Research Article Open Access
Organophosphorus Pesticide Exposure of School Children in Agricultural Villages in Indonesia
| Makiko Sekiyama*, Tetsuo Shimmura, Mineko Nakazaki, Ieva B Akbar, Budhi Gunawan, Oekan Abdoellah, Sadeli Masria, Linda Dewanti,Ohtsuka and Chiho Watanabe | |
| Graduate Program in Sustainability Science – Global Leadership Initiative (GPSS-GLI), Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5, Kashiwanoha, Kashiwa City, Japan | |
| Corresponding Author : | Makiko Sekiyama Graduate Program in Sustainability Science – Global Leadership Initiative (GPSS-GLI) Graduate School of Frontier Sciences The University of Tokyo, 5-1-5, Kashiwanoha Kashiwa City, Japan Tel: +81 4 7136 4859 Fax: +81 4 7136 4878 E-mail: sekiyama@k.u-tokyo.ac.jp |
| Received December 22, 2014; Accepted April 19, 2015; Published April 21, 2015 | |
| Citation: Sekiyama M, Shimmura T, Nakazaki M, Akbar IB, Gunawan B, et al. (2015) Organophosphorus Pesticide Exposure of School Children in Agricultural Villages in Indonesia. J Preg Child Health 2:153. doi: 10.4172/2376-127X.1000153 | |
| Copyright: ©2015 Sekiyama M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
The effects of environmental chemicals on children’s health are important issues in Asian developing countries undergoing rapid urbanization, although little data have been reported. We investigated the organophosphorus (OP) pesticide exposure of 235 elementary school children living in three agricultural villages in the Citarum watershed, Bandung, Indonesia, through urinary and blood biomonitoring. We evaluated four common dialkylphosphate (DAP) metabolites of OP compounds: dimethylphosphate (DMP), dimethylthiophosphate (DMTP), diethylphosphate (DEP), and diethylthiophosphate (DETP). Moreover, blood cell acetylcholinesterase (AChE) activity was assessed using blood samples. The collection of biological samples was accompanied by a questionnaire-based survey on sociodemographic indicators, food consumption, and behavioral patterns. The detection rates of DMP, DMTP, DEP, and DETP were 8.6, 21.7, 17.2, and 29.8%, and the median levels of dimethyl-, diethyl-, and total DAP were 5.8, 3.1, and 11.8nmol /L, respectively. These exposure levels of OP pesticides were lower than those reported in developed countries, possibly due to the low consumption of farm-grown fruits and the infrequent use of OP pesticides. The detection rate of DAP was highest in the village practicing pisciculture, where the use of pesticides was expected to be the lowest among the three villages, although the reason for this was not clear from our interview results.
| Keywords |
| Organophosphorus pesticides; Dialkyl phosphates; Agricultural communities; School children; Indonesia |
| Introduction |
| Developing countries in Asia are being confronted with environmental issues associated with rapid industrial growth and population increase. The effect of pesticides on health is among such issues with high priority, since the improper use of pesticides causes serious problems in developing countries [1], and the majority of the 220,000 deaths per year from pesticide exposure occur in developing countries [2-4]. WHO declared the necessity of protecting children from pesticide exposure in developing countries [5]. Children are particularly susceptible to pesticides because they have greater intake rates on a body-weight basis such as food and water ingestion and inhalation as well as greater relative skin surface area than adults. Moreover, children’s typical behavior such as hand-to-mouth activities and frequent contact with soil and dust can lead to a considerable uptake of pesticides. Although the potential health effects associated with children’s non-occupational exposure to pesticides have been a subject of increasing concern, very few studies have focused on this issue in developing countries in comparison with in developed countries [6,7]. To enable the full protection of children, basic data describing the exposure situation of children from developing countries are required. |
| Organophosphorus (OP) pesticides are of particular concern among the various pesticides because of their widespread use, both for residential and agricultural purposes, and because of their adverse effects on the nervous system [8]. Measurement of dialkyl phosphate (DAP) compounds in urine has been used to assess children’s exposure to OP pesticides in agricultural [9,10] and urban communities [11]. While the risk of OPs to children’s health has been documented in several reports, much effort is required to delineate the risk profile of OPs, including their toxic mechanisms [12]. |
| To the best of our knowledge, the only studies on DAP compounds in developing countries have been on the exposure of children in Ecuador to the OP pesticides. The children’s exposure to pesticides was measured in two ways: acetyl cholinesterase (AChE) measurement using blood samples and DAP compound measurement using spot urine samples [13,14]. These studies, however, examined the effect of prenatal exposure on the current exposure, and the effect of the postnatal living environment and behavior was not discussed. |
| In this study, we examined the OP pesticide exposure of school children living in three villages located in the watershed of the Citarumriver, West Java, Indonesia. This area has been reported to be contaminated with various types of hazardous materials including pesticides and metals from industrial and agricultural runoff as well as domestic sewage [15]. The three villages, although located in the same watershed, were characterized by differences in ecological settings, i.e., altitude, vegetation, temperature, and means of subsistence. Biological monitoring of OP pesticide exposure was conducted using urinary metabolites. Moreover, the activity of AChE, an effect marker for OP pesticides, was measured. Data on sociodemographic indicators, food consumption, and behavioral patterns were collected using questionnaires. By combining these data, we aimed to evaluate the level of OP pesticide exposure among rural school children in Indonesia and to elucidate the possible routes of exposure among them. |
| Subjects and Methods |
| Study village |
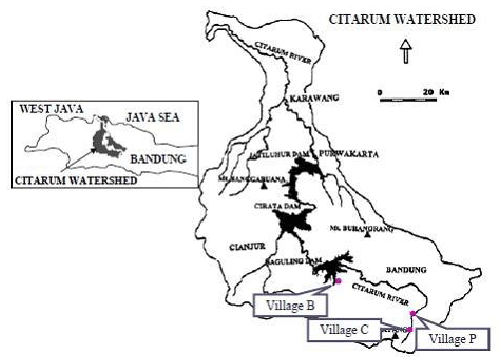
| The subject area is located in the watershed of Citarum river, Bandung district, West Java, Indonesia (Figure 1). The river has a watershed area of 6,000 km2 and three reservoirs. During the past 30 years, the downstream area has experienced rapid industrialization and urbanization, while the upstream area has seen an expansion of cultivated fields (including illegal expansion into the forest area), both of which have contributed to environmental pollution. The villages selected for this study are geographically distinct and differ in terms of ecological settings. |
| The first study village (P) is located in the eastern foothills of Mt. Malabar, 30 km south of Bandung city and 950-1,450 m above sea level (ASL). Sixty percent of the village area consisted of agricultural fields, and 25.4% was forested. The number of households was 3,200 and the population was 9,555 in 2002 (village statistical data). The major means of subsistence were cash crop harvesting and rice cultivation. |
| The second study site (C) is situated in the western foothills of Mt. PuncakCae, 35 km southeast of Bandung and 1,200-1,600 m ASL. Most of the area was forested (state property), while cultivated fields and residential areas occupied only 18 and 3.5%, respectively. The number of households was 1,300 and the population was 5,070 in 2002 (village statistical data). The major means of subsistence was crop cultivation. |
| The third village (B) is located downstream, 30 km west of Bandung city. The number of households was 2,133 and the population was 8,034 in 2001 (village statistical data). In 1985, when the Saguling dam was constructed, the government and related agencies introduced a floating net cage culture (FNCC), a new piscicultural method, to the reservoir. Although the technique was new to the inhabitants, they very quickly became familiar with this practice, and as a result, the number of FNCCs increased. Currently, the livelihoods of many of the households depend on FNCCs. The water quality of the reservoir has been affected by events upstream, and the worsening of the water quality has had a tremendous negative impact on the local pisciculture. |
| Study subjects |
| Villages P, C and B had four or five primary schools. Among them, two primary schools were randomly chosen from each village. An invitation to participate in the study was extended to the parents of all children in grades 1 and 2 through the school headmaster, with a written description of the study. Examinations were scheduled in a room set up in each selected school from 9 to 11 a.m. Before starting the examinations, we explained to the parents the purpose of the survey and procedures for interviewing and sampling, and asked them to sign the informed consent forms. We obtained permission for the examination from all the recruited parents and the numbers of subject children were 77, 81, and 77 in villages P, C, and B, respectively. Examination took around 1.5 h for each subject: thus, we examined around 35 to 40 children each day. |
| Ethical considerations |
| The entire protocol was evaluated and approved by the Ethical Committee of the Graduate School of Medicine, The University of Tokyo, as well as the Ethical Committee of the Faculty of Medicine, Padjadjaran University. |
| Study methods |
| Field survey: Examinations were conducted in September 2003. During the examinations, anthropometric measurement, urine sampling, blood sampling, a health examination, and an interview using a questionnaire for socio-demographic characterization were conducted for all children in this order. The results of the anthropometric measurement and health examination were reported to parents on site. |
| Biological monitoring using blood and urine samples: Urine samples were used to evaluate exposure to pesticides. Single-spot urine samples collected during the health examination in the morning (9 to 11 A.M.) were immediately frozen with dry ice. The samples were brought to Japan, and the major metabolites of various OP pesticides, dimethylphosphate (DMP), dimethylthiophosphate (DMTP), diethylphosphate (DEP), and diethylthiophosphate (DETP), were quantified by1-benzyl-3-p-tolyltriazene (BTT) derivatization [16] and gas chromatography using a flame photometric detector (FPD-GC). Standard materials such as DMP tetramethylammonium salt (99.9% purity), DMTP ammonium salt (98.9%), DEP (98.9%), and DETP ammonium salt (95.2%) were purchased from Hayashi Pure Chemical Ind. (Osaka, Japan), and BTT was purchased from Tokyo Kasei Kogyo (Tokyo, Japan). Owing to their insufficient volume, 37 samples were not used for the measurement of urinary metabolites. Quality control data was used to provide an overall assessment of the precision, accuracy, and overall reliability of the method. Spike sample recoveries and urine blank analysis were conducted for every set of 9 samples. Pooled urine from a healthy volunteer not being treated with any drugs nor exposed to chemicals was used as blanks and for spike recoveries. At a concentration of 20 μg/L, the within-series imprecision (CV%) was 5.5% for DMP, 8.7% for DMTP, 7.1% for DEP, and 11.3% for DETP (n=5). The between-day imprecision during 10 consecutive days was 8.0% for DMP, 6.1% for DMTP, 8.7% for DEP, and 11.3% for DETP. The mean recoveries within series and between days were 94.2 and 109.7% for DMP, 100.6 and 105.7% for DMTP, 93.9 and 94.7% for DEP, and 85.7 and 82.9% for DETP, respectively. The limit of detection (LOD) and quantitative limit were 1.0 and 2.0 μg/L for DMP and 0.5 and 1.0 μg/L for DMTP, DEP, and DETP, respectively, indicating similar or greater sensitivity to those in recent studies on urinary DMP levels [9,10,17]. Values below LOD were assigned a value of LOD/2. Total molar quantities (nmol/L) were calculated by combining individual DAPs for dimethyl and diethyl DAPs separately. Creatinine concentration (μg per dl) was determined by the Jaffe method. |
| Blood was taken from the fingertip with a finger-prick device by one of the authors (LD; an Indonesian physician). Immediately after sampling, the levels of AChE activity and Hb were determined using a commercial kit (EQM Research, Inc., USA). The former has been used as an effect indicator for anticholinesterase agents such as organophosphates and carbamates. |
| Socio-demographic and economic characteristics of the households: Information regarding socio-demographic and economic characteristics of the households of the participating children was obtained through interview. |
| Living environment and behavior of the children: Information regarding the children’s behavioral patterns, frequency of food consumption, and sources of drinking water was obtained through interview. The interview on the children’s behavior included such items as participation in agricultural work and playing near water sources. Food frequency questions included the frequencies of consumption of rice, vegetables, meat, and fish (freshwater fish and salted sea fish) during the past month. These interviews with the children were carried out in the presence of their parent(s) so that when a question or answer was not clear, it was clarified by the parent(s). |
| Statistical analysis: Means were compared by Student’s t-test for two samples. The Χ2-test was used for categorical variables. The means of the three groups were compared by analysis of variance (ANOVA) and a subsequent Tukey HSD comparison if the variance was significant. The distributions of the urinary metabolite levels were not normal, and the Mann-Whitney U-test for independent samples was used to determine significant differences between groups. All the statistical analyses were carried out using the SPSS software package (Version 10.0, SPSS Inc., Chicago). |
| Results |
| Socio-demographic characteristics of the subjects’ households |
| The socio-demographic characteristics of the subjects’ households are shown in Table 1. The percentage of agricultural workers differed among the three communities, and the largest was in village P, followed by C then B; very few agricultural workers were reported in village B. Among the agricultural workers, 20 and 46% of those in villages P and C, respectively, were landed farmers. In village B, a variety of fathers’ occupations were reported, including individual pisciculture operators, merchants, and construction workers, while for the mothers, housewives (65%) and overseas wage laborers (8%) were the most frequent occupations. Significant between-community differences were observed in terms of the educational level of the parents; in village P, the educational level was lower than those of the other two communities. |
| Urinary metabolites of organophosphorus pesticides |
| The major urinary metabolites of the OP pesticides, namely, DMP, DMTP, DEP, and DETP, were determined by gas chromatography. All these major metabolites were detected in at least one sample (Table 2).Detection rates varied between the metabolic species, that is, DMP, DMTP, DEP, and DETP were detected in 8.6, 21.7, 17.2, and 29.8% of the subjects, respectively (Table 2). The median levels of dimethyl-, diethyl-, and total DAP were 5.8, 3.1, and 11.8 nmol/L, respectively. Significant differences were found in terms of the detection rates among the communities, and village C had the lowest detection rates and mean levels except for DEP, for which the rate was comparable to that of village B (Table 2). |
| Acetyl cholinesterase (AChE) activity in blood |
| AChE activity in the blood samples was within the expected range and a significant difference was observed regarding the AChE activities in the blood samples; that is, children from village B (27.4±3.0 U/gHb) had lower values than those of the other two villages (28.7 ± 3.3 and 27.9 ± 3.6 U/gHb for villages P and C, respectively; Table 3). |
| AChE levels were compared between detected and non-detected groups for DMP (n=46 and n=150 for detected and non-detected, respectively), DEP (n=79 and n=117 for detected and non-detected, respectively), and total DAP (n=100 and n=96 for detected and nondetected, respectively). Results showed that children with detected levels of DMP had significantly lower AChE activity (27.2 ± 2.6 U/gHb) than the children whose DMP levels were below the LOD (28.3 ± 3.5 U/gHb). |
| Children’s living environment and behavioral patterns |
| To elucidate the potential sources of pesticide exposure, an interview was conducted on the children’s behavior (play behavior, participation in agricultural work, etc.), food consumption frequency, and sources of drinking water (Table 4). In villages P and C, spring water was mainly used as the source of drinking water, while in village B, well water was the predominant source. Participation in agricultural work was significantly higher in village P, while swimming in rivers, ponds, and lakes and playing near agricultural land were not different among the three villages. Children from village B consumed freshwater fish more frequently than those of the other two villages, although they consumed vegetables less frequently. |
| Discussion |
| The urinary concentration of OP metabolites was examined to assess the exposure to OP pesticides in previous non-occupational exposure studies, particularly in developed countries. In most of these studies, however, the health effect of chronic exposure was not examined. Eskenazi et al. [18] found a significant association between increased dimethylphosphate metabolite level and decreased gestational duration, particularly during late pregnancy among Latino women living in an agricultural community in California. This result indicates that pesticide exposure in late pregnancy may be associated with shorter pregnancies. Young et al. [19] examined the relationship between maternal OP urine metabolites and infant neuronal development using the Brazelton Neonatal Behavioral Assessment Scale for 381infants younger than 62 days of age. They found a significant association between increased total concentration of maternal urinary OP metabolites and increased numbers of abnormal reflexes in the infants. The exposure level in the present study was lower than that in these studies [18,19]; i.e., the median levels of dimethyl DAP (DMP, DMTP, and DMDTP), diethyl DAP (DEP, DETP, and DEDTP) and total DAP were 101, 22, and 136nmol/L in Eskenazi’s study, and 97, 21, and 132 nmol/L in Young’s study, which are higher than the median levels found in the present study, i.e., 5.8, 3.1, and 11.8nmol/L, respectively. |
| To the best of our knowledge, this is the first study on the urinary concentration of DAP compounds as a measure of OP pesticide exposure in rural communities of Indonesia. The exposure level in this study was not high compared with children’s exposure level in several developed countries such as US, Italy, and Canada [9,10,20]. With similar levels of detection sensitivity, the United States CDC’s nationwide survey in 2001 showed that more than 90% of the subjects were positive for at least one of the OP metabolites examined [17].The median levels of dimethyl- and diethyl-DAPs in this study, i.e., 5.8 and 3.1 nmol/L, were approximately 10 times lower than those reported inthe US [10], i.e., 90 and 60 nmol/L, respectively. Possible reasons for this result are considered as follows. First, chronic exposure from the diet is potentially very low in these rural communities. Studies in developed countries showed that children who consumed fresh fruit and related products had higher exposure levels to OP metabolites [21,22]. In our study area, children generally consume only indigenous tropical fruits with no pesticide treatment and rarely consume farmgrown fruits such as apples, oranges, and grapes. Thus, it is considered that exposure to OP pesticides via fruit consumption is potentially low among rural children in developing countries. Second, the use of OP pesticides that are metabolized to DAP compounds is not high in the study area. According to our interview results regarding the pesticide use in village C and its neighboring village, 13.7% of the pesticides used were OP pesticides (profenofos (13.5%) and glyphosate (0.2%)) [23]. According to Wessels et al. [17], metabolites of profenofos are not DAP compounds, which possibly explains the low DAP concentration in urine samples. The AChE activity in the blood samples of children from village B was significantly lower than those of the children from the other two villages. When the AChE activity was compared between positive and negative activity for each OP metabolite, however, the difference was only significant for DMP exposure, and the difference was less than 5%. Because AChE is more of a toxicity marker at high exposure levels [17] than a sensitive response marker at low exposure levels, the results suggested the exposure to OPs in these communities was not sufficiently severe to cause overt toxicity that could be detected by AChE measurement. |
| Before conducting this study, it was assumed that pesticide exposure would be most severe in village C, where vegetable crops were most intensively grown. The results, however, revealed the highest exposure in village B, which relies on piscicultural activities in the Saguling water reservoir. To elucidate possible sources of OPs, we conducted interviews on children’s behavior, food consumption frequency, and sources of drinking water. The results showed that village B had a high consumption of freshwater fish, a low consumption of vegetables, and used well water as drinking water. Many studies in developed countries showed a higher concentration of urinary DAP metabolites among children with high vegetable consumption. However, we could not find such a relation in this study. The intake of OPs from freshwater fish or drinking water does not seem plausible as the bioaccumulation of OPs in ecosystems has been reported to be minor [12]. |
| A limitation of our study is that we collected spot urine samples instead of 24-h total voids or first morning voids. As the study subjects were 1st or 2nd grade elementary school children and some of them did not have a latrine at their house, we judged that it was more reliable to systematically collect urine samples in the health examination spot where a public latrine was provided. Although this may have introduced random variability into the metabolite measurement, these errors should have occured randomly. |
| In conclusion, this study provides the first biological monitoring data on exposure to OP pesticides in a rural area of Indonesia. The concentration of urinary DAP metabolites among rural Indonesian children was lower than that reported in studies on children in other countries. A between-community difference was found in the urinary DAP metabolite level, although the route of OP pesticide intake was not elucidated. More work is required to clarify the potential exposure routes and also to characterize the exposure level of other subgroups of the population such as adults, mother-child pairs, and father-child pairs. |
References
- Dasgupta S,Meisner C, Wheeler D, Xuyen K, Thi Lam N (2007) Pesticide poisoning of farm workers-implications of blood test results from Vietnam. Int J Hyg Environ Health 210: 121-132.
- WHO. Public health impact of pesticides used in agriculture; 1990.
- Rosenstock L, Keifer M, Daniell WE, McConnell R, Claypoole K. Chronic central nervous system effects of acute organophosphate pesticide intoxication. Lancet. 1991; 338: 223-227.
- Pimental D, Acquay H, Biltonen M. Environmental and economic costs of pesticide use. Bioscience. 1992; 42: 750-760.
- FAO, UNEP and WHO. Child pesticide poisoning: information for advocacy and action. UNEP Chemicals, Switzerland; 2004.
- Eskenazi B, Bradman A, Castorina R (1999) Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect 107 Suppl 3: 409-419.
- Lu C, Fenske RA, Simcox NJ, Kalman D (2000) Pesticide exposure of children in an agricultural community: evidence of household proximity to farmland and take home exposure pathways. Environ Res 84: 290-302.
- WHO. Organophosphorous insecticides: A general introduction, Vol. 6, New York: World Health Organization; 1998.
- Aprea C,Strambi M, Novelli MT, Lunghini L, Bozzi N (2000) Biologic monitoring of exposure to organophosphorus pesticides in 195 Italian children. Environ Health Perspect 108: 521-525.
- Curl CL, Fenske RA, Kissel JC, Shirai JH, Moate TF, Griffith W. Evaluation of take-home organophosphorous pesticide exposure among agricultural workers and their children. Environ. Health Perspect. 2002; 110: A787--792.
- Lu C, Knutson DE, Fisker-Anderson J, Fenske RA. Biological monitoring survey of organophosphorus pesticide exposure among preschool children in the Seattle metropolitan area. Environ. Health Perspect.2001; 109: 299--303.
- Costa LG1 (2006) Current issues in organophosphate toxicology. ClinChimActa 366: 1-13.
- Grandjean P,Harari R, Barr DB, Debes F (2006) Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics 117: e546-556.
- Harari R,Julvez J, Murata K, Barr D, Bellinger DC, et al. (2010) Neurobehavioral deficits and increased blood pressure in school-age children prenatally exposed to pesticides. Environ Health Perspect 118: 890-896.
- Parikesit,Salim H, Triharyanto E, Gunawan B, Sunardi, et al. (2005) Multi-source water pollution in the Upper Citarum watershed, Indonesia, with special reference to its spatiotemporal variation. Environ Sci 12: 121-131.
- Daughton CG, Cook AM, Alexander M (1979) Gas chromatographic determination of phosphorus-containing pesticide metabolites via benzylation. Anal Chem 51: 1949-1953.
- Wessels D, Barr DB, Mendola P (2003) Use of biomarkers to indicate exposure of children to organophosphate pesticides: implications for a longitudinal study of children's environmental health. Environ Health Perspect 111: 1939-1946.
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, Furlong CE, Holland NT. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ. Health Perspect. 2004; 11, 1116--1124.
- Young JG,Eskenazi B, Gladstone EA, Bradman A, Pedersen L, et al. (2005) Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology 26: 199-209.
- Valcke M, Samuel O, Bouchard M, Dumas P, Belleville D, et al. (2006) Biological monitoring of exposure to organophosphate pesticides in children living in peri-urban areas of the Province of Quebec, Canada. Int Arch Occup Environ Health 79: 568-577.
- Curl CL,Fenske RA, Elgethun K (2003) Organophosphorus pesticide exposure of urban and suburban preschool children with organic and conventional diets. Environ Health Perspect 111: 377-382.
- Heudorf U, Angerer J, Drexler H (2004) Current internal exposure to pesticides in children and adolescents in Germany: urinary levels of metabolites of pyrethroid and organophosphorus insecticides. Int Arch Occup Environ Health 77: 67-72.
- Sekiyama M, Tanaka M, Gunawan B, Abdoellah O, Watanabe C (2007) Pesticide usage and its association with health symptoms among farmers in rural villages in West Java, Indonesia. Environ Sci 14 Suppl: 23-33.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14791
- [From(publication date):
April-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10138
- PDF downloads : 4653
