Oral Human Papillomavirus (HPV) Infection in Healthy Individuals and Patients with Head and Neck Squamous Cell Carcinoma (HNSCC)
Received: 14-Dec-2014 / Accepted Date: 12-Jan-2015 / Published Date: 15-Jan-2015 DOI: 10.4172/2161-1165.1000180
Abstract
Human papillomavirus (HPV) infection is the most common sexually transmitted disease. It also contributes significantly to infection-associated human malignancies, including cervical, anal, penile, vaginal, and vulvar cancer as well as a subset of head and neck squamous cell carcinoma (HNSCC). Despite our extensive knowledge on the role of HPV in the etiology of cervical cancer, cervical cancer screening, prevention, and clinical management, we have just begun to appreciate the role of HPV in the tumorigenesis of HNSCC. In this paper, we will review our current knowledge on the natural history of oral HPV infection in the general population, risk factors for oral HPV infection, the presence of novel HPV types infecting oral cavity, limitations of existing methods for oral HPV detection and its application in the clinical setting. Finally, we will also discuss the role of HPV vaccine in preventing HNSCC.
Keywords: HPV infection; Head and neck squamous cell carcinoma (HNSCC); Oral HPV; oropharyngeal cancer; Prophylactic vaccine
162549Introduction
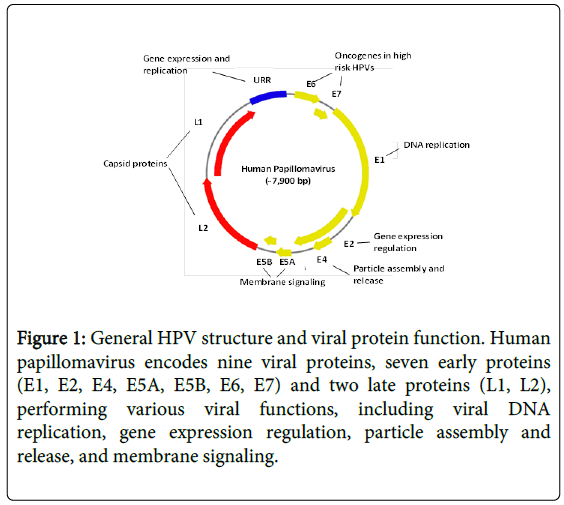
Human papillomavirus (HPV) is an ancient DNA virus present in earliest modern humans millions years ago. It is a small nonenveloped DNA virus with a genome of 8 kb, surrounded by an icosahedral capsid of 52-55 nm in diameter [1,2]. It encodes nine viral proteins, 7 early proteins (E1, E2, E4, E5A, E5B, E6, E7) and 2 late proteins (L1, L2) (Figure 1).
Figure 1: General HPV structure and viral protein function. Human papillomavirus encodes nine viral proteins, seven early proteins (E1, E2, E4, E5A, E5B, E6, E7) and two late proteins (L1, L2), performing various viral functions, including viral DNA replication, gene expression regulation, particle assembly and release, and membrane signaling.
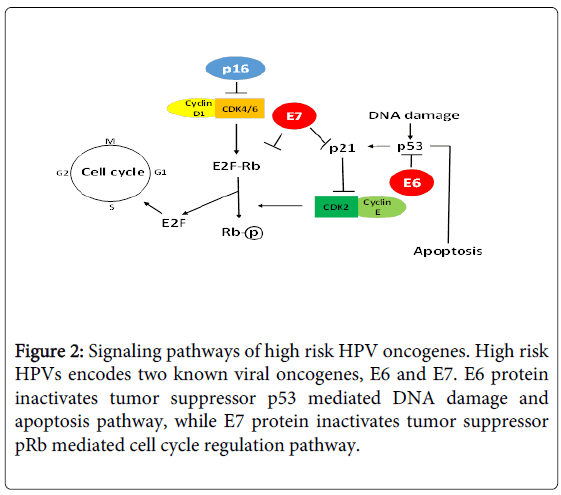
Currently, there are more than 190 full length HPV types being identified and classified into different genera based on genomic sequence, such as alpha-HPV, beta-HPV, and gamma-HPV [3,4] (https://pave.niaid.nih.gov/). HPV types are also classified into highrisk and low-risk types based on their association with cervical cancer [5,6] (Table 1), and their oncogenic mechanisms have been elucidated (Figure 2).
| Types | |
|---|---|
| High-risk | HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 |
| Low-risk | HPV 6, 11, 40, 42, 43, 44, 53, 54, 61, 72, 73, 81 |
| Detection | |
| Generic | Hybrid capture 2 |
| Type-specific | Roche Linear Array, Roche Cobas assay, real-time PCR assays |
Table 1: Genital HPV infection and detection.
HPV can also infect cutaneous epithelial cells and oral mucosal epithelial cells. Cutaneous HPV infection is ubiquitous and associated with increased risk for nonmelanoma skin cancer [7], while oral HPV infection is responsible for 20% of HNSCC [8]. Although oral HPV infection is rare in the general population compared to genital HPV infection, the incidence of HPV-positive HNSCC has increased significantly over the last two decades, with HPV 16 being the most prevalent type [9,10] (Table 2). Detection of HPV 16 infection in oral exfoliated cells increases the odds of oropharyngeal cancer more than 14 fold [11], and HPV-positive HNSCCs have better prognosis compared to HPV-negative HNSCCs [8,12]. Limited natural history studies on oral HPV infection suggest that the most significant risk factor for oral HPV infection is oral sexual behavior, and oral HPV can be transmitted from oral-genital, oral-digital contact [13,14]. Recent studies including ours also suggest the presence of novel HPV types in oral cavity, predominantly beta and gamma HPV types [15-18], though their role in oral carcinogenesis is unclear.
| Types | |
|---|---|
| Oncogenic | HPV 16 |
| Others | HPV 39, 51, 55, 62, 66, 84, novel beta and gamma HPVs |
| Detection | |
| Generic | p16 IHC |
| Type-specific | Roche Linear Array, real-time PCR assays, next generation sequencing |
Table 2: Oral HPV infection and detection.
In this review, we present our current knowledge on the natural history (incidence and risk factors) of oral HPV infection, oral HPV infection in HNSCC, current methods on oral HPV detection and isolation of novel oral HPV types. We will also discuss the potential impact of prophylactic HPV vaccine on the prevention of HNSCC.
Anal-genital HPV infection
Over 40 alpha-HPV types infect human anal-genital tract, representing the most prevalent sexually transmitted disease [19]. Epidemiological studies indicate that most men and women will acquire a sexually transmitted anogenital human papillomavirus (HPV) infection within their lifetime [19,20]. Majority of HPV infections are transient without clinical symptoms although a minority of infections (<5%) progress and develop clinical disease such as warts or malignancies. Almost 100% of cervical cancers, 90-93% of anal canal cancers, 36-40% of penile cancers, 40-64% of vaginal cancers and 40-51% of vulvar cancers are attributable to HPV infection [19,21]. Of the estimated 12.7 million cancers occurring globally in 2008, approximately 5% (600,000 cases) were HPV-associated anogenital cancers [22]. Since the discovery of HPV infection as the etiological agent of cervical cancer, HPV detection has played important roles in clinical management of cervical cancer. Earlier ASCUS/LSIL triage study (ALTS) demonstrated that the reflex HPV testing can be used to triage women with equivocal cytology diagnosis [23,24]. Currently, HPV co-testing is recommended for cytology-based cervical cancer screening to reduce follow-up loss [25]. Most recently, FDA has approved HPV testing for primary cervical cancer screening [26]. HPV testing is also used to monitor treatment response among patients with precancerous lesions [27]. Finally, our knowledge on HPV infection and cervical cancer has allowed the development of highly effective prophylactic HPV vaccines, which will eventually contribute to the elimination of cervical cancer [28,29].
Oral HPV infection incidence and risk factors
Existing epidemiological studies suggest that oral HPV is rare in the general population and its infection is most strongly associated with oral sex [30]. HPV 16 is the most commonly detected oral HPV infection; other high risk HPV types have also been detected [31]. Hang et al determined the prevalence of oral HPV infection among 5410 health individuals in the Chinese population using general PCR and sequencing on oral swab specimens [32]. The prevalence of alpha HPV types is 0.67% with HPV 16 being the most prevalent type, while the prevalence of cutaneous HPV types is 5.46% with HPV 3 being the most prevalent type. Cook et al determined oral HPV prevalence in 1010 healthy women in US using Roche Linear Array assay genotyping 37 mucosal (alpha) HPV types on oral samples [33]. The prevalence of any HPV infection is only 1.9%. A cross-sectional study of oral HPV infection in US men and women, aged 14 to 69 years, determined that the overall prevalence of HPV DNA in oral exfoliated cells was 6.9 percent with HPV 16 comprising 14.5% of total oral HPV infection. Men are three times more likely to have oral HPV infection than women (10.1 versus 3.6 percent) [31]. A recent systemic review of literature reported the prevalence of any oral HPV detected below 5%, and HPV 16 accounted for 28% of all HPV detected in the oral region [34]. Our own study on the incidence and prevalence of oral HPV infection in a cohort of male university students is consistent with these findings: with the prevalence at enrollment of 7.5% and 12- month cumulative incidence of 12.3% [35].
The most well documented risk factors of oral HPV infection are oro-genital sex and high risk sexual behavior [30,35]. Other studies reported the association of oral HPV infection with self-reported history of oral disease, alcohol and tobacco consumption, and marijuana usage [36]. Oral HPV infections are mainly acquired through oral sexual activities, but can also be acquired through selfinoculation or sharing of oral products [13,33]. Infants can get infected during vertical transmission during birth [37,38].
Limited virology studies suggest that most oral HPV infections are transient and cleared within 6 months to a year [39-41]. This is consistent with the observation that oral HPV infection tends to have a much lower viral load when compared to cervical and vaginal HPV infection [16,42,43].
Overall, the role of alpha HPV infection in oral cavity is very limited with HPV 16 being the most significant player. As current evidence indicates that there are many novel beta and gamma HPV types present in the oral cavity [15-18], the true prevalence of HPV infection in the oral cavity is unknown and likely underestimated.
Methods for oral HPV detection
Currently, there are no standardized tests for oral HPV detection, nor are there published guidelines. Methods developed for genital HPV detection have been applied to detect oral HPVs, which include both non-type specific and type-specific DNA-based assays [43,44]. Since HPV 16 represents the majority of HPV present in oral cavity and oral HPV viral loads tend to be much lower than in genital tracks, HPV 16 type-specific assay offers additional advantage by providing viral load information, which could be used to identify clinically significant HPV infection. Either oral swab rinse samples or oral tissue samples can be used for oral HPV detection. The majority of epidemiology studies on natural history of oral HPV have utilized either oral swab or rinse samples. Our study in healthy college students demonstrated good correlation of HPV detection in oral swab and rinse samples, though additional HPV types were also detected in both swab and rinse samples [35]. We recently compared three different methods on oral sample collection for HPV detection, using swab, tooth brush or gargling (unpublished data). Oral epithelial cells can be efficiently collected using all three methods. Since no single method is superior to the others, the optimal method for sample collection is specific to each individual. Future systematic studies are needed to identify and standardize the optimal sample collection method for oral HPV detection.
Incidence and risk factors of HNSCC
Head and neck squamous cell carcinomas (HNSCCs) are tumors arising from one of the five anatomic regions of the head and neck, including oral cavity, pharynx, larynx, nasal cavity and paranasal sinuses, and salivary glands. Worldwide, about 700,000 people are diagnosed of HNSCCs each year with an over 50% mortality rate [45-47].
The well-established risk factors for HNSCCs are tobacco use and alcohol consumption, which are responsible for approximately 75%-80% of all HNSCCs [48-51]. Additional risk factors include betel nut chewing [52], radiation exposure [53], vitamin deficiencies [54], periodontal disease [55], immunosuppression [56], and other environmental and occupational exposures [57,58]. Recently, oral HPV infection has been identified as a significant risk factor for 20% of HNSCCs, mainly for tumors originated from oropharynx (tonsils and base of tongue) [59,60]. The large geographic differences in the incidence and primary site of HNSCCs reflect the prevalence of risk factors of different regions and genetic predispositions associated with different ethnic populations [8]. Although the incidence of HNSCCs is highest among older males, it has been increasing in females as more women use tobacco and in young non-smokers as oral HPV incidence is increasing due to increased rate of oral sex among the young population. Synergistic effect has been reported among different risk factors, such as tobacco and alcohol, tobacco and HPV [61-64].
The management of HNSCC depends mainly on the tumor stage and location [65-67]. Early stage patients (stage I and II) are treated with either surgery or radiation therapy (RT), while advanced stage patients (stage III and IV) require multimodality treatment: surgery followed by radiation, concurrent chemoradiotherapy, or sequential therapy. Because organ preservation is one of the major objectives of treatment, proper staging as well as prognostic markers are required to achieve optimal outcome. HPV infection has been a significant prognostic factor in HNSCC because HPV-positive HNSCCs are more responsive to chemotherapy which translates to better overall survival [68,69].
HPV infection in HNSCC patients
Recently, human papillomavirus (HPV) infection has been identified as an etiologic agent for a subset of HNSCCs, with strongest association with oropharyngeal and tonsillar cancers [47,70,71], which in part explains the rise of incidence rate of HNSCC in European and North American countries [9,72,73], where tobacco-associated HNSCC has been declining. Although oral HPV infection is rare in healthy individuals (<5%) [40,74], the prevalence of HPV infection in HNSCC ranges from 10-20% in oral cavity cancer to 40-60% in oropharyngeal cancer [75-77]. It is estimated that the detection of HPV 16 infection in oral exfoliated cells increases the odds of oropharyngeal cancer more than 14 fold [70,78]. Furthermore, HPV integration and expression of E6 and E7 oncogenes have been detected in HNSCC tissues [60]. In summary, HPV is a significant etiological factor for HNSCC, with potential influence on the prevention, diagnosis and treatment of HNSCC.
Multiple studies suggest that HPV-positive HNSCCs are different from HPV-negative HNSCCs [60]: (1) HPV-positive HNSCC patients are approximately 10 years younger than HPV-negative patients and not associated with tobacco and alcohol consumption; (2) HPVpositive HNSCCs predominantly arise in the base of the tongue or the tonsillar region; (3) HPV-positive HNSCCs display a poorly differentiated basaloid-like histology [75]; (4) although HPV-positive HNSCCs represent biologically more aggressive form of cancer, they have better prognosis: lower incidence of distant metastases, less likelihood to develop second malignancy, and better responsiveness to therapy. In conclusion, the evidence argues for differentiation between HPV-positive and HPV-negative HNSCCs.
HPV detection in HNSCC patients
Recent studies suggest that HPV is a better prognostic marker than tumor stage in HNSCC, especially for oropharyngeal cancer, which spurs clinical interest on HPV testing [79-82]. It is recommended that HPV testing should be used as a routine test in HNSCC management because it not only provides prognosis information, but also offers individualized treatment. For example, HPV-positive patients can be managed with less toxic therapies; HPV-testing can help determine whether a bulky tumor is oropharyngeal origin, and distinguish second primary tumor from metastasis. In clinic, oral HPV infection has been detected using p16 as a surrogate marker [83,84]. p16 is a tumor suppressor gene that inhibits cyclin-dependent kinases, which plays an important role in cell cycle regulation and its expression is negatively regulated by pRb [85]. During HPV infection, oncogenic HPV E7 protein inactivates pRb, leading to p16 overexpression [84,86]. There are several advantages to use p16 as a surrogate marker for HPV infection: p16 overexpression is not HPV type specific, so p16 has the potential to detect novel HPV type infection; p16 overexpression can be detected by immunohistochemistry analysis (IHC) on tissue blocks, which is a routine assay in clinical settings. However, p16 IHC lacks the specificity for HPV infection, because p16 can be overexpressed in certain benign conditions, and p16 overexpression and HPV detection do not show perfect concordance. Future studies are needed to determine the impact of both p16 and HPV detection on patient survival.
Novel markers are being developed that not only detect HPV infection, but also predict the risk of disease progression in uterine cervical cancer. These include detection of HPV viral gene mRNA or protein [87,88], HPV genome methylation [89-91], and host genetic and epigenetic alterations [92,93]. Currently, it is unknown whether such markers have clinical implication in the management of HNSCC.
Novel oral HPV types and HNSCC
Despite the low prevalence (<10%) of oral alpha HPV infection in the general population, our study as well as other published studies strongly indicate the existence of additional HPV types in the oral cavity. Bottalico et al reported the presence of a wide spectrum of beta and gamma HPV types in oral cavity specimens [18], and cloned a novel beta HPV 120 from the oral cavity [17], which subsequently showed that, could be detected in multiple anatomical sites. We identified four and cloned three full length novel gamma HPV types from oral swab and rinse samples collected from healthy young college students using HPV degenerated PCR assays [16]. These data suggest that our understanding on natural history and transmission of oral HPV is still at the early stage.
It is unclear what the best way is to identify these novel HPV types. Traditionally, novel HPV viruses are identified using consensus PCR coupled with cloning. However, this method is labor intensive, low throughput and lacks the sensitivity to isolate low level HPVs. Although DNA microarray-based screening assays improve throughput [94,95], these methods will only detect viruses with extensive sequence homology to known HPV types. Novel HPV viral transcripts can also be detected using various subtractive assays, including representational difference analysis (RDA), computational subtraction, and digital transcript subtraction [96]. However, expression-based techniques rely on the expression of viral genes; therefore it would likely miss latent HPV viral infections or HPV viruses that are not actively transcribing.
We believe that the next generation sequencing technology represents an ideal method to identify novel HPV types present in oral cavity from both healthy individuals and cancer patients. Over the past several years, massive parallel DNA sequencing platforms have dramatically reduced the cost of DNA sequencing by over two orders of magnitude, enabling comprehensive genome analysis by individual investigators [97-99]. Currently there are five commercially available next generation sequencing platforms: the 454 pyro sequencing system from Roche (Branford, CT), the Solexa sequencing by synthesis technology from Illumina (San Diego, CA), the SOLiD sequencing by ligation platform from ABI (Foster City, CA) [97], the Ion Torrent sequencing by ion semiconductor technology from Life Technology (Grand Island, NY) [100], and SMRT single-molecule real-time sequencing technology from Pacific Biosciences (Menlo Park, CA) [101,102]. Each technology uses sophisticated chemical and enzymatic reactions to amplify single strands of fragmented sequencing library in a clonal fashion and perform massive parallel sequencing reactions on the amplified strands. Of these five sequencing technologies, the 454 system and SMRT technology are preferred choice for de novo sequencing, as in the case of discovery of novel viral pathogens [103,104], because it produces long reads (700 bp and 10,000 bp respectively) with extremely low error rate (accuracy of >99.5%), which allows accurate sequence assembly without the reference sequence (scaffold), and discovery of microbial sequences present at low frequency in a complex biological specimen. It has been used extensively in metagenomic analysis of various microbial communities [105-109], including the identification of several novel viruses, such as a novel bovine group A rotavirus associated with diarrhea of cows [110], novel circular DNA viruses from dragonfly larvae [111], a novel paramyxovirus associated with acute febrile disease [112], and a divergent human parainfluenza virus type 4 (HPIV4) [104].
One of the potential challenges is the low HPV viral loads in oral samples. Our unpublished data suggest that the average HPV viral loads in oral samples are at least 1000 fold lower than in genital samples. In some cases, there is less than one HPV virus per cell. The ability to detect HPV at low level depends on the sequencing depth. If HPV is present at 0.01 copies per cell, the probability to detect HPV sequence is 45% if 5x107 bp sequence reads are obtained from a sample, the probability increases to 99.8% if 5x108 bp sequence reads are obtained [96]. Alternatively, HPV present in the samples should be preferentially enriched using sequence independent methods. Our study demonstrated the ability of rolling circle amplification (RCA) can be used to preferentially amplify circular HPV genomic DNA present in the samples [16]. If enough samples are present, circular HPV DNA may be isolated using conventional plasmid isolation methods. However, both methods rely on the premise that HPV DNA is present as the circular form. If HPV is integrated, these methods will not be able to enrich it.
Unlike α-HPV types, it is unclear whether beta and gamma HPV types are oncogenic. There is evidence that beta HPVs might contribute to skin carcinogenesis in conjunction with UVR [113]. Beta HPV 38 E6 and E7 has been shown to act as promoters and progression factors in multi-stage skin carcinogenesis initiated by DNA damage agent DMBA [114,115], both HPV 5 and 8 E6 can increase the tolerance of UVB induced genomic instability [116,117], and HPV 49 E6 and E7 can immortalize primary human keratinocytes and deregulate both p53 and pRb pathways [118]. Very little is known about gamma HPV in tumorigenesis. The complex interaction among HPV, tobacco and alcohol makes it more difficult to elucidate the potential oncogenic mechanism of HPV in oral cancer [119]. This is further complicated by the low viral load of gamma HPV present in oral cavity specimens. It is likely that oral HPV infections induce tumorigenesis via a different mechanism from α-HPVs: they are required for initiation but not progression during oral tumorigenesis, according to the so called “hit and run” theory. If this is the case, it is not expected to detect oral HPV more frequently in tumor tissues than in normal tissues. One of the novel HPV types we identified, HPV 171, was more prevalent in malignant than in normal oral tissues and the viral load was significantly higher in malignant than in normal oral tissues, but it was not significant after adjusting for age [16]. Future studies are needed to determine oncogenic potential of these novel gamma HPV types.
In summary, there are many novel beta and gamma HPV types present in the oral cavity. These novel HPV types are best identified using the high throughput second and third generation sequencing technologies. Both molecular and clinical epidemiological studies are needed to determine their role in the development of HNSCC.
HPV vaccines and HNSCC
It is unknown whether HPV vaccines can be used to prevent and treat oropharyngeal cancer. Prophylactic HPV vaccination has been shown to be highly effective in preventing HPV infection as well as development of cervical diseases caused by the high risk HPV types the vaccine covered [28,29]. Currently, there are two commercially available vaccines: the quadrivalent vaccine Gardasil from Merck covering HPV 6, 11, 16 and 18, and the bivalent vaccine Cervarix from GSK covering HPV 16 and 18. Both are highly immunogenic and produce neutralizing antibodies to prevent HPV infection. Studies in young women demonstrated that seroconversion was almost 100%, the high titer of neutralizing antibodies is maintained even after 6-8 years and are also present in cervical mucus [120]. The vaccines are safe, highly effective in preventing persistent HPV infection as well as development of cervical high grade lesions. A new HPV vaccine is being developed by Merck against nine HPV types (HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58) which will protect over 90% cervical cancer [121]. Currently, there is only one clinical trial demonstrating the effectiveness of bivalent HPV vaccination in reducing the prevalence of oral HPV infection [122], and HPV vaccines are presently not recommended for preventing HNSCC. Because of the low prevalence of oral HPV infection, larger studies are needed to determine the impact of HPV vaccine on the reduction of oral HPV infection and HNSCC incidence.
Because the prophylactic vaccines have no effect on prevalent HPV infection, therapeutic vaccines are needed to generate cellular immunity against HPV infected cells and eliminate HPV-caused diseases. Unlike prophylactic vaccines which are based on HPV L1 VLPs, therapeutic vaccines are based on HPV E6 and E7 oncoproteins. These two proteins are constitutively expressed throughout different stages of HPV infection and play an essential role in the transformation of HPV-associated malignancies. Various different types of vaccines have been developed using either HPV E6/E7 peptides or DNA with different adjuvants, and they are delivered either systematically or locally [123]. Although these vaccines have shown to elicit cellular immune responses against HPV infection, these immune responses do not correlate with clinical outcome in cervical disease [124]. Several clinical trials are undergoing to determine the safety and efficacy of these therapeutic vaccines in eliminating HPV-positive oral cancer lesions. Although HPV therapeutic vaccines are still at the developmental stage, they represent the only hope to cure HNSCC caused by HPV infection.
Future directions
Our understandings on oral HPV in oral cancer have just begun and many questions remain unanswered. What is the optimal method for HPV detection in HNSCC? Can oral HPV detection be used for oral cancer screening? Are novel oral HPVs oncogenic? What is the impact of current prophylactic HPV vaccine on HNSCC incidence? Although much can be learned from our experience with HPV infection in cervical cancer, there are significant differences between these two scenarios: at least 13 high risk HPV types are involved in cervical cancer, while only HPV 16 is significantly involved in HNSCC. The potential HPV type replacement might play a more significant role in HNSCC than in cervical cancer after the implementation of HPV vaccination; the viral load in the oral cavity is significantly lower than in the cervix, which makes it more difficult to prove the role of HPV infection in HNSCC tumorigenesis and the oncogenic mechanism in these two cancers will be different. We truly believe that continued understanding of oral HPV infection in HNSCC will eventually lead to improved survival and reduced mortality in HNSCC patients.
References
- Hagensee ME, Yaegashi N, Galloway DA (1993) Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol 67: 322
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT (1992) Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A 89: 12184.
- Bzhalava D, Guan P, Franceschi S, Dillner J, Clifford G (2013) A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 445: 231.
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H (2004) Classification of papillomaviruses. Virology 324: 27
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S (2007) Human papillomavirus and cervical cancer. Lancet 370: 907.
- Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118: 3044.
- Dubina M, Goldenberg G (2009) Viral-associated nonmelanoma skin cancers: a review. Am J Dermatopathol 31: 573.
- Friedman JM, Stavas MJ, Cmelak AJ (2014) Clinical and scientific impact of human papillomavirus on head and neck cancer. World J Clin Oncol 5: 791.
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML (2008) Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 26: 619.
- Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, et al. (2006) Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer 119: 2620-3
- Gillison ML (2008) Human papillomavirus-related diseases: oropharynx cancers and potential implications for adolescent HPV vaccination. J Adolesc Health 43: S60.
- Blitzer GC, Smith MA, Harris SL, Kimple RJ (2014) Review of the clinical and biologic aspects of human papillomavirus-positive squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys 2014, 88, 770
- Giuliano AR, Nyitray AG, Kreimer AR, Pierce Campbell CM, Goodman MT, et al. (2014) EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer.
- Videla S, Darwich L, Canadas M, Clotet B, Sirera G (2014) Incidence and clinical management of oral human papillomavirus infection in men: a series of key short messages. Expert Rev Anti Infect Ther, 12, 957.
- Ure AE, Forslund O (2014) Characterization of human papillomavirus type 154 and tissue tropism of gammapapillomaviruses. PLoS One 2014, 9, e89342.
- Martin E, Dang J, Bzhalava D, Stern J, Edelstein ZR, et al. (2014) Characterization of three novel human papillomavirus types isolated from oral rinse samples of healthy individuals. J Clin Virol, 59, 37.
- Bottalico D, Chen Z, Kocjan BJ, Seme K, Poljak M, et al. (2012) Characterization of human papillomavirus type 120: a novel betapapillomavirus with tropism for multiple anatomical niches. J Gen Virol, 93, 1779.
- Bottalico D, Chen Z, Dunne A, Ostoloza J, McKinney S, et al. (2011) The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J Infect Dis, 204, 792.
- Bhatia N, Lynde C, Vender R, Bourcier M (2013) Understanding genital warts: epidemiology, pathogenesis, and burden of disease of human papillomavirus. J Cutan Med Surg, 17 Suppl 2, S54.
- Chelimo C, Wouldes TA, Cameron LD, Elwood JM (2013) Risk factors for and prevention of human papillomaviruses (HPV) genital warts and cervical cancer. J Infect, 66, 217.
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, et al. (2012) Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 Suppl 5, F23.
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, et al. (2012) Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol, 13, 615.
- TSA ASCUS-LSIL (2003) Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol, 188, 1392.
- Solomon D, Schiffman M, Tarone R (2001) Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst, 93, 299.
- Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, et al. (2011) Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol, 12, 672.
- Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, et al. (2006) Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer, 119, 1101.
- Jeong NH, Lee NW, Kim HJ, Kim T, Lee KW (2009) High-risk human papillomavirus testing for monitoring patients treated for high-grade cervical intraepithelial neoplasia. J Obstet Gynaecol Res, 35, 711.
- Delere Y, Wichmann O, Klug SJ, van der Sande M, Terhardt M, et al. (2014) The efficacy and duration of vaccine protection against human papillomavirus: a systematic review and meta-analysis. Dtsch Arztebl Int, 111, 591.
- Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, et al. (2012) Human papillomavirus vaccine introduction--the first five years. Vaccine, 30 Suppl 5, F148.
- Chung CH, Bagheri A, D'Souza G (2014) Epidemiology of oral human papillomavirus infection. Oral Oncol 50: 369.
- Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, et al. (2012) Kahle L, Graubard BI, Chaturvedi AK. Prevalence of oral HPV infection in the United States, JAMA 307: 703.
- Hang D, Liu F, Liu M, He Z, Sun M, et al. (2014) Oral Human Papillomavirus Infection and Its Risk Factors among 5,410 Healthy Adults in China, Cancer Epidemiol Biomarkers Prev 23: 2110.
- Cook RL, Thompson EL, Kelso NE, Friary J, Hosford J, et al. (2014) Barkley P, Dodd VJ, Abrahamsen M, Ajinkya S, Obesso PD, Rashid MH, Giuliano AR. Sexual behaviors and other risk factors for oral human papillomavirus infections in young women. Sex Transm Dis 41: 492
- Kreimer AR, Bhatia RK, Messeguer AL, Gonzalez P, Herrero R, et al. (2010) Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex Transm Dis 37: 391.
- Edelstein ZR, Schwartz SM, Hawes S, Hughes JP, Feng Q, et al. (2012) Rates and determinants of oral human papillomavirus infection in young men. Sex Transm Dis 39: 867.
- Gillison ML, Alemany L, Snijders PJ, Chaturvedi A, Steinberg BM, et al. (2012) Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine: 30 Suppl 5: F54.
- Hahn HS, Kee MK, Kim HJ, Kim MY, Kang YS, et al. (2013) Distribution of maternal and infant human papillomavirus: risk factors associated with vertical transmission. Eur J Obstet Gynecol Reprod Biol, 169: 206.
- Smith EM, Parker MA, Rubenstein LM, Haugen TH, Hamsikova E, et al. (2010) Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol: 326369.
- Mooij SH, Boot HJ, Speksnijder AG, Meijer CJ, King AJ, et al. (2014) Six-month incidence and persistence of oral HPV infection in HIV-negative and HIV-infected men who have sex with men. PLoS One 9: e98955.
- Kreimer AR, Pierce Campbell CM, Lin HY, Fulp W, Papenfuss MR, et al. (2013) Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet 382: 887.
- Wright TC Jr (2009) Natural history of HPV infections. J Fam Pract 58: S7.
- Huang CG, Lee LA, Tsao KC, Liao CT, Yang LY, et al. (2014) Human papillomavirus 16/18 E7 viral loads predict distant metastasis in oral cavity squamous cell carcinoma. J Clin Virol 61: 236.
- Chaturvedi AK, Graubard BI, Pickard RK, Xiao W, Gillison ML (2014) High-risk oral human papillomavirus load in the US population, National Health and Nutrition Examination Survey 2009-2010. J Infect Dis 210: 441-7. PMCID: 4110460.
- Pickard RK, Xiao W, Broutian TR, He X, Gillison ML (2012) The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18-30 years. Sex Transm Dis 39: 559-66.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90.
- Syrjanen S (2005) Human papillomavirus (HPV) in head and neck cancer. J Clin Virol 32 Suppl 1: S59-66.
- Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, et al. (2003) Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 95: 1772-83
- Wyss A, Hashibe M, Chuang SC, Lee YC, Zhang ZF, et al. (2013) Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am J Epidemiol 178: 679-90. PMCID: 3755640.
- Marur S, D'Souza G, Westra WH, Forastiere AA (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11: 781-9.
- Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, et al. (2007) Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst 99: 777-89.
- Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S,(1998) Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res 18: 4779-86.
- Guha N, Warnakulasuriya S, Vlaanderen J, Straif K (2014) Betel quid chewing and the risk of oral and oropharyngeal cancers: a meta-analysis with implications for cancer control. Int J Cancer 135: 1443
- Sale KA, Wallace DI, Girod DA, Tsue TT (2004) Radiation-induced malignancy of the head and neck. Otolaryngol Head Neck Surg 131: 645
- Kane MA (2005) The role of folates in squamous cell carcinoma of the head and neck. Cancer Detect Prev 29: 53.
- Spitz MR (1994) Epidemiology and risk factors for head and neck cancer. Semin Oncol; 21: 288.
- Deeb R, Sharma S, Mahan M, Al-Khudari S, Hall F, et al. (2012) Head and neck cancer in transplant recipients. Laryngoscope 122: 1569.
- Fayosse A, Menvielle G, Cyr D, Sanchez M, Stucker I, et al. (2014) 0279 Head and neck cancer and occupational exposure to chlorinated solvents: results from the ICARE study. Occup Environ Med 71 Suppl 1: A100.
- Paget-Bailly S, Cyr D, Carton M, Guida F, Stucker I, et al. (2014) 0234 Head and neck cancer and occupational exposure to asbestos, mineral wools and silica: results from the ICARE study. Occup Environ Med 71 Suppl 1: A90.
- Joseph AW, D'Souza G (2012) Epidemiology of human papillomavirus-related head and neck cancer. Otolaryngol Clin North Am 45: 764.
- Ragin CC, Modugno F, Gollin SM (2007) The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res 86: 114.
- Fakhry C, Gillison ML, D'Souza G (2014) Tobacco use and oral HPV-16 infection. JAMA 312: 1467.
- Prabhu A, Obi KO, Rubenstein JH (2014) The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am J Gastroenterol 109: 827.
- Singh J, Ramamoorthi R, Baxi S, Jayaraj R, Thomas M (2014) The risk factors of head and neck cancer and their general patterns in Australia: a descriptive review and update. J Environ Pathol Toxicol Oncol 33: 57.
- Curado MP, Boyle P (2013) Epidemiology of head and neck squamous cell carcinoma not related to tobacco or alcohol. Curr Opin Oncol 25: 234.
- Siddiqui F, Yao M. Application of fluorodeoxyglucose positron emission tomography in the management of head and neck cancers. World J Radiol 6: 251.
- Garg MK, Glanzman J, Kalnicki S (2012) The evolving role of positron emission tomography-computed tomography in organ-preserving treatment of head and neck cancer. Semin Nucl Med 42: 327.
- Miller ME, Palla B, Chen Q, Elashoff DA, Abemayor E, et al. (2012) A novel classification system for perineural invasion in noncutaneous head and neck squamous cell carcinoma: histologic subcategories and patient outcomes. Am J Otolaryngol 33: 215.
- Minassian M. HPV-Positive Head and Neck Cancers: A Review of the Literature. J Dent Hyg 88: 201.
- Garbuglia AR (2014) Human papillomavirus in head and neck cancer. Cancers (Basel) 6: 1726.
- D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, et al. (2007) Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356: 1956.
- Hobbs CG, Sterne JA, Bailey M, Heyderman RS, Birchall MA, et al. (2006) Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol 31: 266.
- Evans M, Newcombe R, Fiander A, Powell J, Rolles M, Thavaraj S, Robinson M, Powell N. Human Papillomavirus-associated oropharyngeal cancer: an observational study of diagnosis, prevalence and prognosis in a UK population. BMC Cancer 13: 220.
- Cleveland JL, Junger ML, Saraiya M, Markowitz LE, Dunne EF, et al. (2011) The connection between human papillomavirus and oropharyngeal squamous cell carcinomas in the United States: implications for dentistry. J Am Dent Assoc 142: 924.
- Kreimer AR, Villa A, Nyitray AG, Abrahamsen M, Papenfuss M, et al. (2011) The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol Biomarkers Prev 20: 182.
- Hennessey PT, Westra WH, Califano JA (2009) Human papillomavirus and head and neck squamous cell carcinoma: recent evidence and clinical implications. J Dent Res 88: 306.
- Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, et al. (2008) Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 100: 420.
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, et al. (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92: 720.
- D'Souza G, Fakhry C, Sugar EA, Seaberg EC, Weber K, et al. (2007) Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer 121: 150.
- Chung CH, Zhang Q, Kong CS, Harris J, Fertig EJ, et al. (2014) p16 Protein Expression and Human Papillomavirus Status As Prognostic Biomarkers of Nonoropharyngeal Head and Neck Squamous Cell Carcinoma. J Clin Oncol.
- Bogusiak K, Kobos J (2014) The role of human papillomavirus infection in the head and neck region and methods for its detection. Pol J Pathol 65: 14.
- Bussu F, Sali M, Gallus R, Petrone G, Zannoni GF, Autorino R, et al. (2014) Human papillomavirus (HPV) infection in squamous cell carcinomas arising from the oropharynx: detection of HPV DNA and p16 immunohistochemistry as diagnostic and prognostic indicators--a pilot study. Int J Radiat Oncol Biol Phys 89: 1120.
- Deng Z, Hasegawa M, Aoki K, Matayoshi S, Kiyuna A, et al. (2014) A comprehensive evaluation of human papillomavirus positive status and p16INK4a overexpression as a prognostic biomarker in head and neck squamous cell carcinoma. Int J Oncol 45: 76.
- Wang H, Sun R, Lin H, Hu WH (2013) P16INK4A as a surrogate biomarker for human papillomavirus-associated oropharyngeal carcinoma: consideration of some aspects. Cancer Sci 104: 1559.
- Mooren JJ, Gultekin SE, Straetmans JM, Haesevoets A, Peutz-Kootstra CJ, Et al. (2014) P16(INK4A) immunostaining is a strong indicator for high-risk-HPV-associated oropharyngeal carcinomas and dysplasias, but is unreliable to predict low-risk-HPV-infection in head and neck papillomas and laryngeal dysplasias. Int J Cancer 134: 2117.
- Hara E, Smith R, Parry D, Tahara H, Stone S, et al. (1996) Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol 16: 676.
- Gronhoj Larsen C, Gyldenlove M, Jensen DH, Therkildsen MH, Kiss K, et al. (2014) Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: a systematic review. Br J Cancer 110: 1587-94.
- Zappacosta R, Gatta DM, Marinucci P, Capanna S, Lattanzio G, et al. (2014) Role of E6/E7 mRNA test in the diagnostic algorithm of HPV-positive patients showing ASCUS and LSIL: clinical and economic implications in a publicly financed healthcare system. Expert Rev Mol Diagn: 1-14.
- Omran OM, AlSheeha M (2014) Human Papilloma Virus Early Proteins E6 (HPV16/18-E6) and the Cell Cycle Marker P16 (INK4a) are Useful Prognostic Markers in Uterine Cervical Carcinomas in Qassim Region- Saudi Arabia. Pathol Oncol Res.
- Brandsma JL, Harigopal M, Kiviat NB, Sun Y, Deng Y, et al. (2014) Methylation of twelve CpGs in human papillomavirus type 16 (HPV16) as an informative biomarker for the triage of women positive for HPV16 infection. Cancer Prev Res (Phila 7: 533.
- Clarke MA, Wentzensen N, Mirabello L, Ghosh A, Wacholder S, et al. (2012) Human papillomavirus DNA methylation as a potential biomarker for cervical cancer. Cancer Epidemiol Biomarkers Prev 21: 2137.
- Brandsma JL, Sun Y, Lizardi PM, Tuck DP, Zelterman D, et al. (2009) Distinct human papillomavirus type 16 methylomes in cervical cells at different stages of premalignancy. Virology 389: 107.
- Steenbergen RD, Snijders PJ, Heideman DA, Meijer CJ (2014) Clinical implications of (epi)genetic changes in HPV-induced cervical precancerous lesions. Nat Rev Cancer 14: 405.
- Wentzensen N, Sherman ME, Schiffman M, Wang SS (2009) Utility of methylation markers in cervical cancer early detection: appraisal of the state-of-the-science. Gynecol Oncol 112: 299.
- Kistler A, Avila PC, Rouskin S, Wang D, Ward T, et al. (2007) Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis 196: 825.
- Wang D, Urisman A, Liu YT, Springer M, Ksiazek TG, et al. (2003) Viral discovery and sequence recovery using DNA microarrays. PLoS Biol 1: E2.
- Duncan CG, Leary RJ, Lin JC, Cummins J, Di C, et al. (2009) Yan H. Identification of microbial DNA in human cancer. BMC Med Genomics 2: 22.
- Ansorge WJ (2009) Next-generation DNA sequencing techniques. N Biotechnol 25: 203.
- Shendure J, Ji H (2008) Next-generation DNA sequencing. Nat Biotechnol 26: 1145.
- Mardis ER (2008) Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet 9: 402.
- Rothberg JM, Hinz W, Rearick TM, Schultz J, Mileski W, et al. (2011) An integrated semiconductor device enabling non-optical genome sequencing. Nature 475: 352.
- Flusberg BA, Webster DR, Lee JH, Travers KJ, Olivares EC, et al. (2010) Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat Methods 7: 461-5.
- Eid J, Fehr A, Gray J, Luong K, Lyle J, et al. (2009) Real-time DNA sequencing from single polymerase molecules. Science 323: 138.
- Droege M, Hill B (2008) The Genome Sequencer FLX System--longer reads, more applications, straight forward bioinformatics and more complete data sets. J Biotechnol 136: 10.
- Alquezar-Planas DE, Mourier T, Bruhn CA, Hansen AJ, Vitcetz SN, et al. (2013) Discovery of a divergent HPIV4 from respiratory secretions using second and third generation metagenomic sequencing. Sci Rep 3: 2468.
- Patel V, Patel AK, Parmar NR, Patel AB, Reddy B, et al. (2014) Characterization of the rumen microbiome of Indian Kankrej cattle (Bos indicus) adapted to different forage diet. Appl Microbiol Biotechnol.
- Ghai R, Mizuno CM, Picazo A, Camacho A, Rodriguez-Valera F (2014) Key roles for freshwater Actinobacteria revealed by deep metagenomic sequencing. Mol Ecol.
- Moore NE, Wang J, Hewitt J, Croucher D, Williamson DA, et al. (2014) Paine S, Yen S, Greening GE, Hall RJ. Metagenomic analysis of viruses in feces from unsolved outbreaks of gastroenteritis in humans. J Clin Microbiol.
- Hyde ER, Luk B, Cron S, Kusic L, McCue T, et al. (2014) Characterization of the rat oral microbiome and the effects of dietary nitrate. Free Radic Biol Med.
- Schurch AC, Schipper D, Bijl MA, Dau J, Beckmen KB, Schapendonk CM, Raj VS, Osterhaus AD, Haagmans BL, Tryland M, Smits SL. Metagenomic survey for viruses in Western Arctic caribou, Alaska, through iterative assembly of taxonomic units. PLoS One 9: e105227.
- Masuda T, Nagai M, Yamasato H, Tsuchiaka S, Okazaki S, et al. (2014) Identification of novel bovine group A rotavirus G15P[14] strain from epizootic diarrhea of adult cows by de novo sequencing using a next-generation sequencer. Vet Microbiol 171: 73.
- Dayaram A, Galatowitsch M, Harding JS, Arguello-Astorga GR, Varsani A (2014) Novel circular DNA viruses identified in Procordulia grayi and Xanthocnemis zealandica larvae using metagenomic approaches. Infect Genet Evol 22: 141.
- Albarino CG, Foltzer M, Towner JS, Rowe LA, Campbell S, et al. (2014) Novel paramyxovirus associated with severe acute febrile disease, South Sudan and Uganda, 2012. Emerg Infect Dis 20: 216.
- Kiviat NB (1999) Papillomaviruses in non-melanoma skin cancer: epidemiological aspects. Semin Cancer Biol 9: 403
- Viarisio D, Mueller-Decker K, Kloz U, Aengeneyndt B, Kopp-Schneider A, et al. (2011) E6 and E7 from beta HPV38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice. PLoS Pathog 7.
- Viarisio D, Decker KM, Aengeneyndt B, Flechtenmacher C, Gissmann L, et al. (2013) Human papillomavirus type 38 E6 and E7 act as tumour promoters during chemically induced skin carcinogenesis. J Gen Virol 94: 752.
- Wallace NA, Robinson K, Galloway DA (2014) Beta human papillomavirus E6 expression inhibits stabilization of p53 and increases tolerance of genomic instability. J Virol 88: 6127.
- Wallace NA, Robinson K, Howie HL, Galloway DA. HPV 5 and 8 E6 abrogate ATR activity resulting in increased persistence of UVB induced DNA damage. PLoS Pathog 8: e1002807.
- Cornet I, Bouvard V, Campo MS, Thomas M, Banks L, et al. (2012) Comparative analysis of transforming properties of E6 and E7 from different beta human papillomavirus types. J Virol 86: 2366-70.
- Smith EM, Rubenstein LM, Haugen TH, Pawlita M, Turek LP (2012) Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: a case for multifactor disease. J Oncol: 571862.
- Wierzbicka M, Jozefiak A, Jackowska J, Szydlowski J, Gozdzicka-Jozefiak A (2014) HPV vaccination in head and neck HPV-related pathologies. Otolaryngol Pol 68: 173.
- Joura EA, Ault KA, Bosch FX, Brown D, Cuzick J, et al. (2014) Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev 23:2008.
- Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, et al. (2013) Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 8: e68329.
- Meyer SI, Fuglsang K, Blaakaer J (2014) Cell-mediated immune response: a clinical review of the therapeutic potential of human papillomavirus vaccination. Acta Obstet Gynecol Scand 93: 1218.
- Han KT, Sin JI (2013) DNA vaccines targeting human papillomavirus-associated diseases: progresses in animal and clinical studies. Clin Exp Vaccine Res 2: 114.
Citation: Dang J, Manrique HXZ, Veron D, Feng Q (2015) Oral Human Papillomavirus (HPV) Infection in Healthy Individuals and Patients with Head and Neck Squamous Cell Carcinoma (HNSCC). Epidemiology(sunnyvale) 5:180. DOI: 10.4172/2161-1165.1000180
Copyright: © 2015 Dang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 19691
- [From(publication date): 3-2015 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 14881
- PDF downloads: 4810