Research Article Open Access
Optimization of Processing Conditions for Aqueous Pigmented Rice Extracts as Bases for Antioxidant Drinks
| Adyati Putriekasari Handayani, Roselina Karim and Kharidah Muhammad* | ||
| Universiti Putra Malaysia, Serdang 43400, Selangor Darul Ehsan, Malaysia | ||
| Corresponding Author : | Kharidah Muhammad Faculty of Food Science and Technology Universiti Putra Malaysia Serdang 43400, Selangor Darul Ehsan, Malaysia Tel: +603-8946-8394 Fax: +603-8942-3552 E-mail: kharidah@upm.edu.my |
|
| Received December 04, 2014; Accepted February 26, 2015; Published February 29, 2015 | ||
| Citation: Handayani AP, Karim R, Muhammad K (2015) Optimization of Processing Conditions for Aqueous Pigmented Rice Extracts as Bases for Antioxidant Drinks. J Rice Res 3:135. doi: 10.4172/2375-4338.1000135 | ||
| Copyright: © 2015 Handayani AP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Rice Research: Open Access
Abstract
Pigmented rice can be categorized as a functional food due to its various health benefits, mainly from its polar antioxidant content which consists of anthocyanins in black rice and proanthocyanidins in red rice. This rice is usually cooked in excess water and removal of the water will be a waste as it can be further utilized as a base for antioxidant drink. Therefore, the objective of this study was to determine the optimum processing conditions (extraction temperature, time, and water/rice (W/R) ratio) for minimum 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity IC50, maximum total flavonoid (TFC), and Maximum Total Phenolic Content (TPC) in the pigmented rice extracts using response surface methodology (RSM). The optimum hot water extraction conditions for black rice were W/R ratio of 20 ml/g at 95.6°C for 40 minutes, while that for red rice are W/R ratio of 20 ml/g at 97°C for 30 minutes. It can be concluded that RSM is a useful method in optimizing the processing conditions for production of antioxidant drink from pigmented rice and hot water extraction showed great potential in extracting antioxidants from pigmented rice.
| Keywords | |
| Red rice; Black rice; Antioxidant drink; Response surface methodology; Optimization | |
| Introduction | |
| The color pigments on the bran layer of black rice are anthocyanins [1] while that of red rice are proanthocyanidins [2]. Previous investigations have shown that anthocyanins and proanthocyanidins contained possessed anti-oxidative and anti-inflammatory activities [3], anti-cancer [4], and various other healthful advantages. These compounds, however, are water soluble and can leach into cooking water. Finocchiaro et al. [5] found that the cooking water of red rice contained 57.66% of the total antioxidant content of red rice, showing that the majority of the antioxidants in red rice were lost into the cooking water. Compared to fruits and vegetables which were commonly used as raw materials for antioxidant beverages, pigmented rice is less perishable, can be stored for longer time due to its low moisture [6], and is cheaper in price especially compared to anthocyanin-rich berries or blackcurrants [7]. Therefore, it can be seen that the cooking water of pigmented rice, which is in the form of an aqueous extract, has the potential to be the base for an antioxidant drink. | |
| Hot water can be used in the extraction of antioxidant compounds from pigmented rice to be further processed into antioxidant drinks. Hot Water Extraction (HWE) has better extraction efficiency compared to cold water extraction due to the change in diffusivity characteristics, viscosity, permittivity, and surface tension [8]. Furthermore, usage of solvents such as ethanol to extract antioxidants from pigmented rice for application in beverages is not recommended as it may affect the Halal status of the beverages. | |
| Response Surface Methodology (RSM) is an effective statistical technique useful for developing, improving, and optimizing processes [9]. The principle of the approach has been reviewed previously by Henika [10,11] and Giovanni [12]. It is a designed regression analysis used to predict the value of a dependent variable based on the controlled values of the independent variables [13,14]. RSM is preferred in this study for the process of optimization because it is able to lead to the need for an experimental design which can generate multiple samples for consumer evaluation in a short period of time, and thus laboratory level tests are more efficient [15]. From the parameter estimates, it can be determined which variable contributes the most to the prediction model, which enables the user to focus on the variables which are most important to the product [16]. | |
| The objective of this study was to determine the optimum processing conditions (extraction temperature, time, and water/rice ratio) for minimum DPPH radical scavenging activity IC50, maximum Total Flavonoid Content (TFC), and Maximum Total Phenolic Content (TPC) in pigmented rice extracted by HWE for further application in antioxidant drinks. | |
| Materials and Methods | |
| Source of materials | |
| Two types of Malaysian commercial pigmented rice were purchased from local supermarkets. Both rice samples were from indica subspecies, non-glutinous type, and contained intermediate level of amylose (20-25%). | |
| Reagents and chemicals | |
| Gallic acid, sodium acetate (CH3COONa), sodium carbonate (Na2CO3), sodium nitrate (NaNO2), (+)-catechin, and 1,1-diphenyl- 2-picrylhydrazyl (DPPH) were from Sigma-Aldrich (St. Louis, MO, USA). Aluminium chloride (AlCl3), Folin-Ciocalteu phenol reagent, iron (III) chloride hexahydrate (FeCl3.6H2O), and hydrochloric acid (HCl) were from Merck (Germany). NaOH was from R&M Chemicals (Essex, UK). Acetic acid glacial (C2H4O2) was from JT Baker (New Jersey, USA). | |
| Preparation of aqueous pigmented rice extracts | |
| Optimization of processing conditions for production of aqueous pigmented rice extracts was conducted by mixing 5 g of pigmented rice with different volumes of distilled water once the temperature had reached the specified temperature based on the experimental design in a Julabo SW23 shaking waterbath (Julabo Labortechnik GmbH, Seelbach, Germany). After the required time, the aqueous pigmented rice extracts were filtered and centrifuged at 3000 × g for 10 minutes. The supernatant was used for analyses. | |
| Determination of DPPH radical scavenging activity IC50 | |
| The free radical scavenging activity of samples on DPPH radical was carried out according to the procedure described by Brand- Williams et al. [17]. Concentration of the sample which is required to scavenge 50% of the DPPH free radicals (IC50) was estimated using a nonlinear regression algorithm | |
| Determination of total phenolic content (TPC) | |
| Total phenolic content of samples was measured by Folin Ciocalteu reagent assay conveyed by Slinkard and Singleton [18] and Singleton et al. [19]. Results were expressed as milligrams of Gallic Acid Equivalent (GAE) per ml of extract. | |
| Determination of total flavonoid content (TFC) | |
| Determination of total flavonoid content was conducted by using aluminium chloride colorimetric assay as described by Shams Ardekani et al. [20]. Total flavonoid content was expressed as mg catechin equivalents (CE) per one ml of extract. | |
| Experimental design | |
| Central Composite Design (CCD) with three factors (X1, X2, X3) and three levels (-1, 0, +1) was used to design the experiments (Table 1). The factors selected based on the preliminary study were water/rice ratio (X1, ml/g), extraction time (X2, min), and extraction temperature (X3, oC), while the response variables were TPC (mg GAE/ml of aqueous pigmented rice extracts), TFC (μM TE), and DPPH radical scavenging activity IC50. | |
| Each experiment was performed in triplicates and the average values were taken as the response, Y. Experimental data were fitted to second order polynomial model and regression coefficients were obtained. The generalized second-order polynomial model proposed for the response surface analysis was as follows: | |
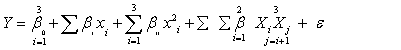 (1) (1) |
|
| where y is the response variable and β0, βi, βii, and βij are the regression coefficients for intercept, linear, quadratic, and interaction terms, respectively. Xi and Xj, are coded values of the independent variables while k equals to the number of the tested factors (k=3). | |
| All analyses were carried out in triplicates and the experimental values were reported as means ± standard deviations. Statistical analysis was performed using the Minitab 14.0 (Minitab Inc., State College, PA, USA) software and fitted to a second-order polynomial regression model containing the coefficient of linear, quadratic, and interaction terms. An Analysis Of Variance (ANOVA) with 95% confidence level was then carried out for each response variable in order to test the significance and suitability. The predicted values were obtained according to the recommended optimum conditions and compared with experimental values in order to determine the validity of the model. | |
| Results and Discussion | |
| Optimization of extraction conditions using RSM | |
| The results for DPPH radical scavenging activity, TPC, and TFC of each run of the experimental design are presented in Table 2. The TPC values for aqueous black rice extract ranged from 0.169 to 0.220 mg GAE/ml, while that for aqueous red rice extract ranged from 0.098 to 0.185 mg GAE/ml. The results for DPPH radical scavenging activity IC50 of aqueous black rice extract ranged from 0.216 to 0.404, and 0.369 to 0.549 for aqueous red rice extract. Additionally, TFC values for aqueous black rice and red rice extracts varied from 0.056 to 0.077 mg CE/ml and 0.028 to 0.059 mg CE/ml, respectively. | |
| Regression coefficients of the second-order polynomial models for DPPH radical scavenging activity, TFC and TPC are summarized in Table 3. It can be seen that the regression parameters of the surface response analysis which had significant effects on the response variables of aqueous black rice extract were those of full quadratic model for DPPH radical scavenging activity, linear+interaction model for TPC, and linear+square model for TFC of aqueous black rice extract. The ones which had significant effects on the response variables of aqueous red rice extract were those of full quadratic model for TPC and DPPH radical scavenging activity, while linear+square model gave significant effects to the TFC of aqueous red rice extract. The models were used to construct three dimensional response surface plots to predict the relationship between process and response variables. | |
| Effects of processing variables on DPPH radical scavenging activity | |
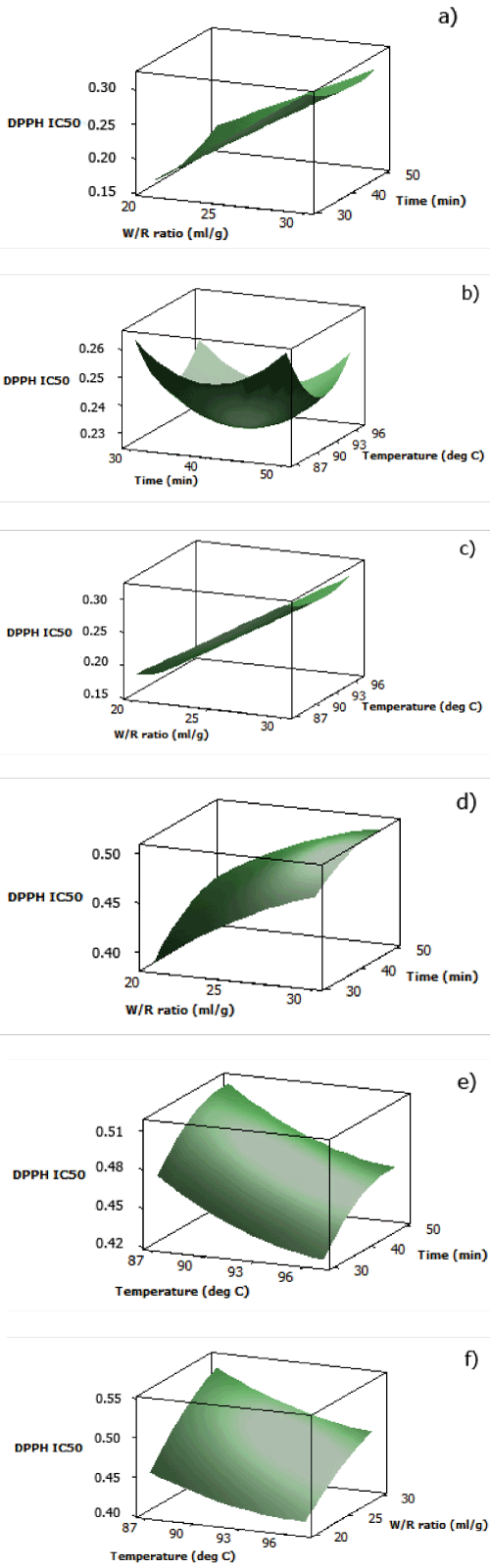
| The analytical results for DPPH radical scavenging activity IC50 of both aqueous black and red rice extracts are displayed in Table 2. As described by the three dimensional surface plots in Figures 1(a) and 1(d), DPPH radical scavenging activity IC50 increased as water/rice ratio increased, which was due to increasing volume of water as solvent for the antioxidants. Cooking time also showed the same pattern, but the increase in IC50 was more noticeable after around 40 minutes. Less time and lower water/rice ratio were favored since water exhibits thermodynamic properties favorable to heat transfer, resulting in better heat transfer with increasing water quantity. Hence, anthocyanins and proanthocyanidins might be chemically degraded when in longer contact with heat [21]. | |
| Response surface plot of aqueous black rice extract for interaction between cooking temperature and time (Figure 1(b)) displayed a negative quadratic function at fixed water/rice ratio. At water/rice ratio of 25 ml/g, minimum DPPH scavenging activity IC50 could be obtained by applying the middle values of optimized cooking time and cooking temperature, which were approximately at 92°C in 40 minutes. The plot showed that at cooking temperature and time before the middle values, antioxidants were probably not fully extracted yet, while the increase in IC50 at cooking temperature and time after the middle values indicated interference of antioxidant stability due to, as previously explained, chemical degradation from longer exposure to heat and application of increasing temperature. In contrast with black rice, at fixed water/ rice ratio (25 ml/g), DPPH scavenging activity IC50 of aqueous red rice extract decreased as cooking time decreased and cooking temperature increased (Figure 1(e)). This might be an indication that higher temperature was required to break the matrix of red rice bran in order to extract the antioxidants in red rice | |
| At a fixed time of 40 minutes, the results indicated that a decrease in water/rice ratio decreased the DPPH radical scavenging activity IC50 for aqueous black rice extract (Figure 1(c)). However, cooking temperature had no significant effect on the IC50 at the range of 87- 97°C, with only a slight decrease in IC50 as the temperature reached approximately 92°C. This illustrated that cooking time had more significant effect on the IC50 than cooking temperature in terms of the interaction with heat and the breaking of bran matrix to release the antioxidants. This might relate to the interaction of antioxidants in the rice with other food constituents which led to different time required to reach equilibrium between the solution in the bran matrix and in the water [22]. For aqueous red rice extract, minimum DPPH radical scavenging activity IC50 could be obtained by applying lower water/ rice ratio and higher cooking temperature (Figure 1(f)). Contrary with aqueous black rice extract, cooking temperature influenced the release of antioxidants in the aqueous red rice extract. | |
| Effects of processing variables on total phenolic content (TPC) | |
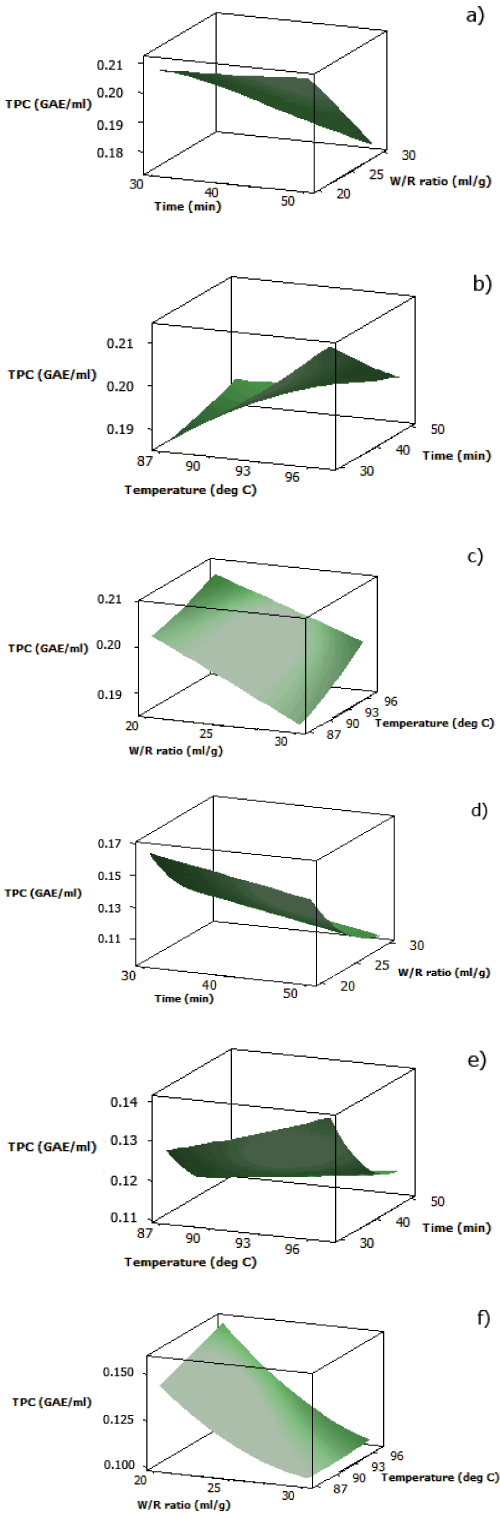
| The analytical results for TPC of both aqueous black and red rice extracts are displayed in Table 2. For the interaction between water/ rice ratio and cooking time, both surface plots (Figure 2(a) and 2(d)) showed increasing TPC as water/rice ratio decreased and cooking time decreased. The pattern of effects from interaction between water/rice ratio and cooking time for TPC of both aqueous black and red rice extracts was similar to that of DPPH radical scavenging activity of both extracts as discussed previously. | |
| At fixed water/rice ratio (25 ml/g), increased TPC for both aqueous pigmented rice extracts was obtained by increasing cooking temperature at less cooking time (Figure 2(b) and 2(e)). This pattern was also seen in the interaction between cooking temperature and cooking time for DPPH radical scavenging activity of aqueous red rice extract. However, TPC of aqueous black rice extract showed a contradictory pattern to the DPPH radical scavenging activity since increasing cooking temperature and less cooking time was required to obtain maximum TPC. This might be because the Folin-Ciocalteu reagent used in this assay was also reactive towards other nonantioxidant compounds such as proteins, carbohydrates, or inorganic ions which might be released from the rice bran matrix as temperature increased [23]. | |
| At fixed cooking time (40 minutes), it was found that for both aqueous black and red rice extracts, a combination of lower water/ rice ratio and higher temperature was required to achieve maximum TPC (Figure 2(c) and 2(f)). Both aqueous pigmented rice extracts also showed the same pattern for their DPPH radical scavenging activity. | |
| Effects of processing variables on total flavonoid content (TFC | |
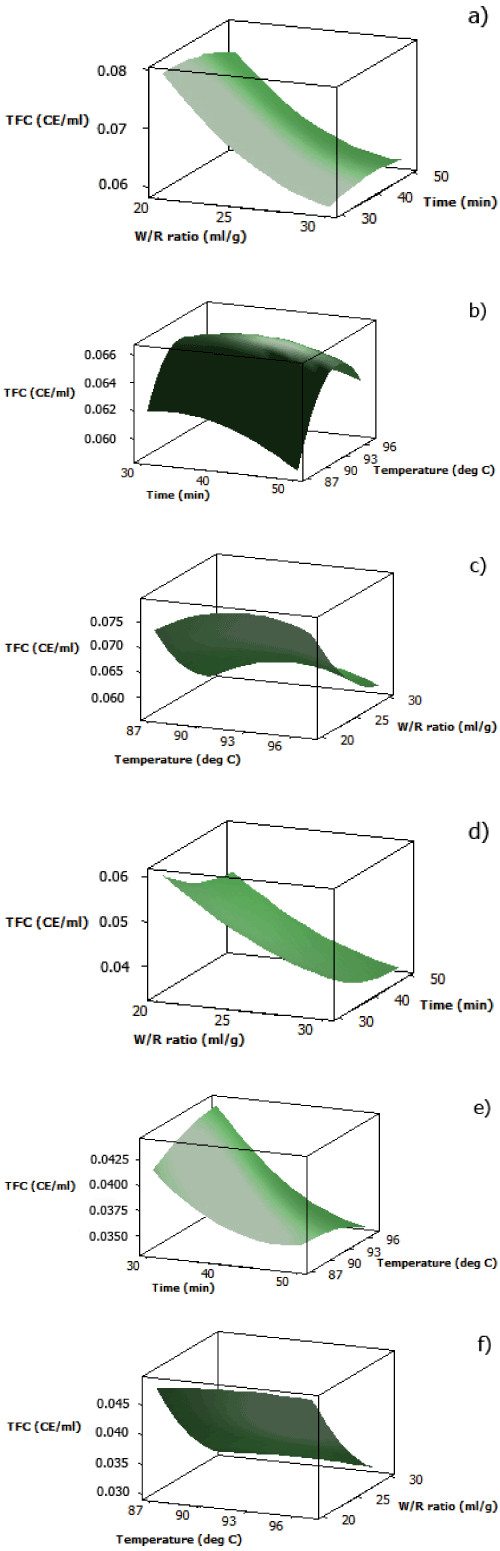
| The analytical results for TFC of both aqueous black and red rice extracts are displayed in Table 2. It was found that at fixed temperature (92 oC), a combination of minimum cooking time and minimum water/rice ratio was desired to obtain maximum TFC of both extracts (Figure 3(a) and 3(d)). This pattern was also observed in the effect of interaction between cooking time and water/rice ratio at fixed temperature on DPPH radical scavenging activity and TPC of both aqueous pigmented rice extracts. | |
| The parabolic shape of the surface plot for the effect of cooking temperature and time on TFC of aqueous black rice extract showed that the middle values for cooking temperature and time at fixed water/ rice ratio (25 ml/g) gave maximum TFC (Figure 3(b)), similar to DPPH radical scavenging activity when subjected under the same variables. This further proved that the major contributors for radical scavenging activity of aqueous black rice extract were flavonoid compounds, specifically anthocyanins, which are the major flavonoid compounds in black rice, together with other non-anthocyanin flavonoid compounds such as quercetin, isorhamnetin, apigenin, and kaempferol [24,25]. The surface plot for effect of cooking temperature and time on TFC of aqueous red rice extract (Figure 3(e)) also showed the same pattern as the one for DPPH radical scavenging activity and TPC, in which maximum TFC was obtained by applying higher temperature at less cooking time. | |
| At fixed cooking time (40 minutes), TFC of aqueous black rice extract increased as water/rice ratio decreased (Figure 3(c)). However, comparable to the trend showed by DPPH radical scavenging activity, a slight increase in TFC was obtained as cooking temperature reached around 92°C. The surface plot of aqueous red rice extract also showed an increase in TFC as the water/rice ratio decreased (Figure 3(f)), with insignificant effect from cooking temperature towards TFC at fixed cooking time, which differed from the one of its DPPH radical scavenging activity. This explains the nature of proanthocyanidins which are heat-stable antioxidant compounds [26]. | |
| Optimum processing conditions of aqueous pigmented rice extracts | |
| Tables 4 and 5 show the optimum conditions for the production of aqueous pigmented rice extracts with regard to the antioxidant content based on response surface methodology. For aqueous black rice extract, maximum TPC (0.215 mg GAE/ml) (d=0.91553), maximum TFC (0.076 mg CE/ml) (d=0.95599), and minimum DPPH scavenging activity IC50 (0.216) (d=1.0000) was achieved by applying the optimum conditions (d=0.95655) which consist of cooking temperature of 92 oC, cooking time of 40 minutes, and water/rice ratio of 20 ml/g. On the other hand, combination of cooking temperature of 97 oC, cooking time of 30 minutes, and water/rice ratio of 20 ml/g were the optimum conditions (d=0.93555) required to obtain maximum TPC (0.178 mg GAE/ml) (d=0.92480), maximum TFC (0.056 mg CE/ml) (d=0.92281) and minimum DPPH scavenging activity IC50 (0.376) (d=0.95948) for aqueous red rice extract. The fitted results had no significant difference (p>0.05) with the experimental results, showing that the RSM model was theoretically validated. The practical validation of the RSM model also showed that the predicted results had no significant difference (p > 0.05) from the experimental results obtained using optimum extraction conditions. | |
| Conclusion | |
| The water attained from cooking pigmented rice in excess water was found to have high potential to be developed into antioxidant drinks because antioxidant compounds in the rice bran tend to seep out into the cooking water. The optimum hot water extraction conditions for black rice were water/rice (W/R) ratio of 20 ml/g at 92 oC for 40 minutes, while that for red rice were W/R ratio of 20 ml/g at 97 oC for 30 minutes. Hence, it can be concluded that RSM is a useful method in optimizing the processing conditions for production of antioxidant drink from pigmented rice and Hot Water Extraction (HWE) showed great potential in extracting antioxidants from pigmented rice, making it suitable for the production of antioxidant drinks. | |
| Acknowledgements | |
| Authors thank Padiberas Nasional Berhad (BERNAS) and Faculty of Food Science and Technology UPM for the financial support of this research. | |
References |
|
|
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
 |
|||
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Basmati Rice
- Drought Tolerence
- Golden Rice
- Leaf Diseases
- Long Grain Rice
- Par Boiled Rice
- Raw Rice
- Rice
- Rice and Aquaculture
- Rice and Nutrition
- Rice Blast
- Rice Bran
- Rice Diseases
- Rice Economics
- Rice Genome
- Rice husk
- Rice production
- Rice research
- Rice Yield
- Sticky Rice
- Stress Resistant Rice
- Unpolished Rice
- White Rice
Recommended Journals
Article Tools
Article Usage
- Total views: 14781
- [From(publication date):
April-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10066
- PDF downloads : 4715
