Review Article Open Access
Orphan Drugs: Getting Arms around Rare Diseases
Duygu Koyuncu Irmak*Department of Clinical Operations, INC Research, Turkey
- *Corresponding Author:
- Duygu Koyuncu Irmak
Associate Director of Clinical Operations, INC Research
Nida Kule Is Merkezi Goztepe Merdivenkoy Mah
Bora Sokak No: 1 Kat: 14 D: 51-52, Goztepe
Kadikoy 34732 Istanbul, Turkey
Tel: 00905327862060
E-mail: Duygu.Irmak@INCResearch.com
Received date: March 17, 2017; Accepted date: April 11, 2017; Published date: April 18, 2017
Citation: Irmak DK (2017) Orphan Drugs: Getting Arms around Rare Diseases. J Comm Pub Health Nurs 3:167. doi:10.4172/2471-9846.1000167
Copyright: © 2017 Irmak DK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community & Public Health Nursing
Abstract
Finding ways to bring new therapies for rare diseases to patients in a timely manner, effectively and affordably is an important public health challenge. The key concern for decision makers in the health authorities for all medicinal products including Orphan Drugs is that the treatment demonstrates efficacy through “substantial evidence” from adequate, well-planned, well-controlled clinical trials
A successful clinical development programs in rare diseases starts with a tailored approach to ensure the right methodology is employed for the target rare disease therapy. The research methodology needs to be evaluated specifically for each rare disease and the target therapy in the light of all available scientific knowledge by all experts acting in all stakeholders.
Keywords
Rare disease; Orphan drug; Methodology; Clinical development
Introduction
Finding ways to bring new therapies for rare diseases to patients in a timely manner, effectively and affordably is an important public health challenge. The key concern for decision makers in the health authorities for all medicinal products including Orphan Drugs is that the treatment demonstrates efficacy through “substantial evidence” from adequate, well-planned, well-controlled clinical trials [1,2].
This common expectation, however, has been more difficult to address in rare disease treatment studies due to the challenges related to the medicinal products, the disease, the availability of resource and the clinical trial itself. In addition, the focus of clinical trials in rare diseases shifts from a focus on public health and population based statistics to the individual patients [3].
Clinical trials studying small populations are necessary to provide the required proof of concept for the efficacy and safety of the candidate Orphan Drug; however, there are difficulties in recruiting enough qualified patients for conventional statistical analyses to provide a powered result that will prove or disprove a hypothesis. Nonetheless, these trials are necessary for the rare diseases studies, as well as specific pediatric, geriatric, individually tailored therapies, regional subpopulations [4].
The level of clinical evidence generated for orphan medicines in European market authorization submissions often is low compared to submissions in more common indications. This is a result of few randomized controlled trials, short treatment follow-up, evolving consensus on the appropriate clinical endpoints. The success rate at centralized market authorization is lower for orphan drugs (62.9%) than for non-orphan medicines (70.7%). The burden of disease of rare diseases is high, affecting the lives of at least 30 million patients in the European Union (EU) [5].
The aim of this review article is to outline the challenges involved with rare disease clinical research and compile recent worldwide efforts to design smart and robust clinical development programs for the rare diseases that will overcome the special challenges. There is no “one size fits all” trial design and development plans must be tailored to each indication. Addressing the unmet medical needs that these orphan treatments address quickly and effectively is the only way of getting our arms around these rare disease patients.
Challenges in Rare Disease Research
The specific difficulties in Rare Disease clinical research can be disease related and therapy related. These challenges must be considered when considering the clinical development strategy, especially the clinical development methodology. Here, we outline various issues that must be incorporated into the strategic and operational thinking when developing a new therapy for a rare disease.
Prevalence (rarity) of disease
By definition, rare diseases occur at a low prevalence. There is no global consensus on definition of “rare” and the criteria vary from country to country. For instance, in the US and Brazil, rare is a prevalence of 6.5 people per 10 000 population, while in Canada and Mexico it’s 5 persons, Japan 4 persons, Russia 1 person, Turkey 0.1 person and the UK 0.2 person all per in 10 000 population [6,7]. Continued epidemiological research is critical to the understanding of these rare diseases, particularly when planning the research and development strategy and clinical development program for orphan drugs.
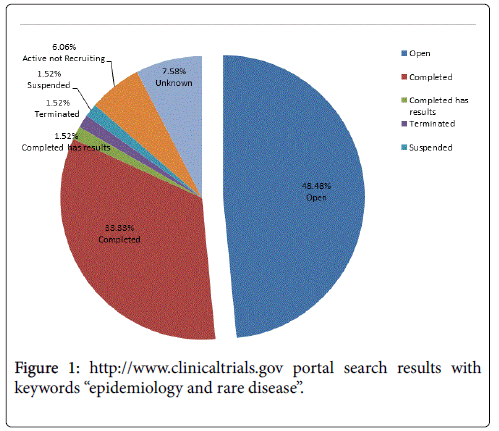
Of the epidemiology studies reported on clinicaltrials.gov, only 2.29% investigate rare diseases [8]. As seen in Figure 1, the 33% of the epidemiology studies’ are closed, however only 1.5% are completed and have available results. A search for rare disease epidemiology studies articles in PubMed results in only 1.23% of the total epidemiology published articles [9]. This reflects the relative paucity of prevalence data in rare diseases that drive some of the difficulties in conducting clinical trials in these indications.
Severity, diversity and unknown natural history of rare diseases
Rare diseases often are life threatening or chronically debilitating as well as emotionally and physically demanding, making the medical need in these indications urgent and severe Besides the obvious physical burdens the affected patients bear, there are also quality of life issues for the patients and their families and care givers, as well as a significant financial burden not only for these families, but also for the health care system [10].
The great majority of rare diseases (estimated 80%) are genetic in origin [11,12]. Many of genetic diseases are caused by defects in a single gene, for example, alpha1-antitrypsin deficiency which may cause serious lung and/or liver disease and Friedreich’s ataxia, a neurological disorder that may be accompanied by cardiac problems. Multiple different mutations in that single gene may result in disease with varying features or severity. Other diseases, such as Fanconi anemia, have several named variants, each caused by a defect in a different gene [13]. Muscular dystrophy, which was once viewed as a single disease, now is described as having nine major forms, of which Duchenne muscular dystrophy may be the best known [14].
Identifiability of the patients of treatment
These rare diseases often present diagnostic difficulties because they are so rare or because they can present in unusual ways, or because they often present on top of comorbidities. For many diseases no diagnostic method exists or diagnostic facilities are unavailable. Consequently validity, coding and reproducibility are the problems [3].
Difficulties in assessing clinical relevance and cost-effectiveness: The methodology for evaluating clinical relevance and cost effectiveness of rare disease treatments is still in an experimental phase, hampering positioning in clinical practice, pricing and reimbursement.
Unmet medical need (i.e., availability of treatment alternatives)
There are estimated 7000-8000 diseases that each affect a small number of patients and approximately 300 of these diseases have medicines approved to treat the patients with that rare disorder [15]. Yet, around 95% of the rare diseases have no available treatment. This therapeutic disparity between the number of known rare diseases and those that have an approved treatment highlights the severe unmet medical need for majority of rare diseases [2]. An orphan drug can be defined as one that is used to treat an orphan disease. For example, haemarginate, used to treat acute intermittent porphyria, variegate porphyria and hereditary coproporphyria is an orphan drug [16]. However, ibuprofen can also be categorized as an orphan drug, because it has been used to treat an orphan disease, patent ductus arteriosus in neonates [17].
Expertise infrastructure
Another challenge is the availability of the clinical and pre-clinical scientists, policy experts, industry professionals and academics who are involved in rare disease R&D, work in a multidisciplinary manner and are supported by an appropriate infrastructure. There are limited physicians who are able to diagnose and treat these rare diseases [18].
Defining the endpoints
A clinical end point is defined as “a characteristic or variable that reflects how a patient or consumer feels, functions, or survives”. A biological marker (biomarker) is “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic response to a therapeutic intervention” [19]. In order to grant approval, regulatory authorities expect a substantial evidence package that demonstrates whether a treatment is clinically effective with an appropriate safety profile. Determining both efficacy and safety is challenging when the disease prevalance is low and the clinical endpoints are not well established.. For disorders with very few and variable patients or lengthy time courses or irreversible disease progression, the use of clinical outcomes or clinically associated biological measures as endpoints can make specific treatment development intractable for practical and ethical reasons [20].
How to Plan ‘Smart’ Clinical Development Programs for Rare Disease Treatments
Role of incentives: Are these sufficient?
Without incentives many orphan drugs would not be developed and authorized as they can present an unfavorable commercial value for the pharmaceutical industry. Orphan drug legislation and policy have been successful in incentivizing the development of therapies in these rare conditions and more medicines for rare diseases have been authorized since the implementation of these policies [21]. An example is the Orphan Drug Act enacted in 1983 by FDA [22].
Following this, European Union implemented the Orphan Drug regulation of 2000 [23]. This was followed by pediatric drugs regulations in 2006 that contributed to increased R&D activity in orphan drugs [24]. Please refer to Table 1 for Incentives provided under national programs in the US, EU, Japan and Australia. The European Medicinal Agency also implemented Guidelines on Clinical Trials in Small Populations in 2006. These regulatory milestones accelerated the orphan drug development dramatically) [25].
| United States | Japan | Australia | European Union | |
|---|---|---|---|---|
| Original Policy | Legislation | Regulation | Regulation | Regulation |
| Issue date | 1983 | 1993 | 1997 | 2000 |
| Market Exclusivity | 7 years | 10 years | 5 years* | 10 years* |
| Grants Program | Exists | Exists | Does Not Exist | Does Not Exist at Union |
| Tax Credits for clinical trials | 50% for clinical costs | 6% for clinical and non-clnical costs | Does Not Exist | Does Not Exist at Union** |
| Protocol or Trial Design Assitance | Exists | Exists | Exists | Exists*** |
| Application Fee Waivers | Exists | Not Exists | Exists | Reduced Fees |
| Adapted from [13] | ||||
| *Same exclusivity with other drugs; **Managed under Member States; ***Partial | ||||
Table 1: Comparison of selected national policy incentives for orphan drug development
Once a potential therapeutic drug or biologic has been discovered, the process of developing the therapeutic for a particular disease, whether rare or not, begins with preclinical development and continues through increasingly complex and demanding phases of clinical testing. Much of this work has traditionally been done within for profit pharmaceutical companies, however, as this research is expensive and risky, the financial incentives that a blockbuster drug in an common indication offers is much more commercially attractive, making the choice to develop therapies in common indications more favorable. As a result, potential therapies for rare diseases have often been abandoned, even with the incentive policies [14].
Orphan drug designation system creates a strong promise for future orphan drugs. Nevertheless, receiving orphan drug designation does not automatically guarentee an orphan drug will be authorised for marketing. The study by Joppi pointed out that in April 2004, only 7.1% of the EU designated potential orphan drugs were approved for marketing, questioning whether the incentives are sufficient to provide the European market with new orphan drugs [26].What are the critical success factors that turn an orphan designation into an authorisation? One of the discussion points, for example, is that US Orphan Drug Act is successful because of tax grants, which are not available in Europe [27]. Other factors that may impact the likelihood of approval are the potential of the sponsor to carry out suitable clinical trials and the level of patient involvement in the development process [28]. However, beyond these suggested factors, there may be additional, less well studied characteristics may be of importance [21].
Accelerated Approval Process and Its Contribution to Rare Disease Research
All drugs must undergo clinical trials to demonstrate safety and clinical efficacy before they can be approved. In disorders with very few affected individuals the use of clinical measures as endpoints makes the development of new drugs difficult for both ethical and practical reasons. In order to incentivize the pursuit of new treatments for serious and life-threatening disorders, the FDA announced the “Accelerated Approval (AA) Regulations” in 1992. AA regulations were released to drive the development of new treatments for serious and life-threatening disorders, primarily motivated by the AIDS crisis and the slow pace of treatment development for HIV infection [29].
These regulations allowed for drug approval based on the use of surrogate endpoints as a proxy for demonstrating substantial clinical benefit. However, because of the challenges in obtaining consensus acceptance of novel surrogate endpoints in very rare diseases, the accelerated regulations have not been utilized for the majority of those conditions [30]. By the analysis of conceived clinical development programs using proposed clinical or surrogate endpoints for fifteen rare diseases Miyamoto and Kakkis could demonstrate that better access to the “Accelerated Approval Regulations” could speed up the process of approval and reduce the costs for the drug development by about 60% [31]. The authors suggested that well-defined qualification criteria are needed for the use of surrogate endpoints in order to facilitate the approval for orphan drugs by using the “Accelerated Approval Regulations” pathway.
Methodology Evolution in Orphan Drug Development Programs
Clinical trial methodology has been evolving dramatically in the last decades as well-established and validated methods are available for the design, conduct and analysis of clinical trials. The randomised, parallel-group controlled clinical trial design remains the gold standard [32]. However, in rare disease treatment studies it may be impossible to run this sort of study because of the very low incidence and prevalence, individually tailored therapies and specific heterogenous trial populations.
As required for the adequately sized trials, for small trials the design and data analysis should enable a reasonable measure of the treatment effect. The design should include an outcome that can be measured to determine clinical change or treatment success, using a baseline value and an ‘under-treatment’ value for the outcome. Here, the most fundamental point is to ensure that any systematic bias is minimized using a validated methodology. Please refer to Table 2 for examples of systematic bias and commonly used methodology that addresses this.
| Bias Types | Meaning | Methods to Avoid Bias | Statistical Approach |
|---|---|---|---|
| Selection Bias | Biased allocation of patients to treatment or placebo groups | Central randomization planned in good quality | Methods for replacement of missing data |
| Performance Bias | Unequal provision of care apart from the treatment under evaluation | Double-blind follow-up and outcome evaluation | Methods for measurements in designs with intra-individual assessments, |
| Detection Bias | Biased assessment of the outcome attrition bias, which is the biased occurrence and handling of deviations from protocol and loss-to-follow-up | Outcome evaluation in a blinded manner | Methods for intention-to-treat analyses |
| Historical Control bias | Biased data obtained not addressing the variability of the patients | Use of concurrent controls for which participants serve as their own control | Include appropriate control of the type I error rate |
Table 2: Major systematic bias types and used prevention methods, (adapted from published articles of Cornu in 2013 [4] and Griggs in 2009 [13].
When the size of the patient population under study is limited because a disease is rare, or a treatment is targeted at a particular genetic subgroup or a small pediatric population, a number of specific statistical difficulties arise that can lead to poorly designed studies with limited probability of success. In 2013, the European Commission issued call for new methodologies for clinical trials for small population groups within the 7th Framework Program to address just this issue. This builds on other initiatives from around the world aimed at improving research, including methodology, in rare diseases and personalized medicine. Three collaborative research projects Asterix, IDeAl and InSPiRe, are funded under this initiative. The three projects focus on a number of methodological challenges in the design, analysis and interpretation of clinical trials in small populations and rare diseases and specific aspects such as patient perspectives and ethical issues [33].
The research under these projects have been adressing the challenges in rare disease clinical research such as:
• Adapting methods to support the planning, analysis and interpretation of a single randomized controlled trial.
• Developing and assessing new methods that are feasible and convenient in small population settings.
• Generalized evidence synthesis approaches that can be applied to pediatric studies and compounds developed for potentially multiple rare indications.
• Evidence synthesis methods across trials (of similar or different design) that take into account the sequential nature of drug development.
Official outputs from these studies have been published and presented in different events [34-45].
Another striking example is the CRESim which is a PrioMedChild European project. Its objective is to optimise the study design selection of a randomised controlled trial for rare diseases and orphan treatments. The CRESim project develops a platform performing trial modelling and simulation initially based on three pathological situations: Dravet Syndrome (rare form of epilepsy), T-cell lymphoblastic lymphoma in children and cystic fibrosis. The common approach is an integrative modelling, using the development and connection between the pathophysiological model of the disease, the pharmacotherapeutic model of the study treatment and the clinical trial model with a specific trial design [46].
In one review article, the authors made a seach in PubMed for the rare disease research methodology and reported the methods for various randomised, comparative trial designs that could be used for the evaluation of therapies in orphan diseases. These are parallel, factorial, cross-over group settings as well as the delayed start design, designs minimising time on inactive treatment or placebo (randomised withdrawal, early escape, randomised, placebo phase, stepped wedge designs) and adaptive randomisation (play the winner, drop the looser designs). Cornu and co-authors, in this approach, proposed an algorithm for choosing an experimental design for small randomized clinical trials that also involves judging
• Whether the outcome is reversible,
• Whether the treatment response is likely to be rapid and
• Whether investigators seek to minimize the time participants are receiving placebo [4].
Amongs the alternative designs, use of external or historical controls, use of concurrent controls for which participants serve as their own control, use of case-control design where individuals in whom a certain outcome has been observed matched to controls that did not have such an outcome prospectively or retrospectively can be considered [47].
Additional factors, such as the objectives of the trial, the number of patients needed, the length of trial and how the variability is handled, should be considered when choosing the most suitable trial design. There is no “one size fits all” design for rare diseases. There are a number of trial designs that can be employed, each with its specific advantages and specific limitations [4]. Often the regulatory agencies can be engaged as partners throughout the development process to best leverage the various incentives and designations [48,49].
On 23 Dec 2016, FDA approved the first drug Spiranza (Nusinersen) or Spinal Muscular Atrophy. The FDA worked closely with the sponsor during development to help design and implement the analysis upon which this approval was based. The FDA granted this application fast track designation and priority review. The drug also received orphan drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases [50].
Conclusion and Future Insight
Developing novel therapies for rare diseases is not often a priority for pharmaceutical companies when a favorable return on R&D investment is difficult to achieve. Therefore, all stakeholders, including but not limited to the regulators, pharma industry, researchers and physicians need to keep on working on exploring the innovative ways to achieve effective, fast, affordable and successful development of treatment options for patients suffering from rare diseases without available treatment options.
A successful clinical development programs in rare diseases starts with a tailored approach to ensure the right methodology is employed for the target rare disease therapy. The research methodology needs to be evaluated specifically for each rare disease and the target therapy in the light of all available scientific knowledge by all experts acting in all stakeholders. There is a ‘need’, still for:
1. The scientific availability of the outcome of the previous research on the disease natural history and the investigational drug trials
2. Consideration of specific the drug related issues and the disease related challenges
3. Commitment to disease-centered development with welldesigned trial protocols
By translating of all the knowledge and expertise in the field into accelerated availability of effective treatment options is how the rare disease research community will get their arms around patients suffering from rare diseases and waiting for treatment options.
Competing Interest
The author declares that there are no competing interests.
References
- Committee for Medicinal Products for Human Use (2006) EMA guideline in clinical trials in small populations.
- Sasinowski FJ (2011) Quantum of effectiveness evidence in FDA's approval of orphan drugs. National Organization for Rare Disorders
- Stolk P, Willemen MJ, Leufkens HG (2006) Rare essentials: Drugs for rare diseases as essential medicines. Bull World Health Organ 84: 745-751.
- Cornu C, Kassai B, Fisch R, Chiron C, Alberti C, et al. (2013) Experimental designs for small randomised clinical trials: An algorithm for choice. Orphanet J Rare Dis 8: 48.
- Dupont AG, Van Wilder PB (2011) Access to orphan drugs despite poor quality of clinical evidence. Br J Clin Pharmacol 71: 488-496.
- Rodwell C, Aym√?¬© S (2015) Rare disease policies to improve care for patients in Europe. Biochim Biophys Acta 1852: 2329-2335.
- Dharssi S (2016) Analysis of rare disease global policies and programs to advance access to care and treatment. RareX Conference.
- https://clinicaltrials.gov/
- https://www.ncbi.nlm.nih.gov/pubmed
- Borski K (2015) Economic aspects of rare diseases. Dev Period Med 19: 528-532.
- NORD (2003) Guide to rare disorders. Lippincott Williams & Wilkins, Philadelphia, PA.
- Kandi V (2015) Human nocardia infections: A review of pulmonary nocardiosis, Cureus 7: e304.
- D'Andrea AD (2010) Susceptibility pathways in Fanconi's anemia and breast cancer. N Engl J Med 362: 1909-1919.
- Field M, Boat TF (2010) Rare diseases and orphan products, accelerating research and development. The National Academies Press, Washington (DC).
- Inventory of Union and member state incentives to support research into and the development and availability of orphan medicinal products state of play (2015) Europen Comission Report.
- Hift RJ, Meissner PN (2005) An analysis of 112 acute porphyric attacks in Cape Town, South Africa: Evidence that acute intermittent porphyria and variegate porphyria differ in susceptibility and severity. Medicine (Baltimore) 84: 48√ʬ?¬?60.
- Aronson JK (2006) Editors√ʬ?¬? view: Rare diseases and orphan drugs Br J Clin Pharmacol 61: 243√ʬ?¬?245.
- Ilbars H, Koyuncu Irmak D, Akan H (2014) Orphan drugs: R&D Challenges with updates from Turkey and middle east countries. J Clin Stud 6: 2.
- Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther 69: 89-95.
- Onakpoya IJ, Spencer EA, Thompson M, Henegan CJ (2015) Effectiveness, safety and costs of orphan drugs: An evidence-based review. BMJ Open 5: e007199.
- Heemstra HE, de Vrueh L, van Weely S, B√?¬ľller HA, Leufkens HGM (2008) Predictors of orphan drug approval in the European Union. Eur J Clin Pharmacol 64: 545√ʬ?¬?552.
- Orphan Drug Act of US (1983) Public Law 97-414.
- Commission Regulation (EC) No 847/2000 (2000) Laying down the provisions for implementation of the criteria for designation of a medicinal product as an orphan medicinal product and definitions of the concepts √ʬ?¬?similar medicinal product√ʬ?¬? and √ʬ?¬?clinical superiority√ʬ?¬?. Official Journal of the European Communities L 103: 5-8.
- Paediatric regulation (2007) European medicines agency.
- https://globalgenes.org/rare-diseases-facts-statistics/
- Joppi R, Bertele V, Garattini S (2006) Orphan drug development is progressing too slowly. Br J Clin Pharmacol 61: 355√ʬ?¬?360.
- Dear JW, Lilitkarntakul P, Webb DJ (2006) Are rare diseases still orphans or happily adopted? The challenges of developing and using orphan medicinal products. Br J Clin Pharmacol 62: 264√ʬ?¬?271.
- W√?¬§stfelt M, Fadeel B, Henter JI (2006) A journey of hope: Lessons learned from studies on rare diseases and orphan drugs. J Intern Med 260: 1√ʬ?¬?10.
- Code of federal regulations (2016) Title 21.
- Beck M (2012) Rare and ultra rare diseases. J Develop Drugs 1: 2.
- Miyamoto BE, Kakkis ED (2011) The potential investment impact of improved access to accelerated approval on the development of treatments for low prevalence rare diseases. Orphanet J Rare Dis 6: 49.
- Diniz BJ, Fossaluza V, de Bracanga CA, Wechsler S (2016) Rain dance: The role of randomization in clinical trials. Open Access J Clin Trials 8: 21√ʬ?¬?32.
- Hilgers R, Roes K, Stallard N, InSPiRe project groups (2016) Directions for new developments on statistical design and analysis of small population group trials. Orphanet J Rare Dis 11: 78.
- Pontes C, Rios J, Vives R, Torres F (2014) Systematic approach to the description of the evidence supporting EMA regulatory opinions on orphan medicinal products. Conference Paper in Basic and Clinical Pharmacology and Toxicology.
- Koening F, Slattery J, Groves T, Lang T, Benjamini Y, et al. (2015) Sharing clinical trial data on patient level: Opportunities and challenges. Biom J 57: 8√ʬ?¬?26.
- Nikolakopoulos S, Roes KCB, van der Tweel I (2016) Sequential designs with small samples: Evaluation and recommendations for normal responses. Stat Methods Med Res 0: 1√ʬ?¬?13.
- Lodha A (2016) Clinical analytics √ʬ?¬? Transforming clinical development through big data. Imperial Journal of Interdisciplinary Research 2.
- Reetz K, Dogan I, Hilgers RD, Giunti P, Mariotti C, et al. (2016) Progression characteristics of the European friedreich√ʬ?¬?s ataxia consortium for translational studies (EFACTS): A 2 year cohort study. Lancet Neurol 15: 1346√ʬ?¬?1354.
- Bauer P, Bretz F, Dragalin V, Koning F, Wasmer G (2016) Twenty-five years of confirmatory adaptive designs: Opportunities and pitfalls. Statist Med 35: 325√ʬ?¬?347.
- Van der Elst W, Molenberghs G, Hilgers RD, Verbeke G, Heussen N (2016) Estimating the reliability of repeatedly measured endpoints based on linear mixed-effects models. A tutorial. Pharmaceut Statist.
- Petit C, Samson A, Morita S, Ursino M, Guedj J, et al. (2016) Unified approach for extrapolation and bridging of adult information in early-phase dose-finding paediatric studies. Stat Methods Med Res 0: 1√ʬ?¬?18.
- Ondra T, Jobjornsson S, Beckman RA, Burman CF, Konig F, et al. (2016) Optimizing trial designs for targeted therapies. PLoS ONE 11: e0163726.
- Friede T, Rover C, Wandel S, Neuenschwander B (2016) Meta-analysis of two studies in the presence of heterogeneity with applications in rare diseases. Biom J 00: 1√ʬ?¬?14.
- Wan Hee S, Hamborg T, Day S, Madan J, Miller F, et al. (2016) Decision-theoretic designs for small trials and pilot studies: A review. Stat Methods Med Res 25: 1022√ʬ?¬?1038.
- Graf AC, Posch M, Koening F(2015) Adaptive designs for subpopulation analysis optimizing utility functions. Biom J 57: 76√ʬ?¬?89.
- Nony P, Kurbatova P, Bajard A, Malik S, Castellan C, et al. (2014) A methodological framework for drug development in rare diseases. Orphanet J Rare Dis 9: 164.
- Griggs RC, Batshaw M, Duknle M, Gopal-Srivastava R, Kaye, E, et al. (2009) Clinical research for rare disease: Opportunuties, challenges and solutions. Mol Genet Metab 96: 20√ʬ?¬?26.
- Lagakos SW (2003) Clinical trials and rare diseases. N Engl J Med 348: 2455√ʬ?¬?2456.
- Hughes DA, Tunnage B, Yeo ST (2005) Drugs for exceptionally rare diseases: Do they deserve special status for funding? QJM 98: 829√ʬ?¬?836.
- FDA News Release (2016) FDA approves first drug for spinal muscular atrophy. New therapy addresses unmet medical need for rare disease.
Relevant Topics
- Chronic Disease Management
- Community Based Nursing
- Community Health Assessment
- Community Health Nursing Care
- Community Nursing
- Community Nursing Care
- Community Nursing Diagnosis
- Community Nursing Intervention
- Core Functions Of Public Health Nursing
- Epidemiology
- Epidemiology in community nursing
- Health education
- Health Equity
- Health Promotion
- History Of Public Health Nursing
- Nursing Public Health
- Public Health Nursing
- Risk Factors And Burnout And Public Health Nursing
- Risk Factors and Burnout and Public Health Nursing
Recommended Journals
- Epidemiology journal
- Global Journal of Nursing & Forensic Studies
- Global Nursing & Forensic Studies Journal
- global journal of nursing & forensic studies
- journal of community medicine& health education
- journal of community medicine& health education
- Palliative Care & Medicine journal
- journal of pregnancy and child health
Article Tools
Article Usage
- Total views: 2580
- [From(publication date):
May-2017 - Dec 19, 2024] - Breakdown by view type
- HTML page views : 1949
- PDF downloads : 631