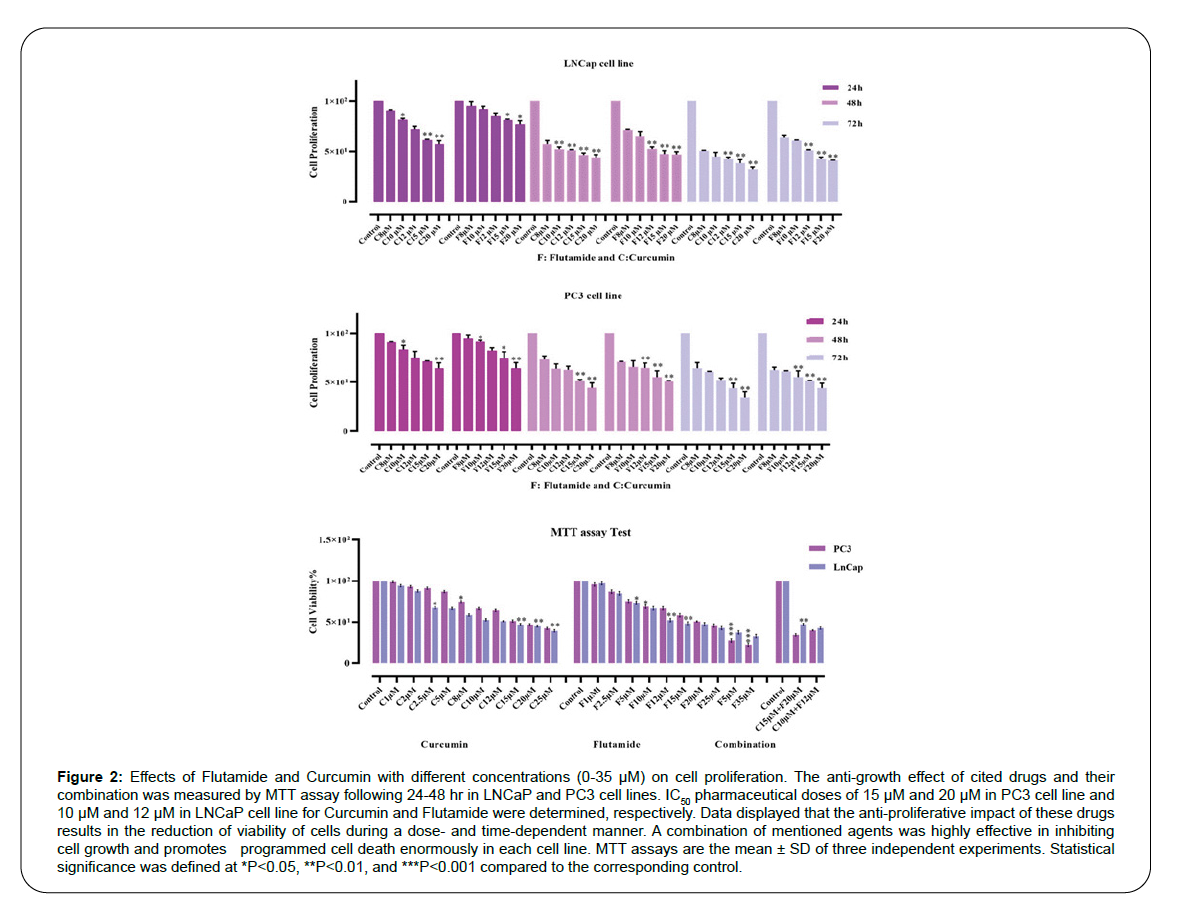
MTT assay was used to evaluate the effect of Flutamide and Curcumin on LNCaP and PC3 cells. First, a lethal concentration of 50% of cells or LD50 was obtained for Flutamide and Curcumin. For cell death to be controlled and significant, a concentration lower than LD50 was used. Figure 2 shows the effect of these two drugs on LNCaP and PC3 cell lines in three time periods of 24, 48, and 72 hours. Cell culture was used for control at the same time without any treatment. The results of this study indicate that Flutamide and Curcumin reduced cell survival in all three time periods. This indicates dose- and timedependent cell survival (Table 2). However, at 72 hours, the reduction in cell survival was more severe than the other two times and was statistically significant. The results are shown as the average of two independent experiments.
Figure 2:Effects of Flutamide and Curcumin with different concentrations (0-35 μM) on cell proliferation. The anti-growth effect of cited drugs and their combination was measured by MTT assay following 24-48 hr in LNCaP and PC3 cell lines. IC50 pharmaceutical doses of 15 μM and 20 μM in PC3 cell line and 10 μM and 12 μM in LNCaP cell line for Curcumin and Flutamide were determined, respectively. Data displayed that the anti-proliferative impact of these drugs results in the reduction of viability of cells during a dose- and time-dependent manner. A combination of mentioned agents was highly effective in inhibiting cell growth and promotes programmed cell death enormously in each cell line. MTT assays are the mean ± SD of three independent experiments. Statistical significance was defined at *P<0.05, **P<0.01, and ***P<0.001 compared to the corresponding control.
| Cells Treated for 24 hr | |||||
|---|---|---|---|---|---|
| Cell line / Dose | 8µM | 10 µM | 12 µM | 15 µM | 20 µM |
| LNCaP+Flutamide | 98.3 ± 2.2 | 94.1 ± 2.3 | 87.2 ± 2.7 | 81.6 ± 2.6 | 79.3 ± 1.7 |
| LNCaP +Curcumin | 91.3 ± 1.1 | 82.2 ± 3.1 | 74.1 ± 2.2 | 61.5 ± 3.4 | 59.4 ± 1.4 |
| PC3+Flutamide | 97.1 ± 2.4 | 92.1 ± 2.5 | 84.2 ± 3.2 | 79.1 ± 1.1 | 68.2 ± 4.1 |
| PC3+Curcumin | 90.4 ± 3.4 | 86.3 ± 3.5 | 79.5 ± 2.4 | 71.4 ± 3.3 | 68 ± 4.1 |
| Cells Treated for 48 hr | |||||
| LNCaP+Flutamide | 71.8 ± 1.9 | 68.4 ± 2.3 | 53.8 ± 1.1 | 49.7 ± 2.3 | 48.8 ± 1.6 |
| LNCaP+Curcumin | 59.5 ± 1.4 | 53.7 ± 1.4 | 51.5 ± 1.3 | 47.8 ± 2.3 | 45.9 ± 1.2 |
| PC3+Flutamide | 70.1 ± 2.0 | 69.9 ± 2.1 | 67.9 ± 1.3 | 59.7 ± 1.7 | 51.2 ± 2.1 |
| PC3+Curcumin | 75.7 ± 2.0 | 67.1 ± 1.9 | 65.1 ± 1.6 | 52.3 ± 1.4 | 47.7 ± 1.0 |
| Cells Treated for 72 hr | |||||
| LNCaP+Flutamide | 65.4 ± 3.3 | 60.2 ± 2.8 | 51.4 ± 3.4 | 43.7 ± 1.1 | 40.6 ± 2.3 |
| LNCaP+Curcumin | 50.1 ± 2.3 | 47.7 ± 2.7 | 43.5 ± 3.9 | 40.6 ± 4.4 | 33.5 ± 2.3 |
| PC3+Flutamide | 64.3 ± 2.7 | 61.5 ± 2.9 | 59.8 ± 3.5 | 51.7 ± 2.3 | 47.4 ± 2.2 |
| PC3+Curcumin | 68.2 ± 1.0 | 60.3 ± 3.4 | 53.7 ± 2.6 | 47.3 ± 3.5 | 38.7 ± 2.4 |
Table 2: Data obtained from MTT assay on the cell viability of PC3 and LNCaP cell lines treated with Flutamide and Curcumin (8, 10, 12, 15, and 20 μM) for 24, 48, and 72 hr.
Flutamide and Curcumin kill about 30% of cells in the first 24 hours of treatment. In comparison, the rate of this effect in 72 hours is more than 60%.
Investigation of apoptosis
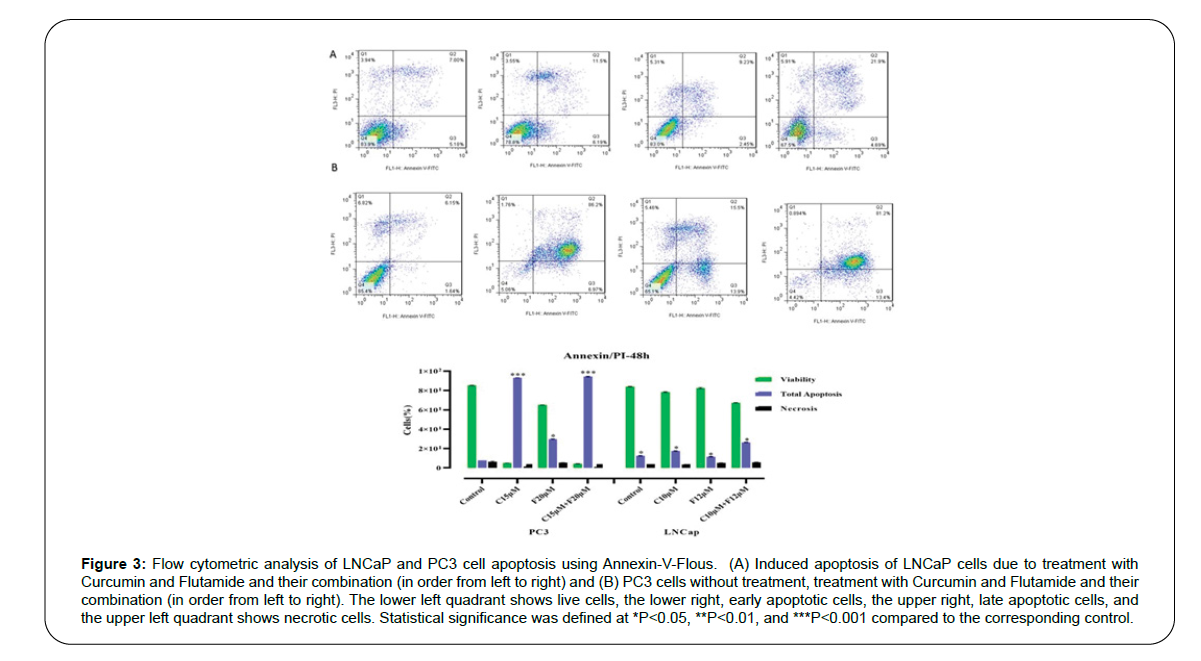
Apoptosis was determined by staining the cells using the AnnexinVFlous kit and flow cytometry. Primary apoptosis was detected by positive staining for Annexin-V-FITC and secondary apoptosis by positive staining for both Annexin-V and PI. LNCaP and PC3 cells were treated with Flutamide and Curcumin for 48 hours before flow cytometric analysis. Cells without any treatment were considered as controls. As shown in Figure 3, the untreated control cells showed nearly 2% primary apoptosis and 6% secondary apoptosis, respectively; while, Flutamide and Curcumin-treated cells showed 60% primary apoptosis and 70% secondary apoptosis, respectively. This indicates that treatment of LNCaP and PC3 cells with Flutamide and Curcumin induced apoptosis.
Figure 3:Flow cytometric analysis of LNCaP and PC3 cell apoptosis using Annexin-V-Flous. (A) Induced apoptosis of LNCaP cells due to treatment with Curcumin and Flutamide and their combination (in order from left to right) and (B) PC3 cells without treatment, treatment with Curcumin and Flutamide and their combination (in order from left to right). The lower left quadrant shows live cells, the lower right, early apoptotic cells, the upper right, late apoptotic cells, and the upper left quadrant shows necrotic cells. Statistical significance was defined at *P<0.05, **P<0.01, and ***P<0.001 compared to the corresponding control.
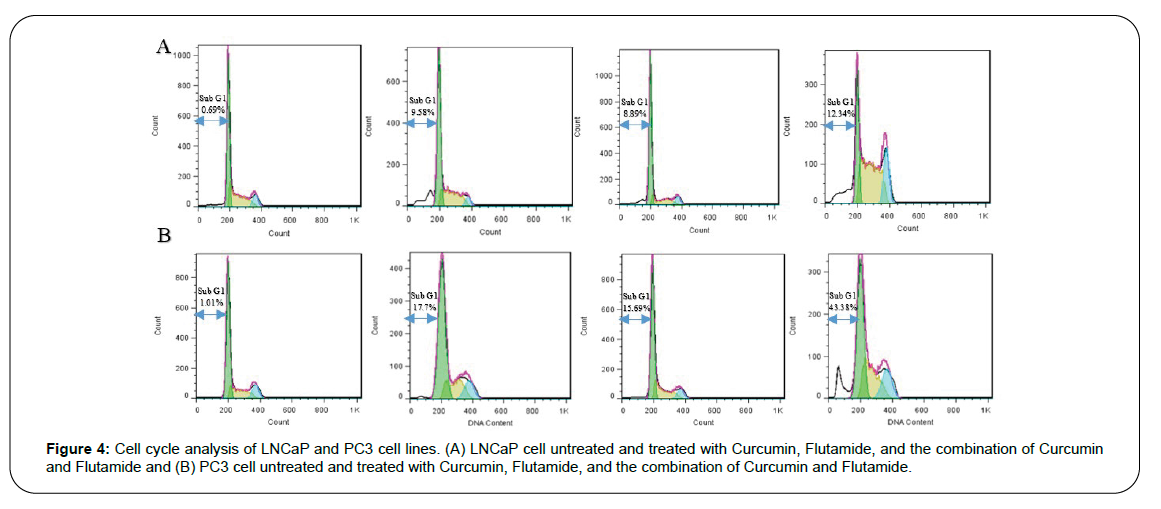
Since cell cycle modification and inhibition are generally the preludes to most processes, including cell growth inhibition and cell apoptosis, the effects of Curcumin and Flutamide and their combination on the cell cycle of PI-stained treated prostate cancer cell lines were investigated by flow cytometry.
Among the control PC3 cell line, cells in stages sub-G1, G1, S, and G2 accounted for 1.01%, 64.71%, 23.22%, and 13.23% of the total cell population, respectively. The Curcumin treatment led to an increase in percentages of cells in the sub-G1 phase (1.01% to 17.70%) and a decrease in G1 (64.71% to 51.01%), S (23.22% to 21.12%), and G2 (13.23% to 10.20%) phases.
Furthermore, the Flutamide therapy increased sub-G1 cells (1.01% to 15.69%) and decreased G1, S, and G2 cells. The results regarding the combination therapy with Curcumin and Flutamide were more prominent with a significant increase in sub-G1 cells (1.01% to 46.38%), and decline in G1 (64.71% to 28.51%), S (23.22% to 20.0%), and G2 (13.23% to 5.11%) cells (Figure 4).
Similar results were obtained concerning the LNCaP cells. In this cell line, the control group, the Curcumin treated group, the Flutamide treated group, and the group with combination therapy had 0.69%, 9.58%, 8.89%, and 12.34% of the cell population in the sub-G1 stage, respectively. The presence of this sub-G1 peak after drug administration indicates the occurrence of apoptosis. Thus, the results revealed that LNCaP and PC3 cells experience apoptosis due to cessation of the cell cycle in the sub-G1/G1 phase following the treatment with Curcumin, Flutamide, and their combination compared to the control group.
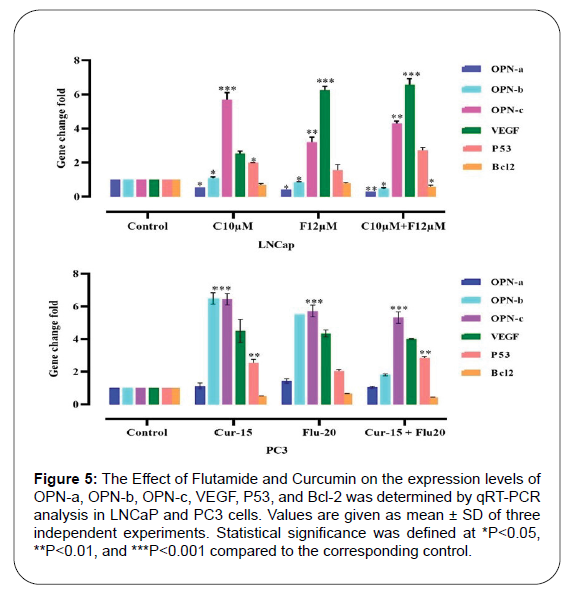
LNCaP and PC3 cells were treated with Flutamide and Curcumin for 48 h and then examined regarding the expression of OPN isoforms by real-time PCR. The OPN-a isoform gene expression level was decreased in the LNCaP cell line treated with Curcumin compared to the untreated cells. Also, Flutamide significantly reduced the expression of both OPN-a and OPN-b isoforms in this cell line. In contrast, OPN-c isoform and VEGF gene expression showed a significant increase in LNCaP cells treated with Flutamide and Curcumin alone and in combination. In contrast to the LNCaP cell line, a significant increase in expression of the two OPN-c and OPN-b isoforms and VEGF was observed in PC3 cells treated with Flutamide and Curcumin alone and in combination (Figure 5). Concerning the tumor suppressor P53 gene, both cell lines demonstrated significant upregulation following administration of Curcumin, Flutamide, and their combination. Moreover, the anti-apoptotic Bcl-2 gene was downregulated in LNCaP and PC3 cell lines after treatment with these two agents and their combination.
Figure 5:The Effect of Flutamide and Curcumin on the expression levels of OPN-a, OPN-b, OPN-c, VEGF, P53, and Bcl-2 was determined by qRT-PCR analysis in LNCaP and PC3 cells. Values are given as mean ± SD of three> independent experiments. Statistical significance was defined at *P<0.05, **P<0.01, and ***P<0.001 compared to the corresponding control.
Discussion
Curcumin was previously demonstrated to inhibit the proliferation and accelerate the apoptosis of prostate cancer cells in both in-vitro and xenograft models [19,28,29]. Numerous pathways have been identified for this anti-cancer effect of Curcumin. At the molecular level, Curcumin inhibits the expression of oncogenes Cyclin D1, Epidermal Growth Factor Receptor (EGFR), AP-1, Matrix Metalloproteinase (MMP), Signal Transducer And Activator Of Transcription 3 (STAT3), NF-κB, and multiple other pathways, e.g., PI3K/Akt [30]. Moreover, Curcumin can sensitize prostate cancer cells to TRAIL-associated apoptosis [29].
Our results also denoted that Curcumin alone and in combination with the anti-androgen drug Flutamide can affect the survival of androgen-dependent (LNCaP) and, more prominently, androgenindependent (PC3) prostate cancer cells through induced apoptosis and cell cycle arrest at sub G1/G1 phase. In addition, our findings indicated the overexpression of the pro-apoptotic P53 gene alongside the downregulation of the anti-apoptotic Bcl-2 gene after administration of Curcumin and its combination with Flutamide, all in favor of increased apoptosis.
Notably, we observed these anti-proliferative effects and apoptosis with few necrotic effects (Figure 3), further implying the safety of this therapeutic regimen. Although androgen depletion therapy provides promising therapeutic properties on androgen-dependent prostate cancers, virtually all prostate cancers ultimately progress to an androgen-independent state due to alteration in androgen receptor signaling and expression [31]. Currently, no established treatment for these androgen-insensitive tumors has been recognized [17]; thus, Curcumin, with its proven safety and few side effects [32], offers great anti-proliferative potential for the management of androgenindependent prostate cancers [33,34].
Additionally, Curcumin was suggested to downregulate the pathologic angiogenesis by direct inhibitory effects on endothelial cells [35] and negative regulation of a plethora of transcription factors such as NF-κB pathway and multiple proangiogenic factors, e.g., MMP, VEGF, and OPN [36]. In this regard, OPN plays a pivotal role in tumor growth, progression, metastasis, and angiogenesis via mediating several autocrine and paracrine pathways by interaction with integrins and CD44 [37]. Previous observations have suggested that cooperative mechanisms implicating OPN and integrin αvβ3 signaling pathways are involved in VEGF-mediated cell migration and angiogenesis [26,38,39]. In the past decade, more and more evidence has accumulated indicating that high expression of OPN in plasma and tumor tissue of patients with advanced cancers is correlated with neoplastic proliferation signature and poor prognosis [40,41]. A recent meta-analysis also showed that OPN overexpression is related to higher Gleason score, higher clinical stage, lymph node and distant metastasis, and shorter relapse-free and overall survival in patients with prostate cancers [42].
However, few studies have addressed the role of OPN splice variants in prostate cancer. To the best of our knowledge, no prior studies have evaluated the effect of Curcumin on these isoforms. We verified that the combination of Curcumin and Flutamide was able to downregulate OPN-a and OPN-b isoforms in androgen-dependent (LNCaP) cell lines, and OPN-a isoform gene in androgen-independent (PC3) cells.
Variations in splicing of OPN mRNA modulate proteome diversity and leads to the following three isoforms: OPN-a is the full-length variant, while OPN-b and OPN-c lack exon 5 and exon 4, respectively [43]. Tilli et al. demonstrated that all three of these isoforms were upregulated in prostate cancer tissues in benign prostatic hyperplasia samples; however, among these, OPN-c was the most overexpressed isoform, with the diagnostic accuracy outperforming even the prostatespecific antigen (PSA) serum levels for prostate cancer. It was postulated that OPN-c upregulation is involved in crucial pro-survival pathways promoting the neoplastic transformation of prostate cells [14].
OPN-b and OPN-c splice isoforms enhance the proliferation, invasion, and metastasis of prostate cancer through PI3K-mediated signaling, as well as the expression of MMP-2, MMP-9, and VEGF (43). Moreover, OPN-c activates the androgen receptor signaling pathway leading to prostate cancer progression [44]. Besides, the overexpression of the OPN-b and OPN-c isoforms are involved in the upregulation of mesenchymal markers and downregulation of epithelial markers in prostate cancer cells leading to chemotherapy resistance and increased neoplastic cell survival [45-47]. Interestingly, our data show OPN-c isoform upregulation was independent of androgen dependency status while OPN-b isoform was upregulated in androgen-independent (PC3) prostate cancer cell line only, suggesting stronger evading mechanisms by OPN-b and OPN-c isoforms in more aggressive variants, warranting future studies.
Such rationale and our results also corroborate findings of e previous studies regarding the role of OPN-b and OPN-c splice variants in prostate cancer progression. Although anti-carcinogenic effects of Curcumin on prostate cancer cells have been described in several preclinical reports, randomized controlled trials investigating the oral supplementation of Curcumin and its derivatives have failed to detect differences regarding the treatment outcomes in patients with prostate cancer [48,49]. Our findings concerning the overexpression of OPN-b and OPN-c isoforms following the administration of Curcumin and anti-androgen drugs can provide new insights on the possible role of OPN and its splice isoforms in the resistance of prostate cancers to androgen targeted and non-androgen targeted therapy.
The role of OPN in cancer chemoresistance has been investigated in several malignancies, including pancreatic cancer [50], hepatocellular carcinoma [51], and breast cancer [52,53]. Regarding this chemoresistance, two possible mechanisms have been proposed. First, upregulation of autophagy by OPN can paradoxically promote resistance to chemotherapy. Yang et al. demonstrated that autophagy induced by OPN results in chemoresistance of pancreatic cancer cells to gemcitabine through activation of the OPN/NF-κB pathway. The OPN knockdown via lentiviral transfection and autophagy blockade contributed to enhanced chemotherapy response and cytotoxic effects [50].
Similar findings concerning the OPN-induced autophagy have been detected in hepatocellular carcinoma cell lines with the involvement of the integrin αvβ3/MEK/ERK1/2 pathway [51]. Second, OPN may play an anti-apoptotic function resulting in drug resistance. By silencing OPN expression, Pang et al. detected a higher apoptotic function of cyclophosphamide in breast cancer cells compared to the normal breast cancer cells [53].
Likewise, downregulation of OPN conferred increased sensitivity of breast cancer cells to doxorubicin-induced apoptosis [52]. These anti-apoptotic effects of OPN, which can lead to chemoresistance, are mediated through p38/MAPK and PI3K/Akt pathways [52,53]. Combining these findings with our results, we hypothesize that OPN, especially OPN-b and OPN-c isoforms, may be involved in the resistance of prostate cancer cells to therapeutic agents.
While involving multiple well-established techniques and diverse cell lines representing androgen-independent and androgen-dependent prostate cancer, future investigations should expand our study to the in vivo microenvironment and to the clinical scenario.
Conclusion
Our results suggest that despite the anti-carcinogenic effects of Curcumin, prostate cancer cells probably evade these effects via induction of OPN-b and OPN-c isoforms and related molecular pathways. The correlation between androgen-independency and OPN-b isoform needs further exploration and targeting these pathways in future studies is of utmost importance to open up possibilities for developing an improved therapeutic response in patients with prostate cancer.
Conflict of Interests
All authors claim that there is no competing interest.
Funding
There was no founding.
Ethical Statements
Not applicable.
References
- Cassell, A., Yunusa, B., Jalloh, M., Ndoye, M., Mbodji, M. M., Diallo, A., et al.nManagement of Advanced and Metastatic Prostate Cancer: A Need for a Sub-Saharan Guideline.nJ Oncol., 2019;2019: 1-9.
- Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A.nCancer Statistics., 2021.nCA Cancer J Clin., 2021;71(1): 7-33.
- Nader, R., El-Amm, J., Aragon-Ching, J. B.nRole of chemotherapy in prostate cancer.nAsian J Androl., 2018;20(3): 221-229.
- Aghamir, S. M. K., Hamidi, M., Salavati, A., Mohammadi, A., Farahmand, H., Meysamie, A. P., et al.nIs antibiotic prophylaxis necessary in patients undergoing ureterolithotripsy?nActa Medica Iran., 2011;49(8): 513-516.
- Zhang, H. N., Yu, C. X., Zhang, P. J., Chen, W. W., Jiang, A. L., Kong, F., et al.nCurcumin downregulates homeobox gene NKX3.1 in prostate cancer cell LNCaP.nActa pharmacol Sin., 2007;28(3): 423-430.
- Folkman, J., & Shing, Y.nAngiogenesis.nJ Biol Chem., 1992;267(16): 10931-10934.
- Mirzaei, A., Zendehdel, K., Rashidian, H., Aghaii, M., Ghahestani, S. M., & Roudgari, H.nThe Impact of OPIUM and Its Derivatives on Cell Apoptosis and Angiogenesis.nTransl Res Urol., 2020;2(4): 110-117.
- Ferrer, F. A., Miller, L. J., Andrawis, R. I., Kurtzman, S. H., Albertsen, P. C., Laudone, V. P., et al.nVascular endothelial growth factor (VEGF) expression in human prostate cancer: in situ and in vitro expression of VEGF by human prostate cancer cells.nJ Urol., 1997;157(6): 2329-2333.
- Wu, T. T., Wang, J. S., Jiann, B. P., Yu, C. C., Tsai, J. Y., Lin, J. T., et al.nExpression of vascular endothelial growth factor in Taiwanese benign and malignant prostate tissues.nJ Chin Med Assoc., 2007;70(9): 380-384.
- Bruni-Cardoso, A., Johnson, L. C., Vessella, R. L., Peterson, T. E., & Lynch, C. C.nOsteoclast-derived matrix metalloproteinase-9 directly affects angiogenesis in the prostate tumor-bone microenvironment.nMol Cancer Res., 2010;8(4): 459-470.
- Rennel, E., Waine, E., Guan, H., Schüler, Y., Leenders, W., Woolard, J., et al.nThe endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice.nBritish J Can., 2008;98(7): 1250-1257.
- Chakraborty, G., Jain, S., Behera, R., Ahmed, M., Sharma, P., Kumar, V., et al.nThe multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis.nCurr Mol Med., 2006;6(8): 819-830.
- Huang, Z., & Bao, S. D.nRoles of main pro- and anti-angiogenic factors in tumor angiogenesis.nWorld J Gastroenterol., 2004;10(4): 463-470.
- Tilli, T. M., Thuler, L. C., Matos, A. R., Coutinho-Camillo, C. M., Soares, F. A., da Silva, E. A., et al.nExpression analysis of osteopontin mRNA splice variants in prostate cancer and benign prostatic hyperplasia.nExp Mol Pathol., 2012;92(1):13-19.
- Tilli, T. M., Bellahcène, A., Castronovo, V., & Gimba, E. R.nChanges in the transcriptional profile in response to overexpression of the osteopontin-c splice isoform in ovarian (OvCar-3) and prostate (PC-3) cancer cell lines.nBMC Can., 2014;14: 433.
- Rashedi, S.nLandscape of Circular Ribonucleic Acids in Urological Cancers.nTransl Res Urol., 2021;3(2): 45-47.
- Abd Wahab, N. A., Lajis, N. H., Abas, F., Othman, I., & Naidu, R.nMechanism of Anti-Cancer Activity of Curcumin on Androgen-Dependent and Androgen-Independent Prostate Cancer.nNutrients., 2020;12(3): 679.
- Mohan, R., Sivak, J., Ashton, P., Russo, L. A., Pham, B. Q., Kasahara, N., et al.nCurcuminoids inhibit the angiogenic response stimulated by fibroblast growth factor-2, including expression of matrix metalloproteinase gelatinase B.nJ Biol Chem., 2000;275(14): 10405-10412.
- Deeb, D., Jiang, H., Gao, X., Al-Holou, S., Danyluk, A. L., Dulchavsky, S. A., et al.nCurcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway.nJ Pharmacol Exp Ther., 2007;321(2): 616-625.
- Nakamura, K., Yasunaga, Y., Segawa, T., Ko, D., Moul, J. W., Srivastava, S., et al.nCurcumin down-regulates AR gene expression and activation in prostate cancer cell lines.nInt J Oncol., 2002;21(4): 825-830.
- Shankar, S., & Srivastava, R. K.nInvolvement of Bcl-2 family members, phosphatidylinositol 3'-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer.nInt J Oncol., 2007;30(4): 905-918.
- Sha, J., Li, J., Wang, W., Pan, L., Cheng, J., Li, L., et al.nCurcumin induces G0/G1 arrest and apoptosis in hormone independent prostate cancer DU-145 cells by down regulating Notch signaling.nBiomed Pharmacother., 2016;84: 177-184.
- Teiten, M. H., Gaascht, F., Cronauer, M., Henry, E., Dicato, M., & Diederich, M.nAnti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway.nInt J Oncol., 2011;38(3): 603-611.
- Tsui, K. H., Feng, T. H., Lin, C. M., Chang, P. L., & Juang, H. H.nCurcumin blocks the activation of androgen and interlukin-6 on prostate-specific antigen expression in human prostatic carcinoma cells.nJ Androl., 2008;29(6): 661-668.
- Saadati, M., Tamehri, S., Kamali, M. P., & Taheri, D.nPhosphatase and tensin gene associated with features of aggressive prostate cancer.nTransl Res In Urol., 2021;3(1): 32-37.
- Gupta, A., Zhou, C. Q., & Chellaiah, M. A.nOsteopontin and MMP9: Associations with VEGF Expression/Secretion and Angiogenesis in PC3 Prostate Cancer Cells.nCancers., 2013;5(2): 617-638.
- Arur, S., Uche, U. E., Rezaul, K., Fong, M., Scranton, V., Cowan, A. E, et al.nAnnexin I is an endogenous ligand that mediates apoptotic cell engulfment.nDev Cell., 2003;4(4): 587-598.
- Dorai, T., Cao, Y. C., Dorai, B., Buttyan, R., Katz, A. E.nTherapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo.nProstate., 2001;47(4): 293-303.
- Shankar, S., Ganapathy, S., Chen, Q., & Srivastava, R. K.nCurcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis.nMol Can., 2008;7: 16.
- Mukhopadhyay, A., Bueso-Ramos, C., Chatterjee, D., Pantazis, P., & Aggarwal, B. B.nCurcumin downregulates cell survival mechanisms in human prostate cancer cell lines.nOncogene., 2001;20(52): 7597-7609.
- Aragon-Ching, J. B., & Dahut, W. L.nChemotherapy in Androgen-Independent Prostate Cancer (AIPC): What's next after taxane progression?nCancer Ther., 2007;5A(A): 151-160.
- Lao, C. D., Ruffin, M. T. T., Normolle, D., Heath, D. D., Murray, S. I., Bailey, J. M., et al.nDose escalation of a curcuminoid formulation.nBMC Complement Altern Med., 2006;6: 10.
- Mohseni, M., Khazaeli, M. H., Aghamir, S. M. K., & Biniaz, A.nChanges in intrarenal resistive index following electromagnetic extracorporeal shock wave lithotripsy.nUrol J., 2007;4(4): 217-220.
- Aghamir, S. M. K., Heshmat, R., Ebrahimi, M., & Khatami, F.nLiquid biopsy: the unique test for chasing the genetics of solid tumors.nEpigenet Insights., 2020;13: 2516865720904052.
- Arbiser, J. L., Klauber, N., Rohan, R., Van, L. R., Huang, M. T., Fisher, C., et al.nCurcumin is an in vivo inhibitor of angiogenesis.nMol Med., 1998;4(6): 376-383.
- Wang, T. Y., & Chen, J. X.nEffects of Curcumin on Vessel Formation Insight into the Pro- and Antiangiogenesis of Curcumin.nEvid Based Complement Alternat Med., 2019;2019: 1390795.
- Shevde, L. A., & Samant, R. S.nRole of osteopontin in the pathophysiology of cancer.nMatrix Biol., 2014;37: 131-141.
- Desai, B., Rogers, M. J., & Chellaiah, M. A.nMechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells.nMol Can., 2007;6: 18.
- Wang, Y., Yan, W., Lu, X., Qian, C., Zhan, G. J., Li, P., et al.nOverexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the avβ3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells.nEur J Cell Biol., 2011;90(8): 642-648.
- Castellano, G., Malaponte, G., Mazzarino, M. C., Figini, M., Marchese, F., Gangemi, P., et al.n Activation of the osteopontin/matrix metalloproteinase-9 pathway correlates with prostate cancer progression.nClin Cancer Res., 2008;14(22): 7470-7480.
- Pang, X., Gong, K., Zhang, X., Wu, S., Cui, Y., & Qian, B. Z.nOsteopontin as a multifaceted driver of bone metastasis and drug resistance.nPharmacol Res., 2019;144: 235-244.
- Yu, A., Xie, K., Xing, C., Qin, Q., Liu, L., & Zu, X.nOsteopontin as a novel biomarker for the prognosis and clinical pathology of prostate cancer: a systematic review and meta-analysis.nResearch Square., 2020.
- Tilli, T. M., Mello, K. D., Ferreira, L. B., Matos, A. R., Accioly, M. T., Faria, P. A., et al.nBoth osteopontin-c and osteopontin-b splicing isoforms exert pro-tumorigenic roles in prostate cancer cells.nProstate., 2012;72(15): 1688-1699.
- Tilli, T. M., Ferreira, L. B., & Gimba, E. R.n Osteopontin-c mediates the upregulation of androgen responsive genes in LNCaP cells through PI3K/Akt and androgen receptor signaling.nOncol Lett., 2015;9(4): 1845-1850.
- Nakamura, K. D., Tilli, T. M., Wanderley, J. L., Palumbo, A., Mattos, R. M., Ferreira, A. C., et al.nOsteopontin splice variants expression is involved on docetaxel resistance in PC3 prostate cancer cells.nTumour Biol., 2016;37(2): 2655-2663.
- Tamehri, Z. S. S., Taheri, D., Shivarani, S., Khatami, F., & Kazemi, R.nLiquid Biopsy in Prostate Cancer Diagnosis and Prognosis: A Narrative Review.nTransl Res Urol., 2020;2(4): 139-146.
- Khatami, F., Aghamir, S. M. K., Salmaninejad, A., Shivarani, S., & Khorrami, M. H.n Biomarkers for Prostate Cancer Diagnosis from Genetic Perspectives.nTransl Res Urol., 2020;2(2): 51-58.
- Choi, Y. H., Han, D. H., Kim, S. W., Kim, M. J., Sung, H. H., Jeon, H. G., et al.nA randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation.nProstate., 2019;79(6): 614-621.
- Hejazi, J., Rastmanesh, R., Taleban, F. A., Molana, S. H., Hejazi, E., Ehtejab, G., et al.nEffect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study.nNutr Can., 2016;68(1): 77-85.
- Yang, M. C., Wang, H. C., Hou, Y. C., Tung, H. L., Chiu, T. J., & Shan, Y. S.nBlockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine.nMol Can., 2015;14: 179.
- Liu, G., Fan, X., Tang, M., Chen, R., Wang, H., Jia, R., et al.nOsteopontin induces autophagy to promote chemo-resistance in human hepatocellular carcinoma cells.nCancer Lett., 2016;383(2): 171-182.
- Yang, L., Wei, L., Zhao, W., Wang, X., Zheng, G., Zheng, M., et al.nDown-regulation of osteopontin expression by RNA interference affects cell proliferation and chemotherapy sensitivity of breast cancer MDA-MB-231 cells.nMol Med Rep., 2012;5(2): 373-376.
- Pang, H., Cai, L., Yang, Y., Chen, X., Sui, G., & Zhao, C.nKnockdown of osteopontin chemosensitizes MDA-MB-231 cells to cyclophosphamide by enhancing apoptosis through activating p38 MAPK pathway.nCan Biother Radiopharm., 2011;26(2): 165-173.
Citation: Mirzaei A, Rashedi S, Mashhadi R, Ghahestani SM, Gorji A, et al. (2021) Osteopontin Isoforms b and c Mediate Prostate Cancer Cell Evasion. Cell Mol Biol 67: 207. DOI: 10.4172/1165-158X.1000207
Copyright: © 2021 Mirzaei A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3023
- [From(publication date): 0-2021 - Nov 07, 2025]
- Breakdown by view type
- HTML page views: 2315
- PDF downloads: 708