Optimization of D-Amino Acid Oxidase Production by Trigonopsis variabilis Using Glucose Syrup from Cassava as Carbon Source
Received: 11-May-2018 / Accepted Date: 06-Jun-2018 / Published Date: 08-Jun-2018 DOI: 10.4172/2155-6199.1000445
Keywords: Glucose syrup from cassava; D-amino acid oxidase; Trigonopsis variabilis ; Optimization; Face-centered response surface methodology
Introduction
The production of pure chiral compounds via cost-effective method has become an important approach to the fine chemical, agrochemical and pharmaceutical industries. The used of biocatalysts for the production of pure chiral compounds has found existing all over a large area application and was becoming a cost-effective approach. The recent development of biocatalysts processes was to employ on a largescale an inexpensive enzyme with good properties (i.e., high activity, stability and selectivity) [1]. Over the last few years, the researchers has focused on several enzyme (e.g., proteases, oxidases and hydrolases) by investigating whether on protein scaffold can be changed and got better to fulfill different applications and be taken advantage for use in various fields.
Chiral compounds containing enantiomeric amine or amino acid groups can be produced using a variety of enzymes, such as amino acid dehydrogenases (EC 1.4.1.X), amine oxidases (EC 1.4.3.22), amino acid oxidases (EC 1.4.3.X), aminotransferases (EC 2.6.4.X), ammonia lyase (EC 4.3.1.X), and lipases (EC 3.1.1.X) [2]. Deracemise a DLmixture to the L-isomer can be done by employing a D-amino acid oxidase (DAAO, EC 1.4.3.3) and a non-selective chemical reduction step to afford enantiopure L-amino acid after numerous cycles [3]. DAAO catalyzes the oxidative deamination of D-amino acids to produce α-keto and ammonia.
DAAO are used in the first step in bioconversion of cephalosporin C (CPC) to 7-ACA, whereby the 7-ACA is used as a precursor for the synthesis of the active pharmaceutical ingredient (API) [4]. This process proposed two-step cleavage with D-amino acid oxidase (DAAO) and glutaryl acylase (GAC) [5]. The recent innovation has been used a one-step mono-enzymatic process using cephalosporin C acylase [6]. DAAO was also used in biosensors, in the resolution of natural (such as methionine and phenylalanine) and synthetic (such as naphthylalanine) amino acid racemic solutions [7], and in keto acids production [8]. DAAO can be found in mammalian organs, mainly in the kidneys. DAAO can also be produced from the following types of microorganism by fermentation, such as the yeasts Trigonopsis variabilis, Rhodotorula gracillis, Candida tropicalis, and fungi Neurospora crassa [9]. Trigonopsis variabilis DAAO has the high catalytic activity for CPC oxidation to produce intermediate compound glutaryl-7 amino cephalosporanic acid.
DAAO can be produced through fermentation using medium containing carbon source, nitrogen, mineral, etc. For industrial scale, the utilization of raw materials based on local resources for extractive and fermentative process has to be considered to achieve economic aspect [10]. Indonesia has an abundant agro-industrial product that can be used as raw material for specific purpose of product. Agro industrial products (molasses, cassava, etc.) have potentially been used as substrate in fermentation industry. Based on preliminary studies, it is known that glucose syrup from cassava is better when compared with molasses or fructose syrup from sorghum, so it can be used as an alternative carbon source. Glucose syrup from cassava (GSC) contains glucose as the main sugar [11], where glucose is the best carbon source for DAAO production [12]. GSC can act as a source of carbon and it also contain a little amount of nitrogen which is needed during culture cultivation and also enzyme biosynthesis, that makes DAAO production economist and efficient. As agricultural country, in 2015, Indonesia is among the 3 largest cassava producing countries in the world, producing more than 24 million tons and increasing every year [13], so the availability of raw materials can be guaranteed for mass scale production.
Statistical technique, including combination of Plackett-Burman design (PBD) and central composite design (CCD) are suitable and the most widely method used for optimization of biological processes [14,15]. Plackett-Burman design (PBD), developed by Plackett and Burman. It was designed to improve the quality control process that could be used to study the effects of design parameters on the system state so that intelligent decisions can be made. Plackett and Burman (PB) devised orthogonal arrays are useful for screening, which yield unbiased estimates of all main effects in the smallest design possible [16]. CCD is the most popular of the many class of RSM. It is widely used for estimating second order response surfaces. The choice of axial distance α is based on the region of interest. Choosing the appropriate values of α specifies the type of CCD. Suppose it is of interest to build a second-order response surface model and the biologist is interested in predicting growth of the organism inside and on the perimeter of the cuboidal region produced by the cube. In addition, for biological reasons, one cannot experiment outside the cube, though experimentation at the extremes in the region is permissible and, in fact, desirable. This scenario, which occurs frequently in many scientific areas, suggests a central composite design in which the eight corners of the cube are centered and α=1, often called the facecentered central composite design (FCCD) [17]. FCCD has been widely used in enzyme production [18,19].
The aim of this study is to optimize the production of DAAO by Trigonopsis variabilis using GSC as carbon source. The optimization was done by response surface methodology (RSM) to enhance the production. Important production parameters screened by Plackett- Burman design and the optimization of significant factors were done using Face-centered Central Composite Design (FCCD).
Experimental Sections
Materials
Trigonopsis variabilis was used as source of the enzyme, obtained from Biotech Center- BPPT (Serpong, Tangerang, Indonesia). Glucose syrup from cassava (GSC) obtained from PT. Rejo Madusari (Pati, Central Java, Indonesia). DL-alanine were purchased from HiMedia. K2HPO4, KH2PO4, MgSO4.7H2O, NaCl, CaCl2, MnCl2.4H2O, ZnSO4.7H2O, CuCl2.3H2O, H3BO3, FeCl3.6H2O were purchased from Merck (Darmstadt, Germany). The other chemicals, such as thiamin, biotin, o-phenylenediamine, horseradish peroxidase were purchased from Sigma-Aldrich Corp. (St. Luois, MO, USA). All experiments were carried out in duplicate.
Inoculum preparation
The strain was maintained in yeast malt medium. The cultures were kept at 4°C and sub cultured at regular intervals of 30 days. Pre culture medium (pH 6) contained: 22 g/L CGS, 4 g/L DL-alanine, 2 g/L K2HPO4, 0.21 g/L K2HPO4, 0.5 g/L MgSO4.7H2O, 0.1 g/L NaCl, 0.1 g/L CaCl2, 0.105 g/L MnCl2.4H2O, 0.0231 g/L ZnSO4.7H2O, 0.042 g/L CuCl2.3H2O, 0.105 g/L H3BO3, 0.0714 g/L FeCl3.6H2O, 0.24 mg/L thiamin and 0.02 mg/L biotin. GSC and DL-alanine was sterilized using 0.2 μm filter. The other components were sterilized in the autoclave at 121°C for 15 min. Inoculum was prepared by taking a loop full of fresh culture (72 h) and suspended in 5 mL of sterile saline water to get an appropriate suspension (OD600=0.1–0.2), then 1 mL of culture suspension was inoculated to 50 ml pre culture medium in 250 ml Erlenmeyer flask. The culture was then incubated in an orbital shaker at 30°C and 200 rpm for 24 h for inoculum development.
Production of DAAO
Production medium (pH 6) contained: 32 g/L GSC, 6.2 g/L DLalanine, 2 g/L K2HPO4, 0.21 g/L K2HPO4, 0.5 g/L MgSO4.7H2O, 0.1 g/L NaCl, 0.1 g/L CaCl2, 0.105 g/L MnCl2.4H2O, 0.0231 g/L ZnSO4.7H2O, 0.042 g/L CuCl2.3 H2O, 0.105 g/L H3BO3, 0.0714 g/L FeCl3.6H2O, 0.24 mg/L thiamin and 0.02 mg/L biotin. GSC and DLalanine was sterilized using 0.2 μm filter. The other components were sterilized in the autoclave at 121°C for 15 min.
Production of DAAO were carried out by transferring 10% inoculum (OD600=0.7–0.8) to the production medium and then incubated in an orbital shaker at 30°C and 140 rpm for 72 h.
Permeabilization of cell
The cells were harvested and neutralized to pH 6.0 using potassium hydroxide, then centrifuged at 10,000 g at 4°C for 10 min to obtain the cell, and then washing with potassium phosphate buffer (pH 8.0) twice. Certain volume of cells were taken and centrifuged, washed with distilled water and dried at 105°C to reach a constant mass to determined dry cells weight [20]. The rest of washed cells were suspended using the same buffer. The suspension cells were permeabilized using 5% toluene-ethanol (1:1) and held for 1 h at 37°C. The permeabilized cells were used for enzyme assay.
Enzyme assay
The DAAO activity were measured by o-phenylenediamine/ horseradish peroxidase coupling assay [21]. The reaction mixture contained 30 mM D-alanine, 0.03% o-phenylenediamine, 1700 U horseradish peroxidase and DAAO of interest in 100 mM potassium phosphate buffer (pH 8.0). The reaction was monitored by an increase in absorbance at 450 nm for 3 min at 25°C.
One unit of enzyme activity was defined as the enzyme needed to produce 1 micromole of H2O2 per min at 25°C and pH 8.0.
Experimental design for optimization
Optimization were applied in two stages, first to identify the most significant factors for DAAO production using Plackett-Burman design and later the significant factors were optimized by using RSM with face-centered central composite design. The experimental design and statistical analysis were done using Design Expert software (version 7.1)
Plackett-Burman design
The Plackett-Burman design is very useful for screening the most important factors with respect to their main effects. This model does not describe interaction among factors, it is used to screen and evaluate the important factors that influence the response. Six different independent variables including carbon (glucose syrup from cassava) concentration, (DL-alanine) concentration, pH, incubation time (period), temperature and inoculum quantity were selected for screening. Each variable was tested at two levels, namely a high level denoted by (+1) and a low level denoted by (-1) as listed in Table 1. Six variables were screened by twelve experiments conducted by Plackett- Burman design (Table 2). All experiments were carried out in duplicate and the average value of DAAO activity was used for statistical analysis.
| Code | Variables | Low level (-1) | High Level (+1) |
|---|---|---|---|
| A | GSC (%) | 10 | 15 |
| B | DL-Alanine (%) | 0.3 | 0.7 |
| C | pH | 6 | 8 |
| D | Temperature (°C) | 28 | 32 |
| E | Incubation time (Hours) | 48 | 72 |
| F | Inoculum (%) | 5 | 10 |
Table 1: Six factors and their levels used in Plackett-Burman design.
| Run No. | A | B | C |
| 1 | 1 | 1 | -1 |
| 2 | -1 | 0 | 0 |
| 3 | -1 | 1 | -1 |
| 4 | 0 | 0 | -1 |
| 5 | -1 | -1 | 1 |
| 6 | 1 | 1 | 1 |
| 7 | -1 | 1 | 1 |
| 8 | 0 | 0 | 0 |
| 9 | 1 | 0 | 0 |
| 10 | 0 | 0 | 0 |
| 11 | 0 | 0 | 1 |
| 12 | 0 | 0 | 0 |
| 13 | 0 | -1 | 0 |
| 14 | 1 | -1 | -1 |
| 15 | 0 | 0 | 0 |
| 16 | 1 | -1 | 1 |
| 17 | 0 | 0 | 0 |
| 18 | -1 | -1 | -1 |
| 19 | 0 | 0 | 0 |
| 20 | 0 | 1 | 0 |
Table 2: Matrix of Face-centered central composite design.
Face-centered central composite design (FCCD)
This step involved optimization of the levels and the interaction effects between various significant variables. In this study, the experimental plan consisted of 20 trials and the independent variables were studied at three different levels: low (-1), middle (0), and high (+1) [22]. The center points were repeated six times in order to evaluate the curvature and the experiment replication facilitated the pure error estimation, so that the significant lack of fit of the models could be predicted. All the experiments were done by duplicate and the average of DAAO activity (U/g yeast cell dry weight) obtained were taken as the dependent variable or response (Y).
The levels of factors used for experimental design are given in Table 3. For three factors, a fractional factorial design with six replications of center points to have a total number of 20 runs.
| Run no. | A | B | C | D | E | F | DAAO activity (U/g YCDW) |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 0.3 | 8 | 28 | 72 | 10 | 87.6509 |
| 2 | 10 | 0.3 | 6 | 28 | 48 | 5 | 85.8477 |
| 3 | 15 | 0.3 | 8 | 32 | 72 | 5 | 43.8850 |
| 4 | 15 | 0.7 | 8 | 28 | 48 | 5 | 66.3733 |
| 5 | 10 | 0.7 | 8 | 32 | 48 | 5 | 68.5606 |
| 6 | 15 | 0.7 | 6 | 28 | 48 | 10 | 66.6269 |
| 7 | 15 | 0.7 | 6 | 32 | 72 | 10 | 32.6922 |
| 8 | 10 | 0.7 | 6 | 32 | 72 | 5 | 41.7822 |
| 9 | 10 | 0.7 | 8 | 28 | 72 | 10 | 50.2795 |
| 10 | 15 | 0.3 | 6 | 28 | 72 | 5 | 52.4000 |
| 11 | 15 | 0.3 | 8 | 32 | 48 | 10 | 79.5999 |
| 12 | 10 | 0.3 | 6 | 32 | 48 | 10 | 87.0552 |
Table 3: Plackett-Burman screening, representing the response of DAAO production as influenced by GSC (A), DL-alanine (B), pH (C), temperature (D), incubation time (E), and inoculum (F).
Results and Discussion
Screening by Plackett-Burman design
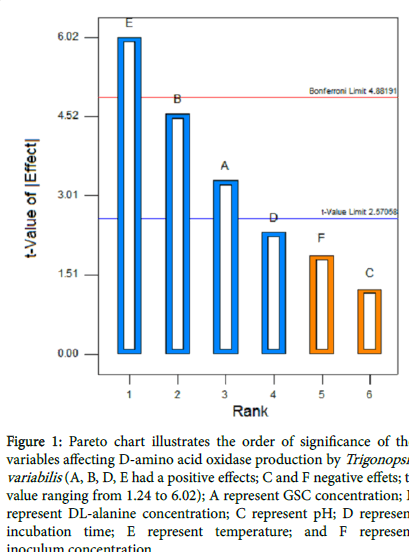
Plackett-Burman design was used to identify the important parameters for DAAO production. Table 3 represents the results of the screening of significant variable for D-amino acid oxidase production and the corresponding response (Y) using Plackett-Burman design. Statistical analysis of the DAAO activity were performed and represented in Table 3. With respect to the main effect of each variable, it shows that two variables named pH and inoculum concentration positively effect on DAAO production, where the other four variables named GSC and DL-alanine concentration, temperature and incubation time negatively effect on DAAO production. The T and P values were used to identify the effect of each factor on DAAO production as shown in Table 4. Incubation time, GSC and DL-alanine concentration had a significant effect on DAAO activity (P<0.05). The R2 values provide a measure of how much variability in the observed response values can be explained by the experimental factors. In this study, the value of the R2 were 0.9400, indicates that 94% of the variability in the response were attributed to the given independent variables and only 6% of the total variations are not explained by the independent variables. The Pareto chart (Figure 1) illustrate the order of significance variables affecting DAAO production in Plackett- Burman experimental design, showed the incubation period, concentration of GSC and DL-alanine were the significant parameters.
| Variables | Main effect | Coef. | T | P value | Source | Sum of Squares | df | Mean Square | F Value | p-value Prob > F |
|---|---|---|---|---|---|---|---|---|---|---|
| A-GSC | -13.27 | -6.63 | -3.3 | 0.0215 | Model | 344.27 | 6 | 57.38 | 12.69 | 0.0068* |
| B-DL-alanine | -18.35 | -9.18 | -4.56 | 0.006 | GSC | 53.87 | 1 | 53.87 | 11.91 | 0.0182* |
| C-pH | 4.99 | 2.5 | 1.24 | 0.2696 | Inducer | 87.61 | 1 | 87.61 | 19.37 | 0.0070* |
| D-Temperature | -55.6 | -4.63 | -2.3 | 0.0694 | pH | 2.75 | 1 | 2.75 | 0.61 | 0.4709 |
| E-Incubation time | -4.04 | -12.11 | -6.02 | 0.0018 | Temperature | 24.71 | 1 | 24.71 | 5.46 | 0.0666 |
| F-Inoculum | 7.51 | 3.75 | 1.87 | 0.1208 | Incubation time | 161.62 | 1 | 161.62 | 35.73 | 0.0019* |
| Inoculum | 13.7 | 1 | 13.7 | 3.03 | 0.1422 | |||||
| Residual | 22.62 | 5 | 4.52 | |||||||
| Cor Total | 366.89 | 11 |
Table 4: Statistical analysis results from Plackett-Burman design showing major effects, coefficient values, t-test (T), P-values for each variable affecting on DAAO production and analysis of variance. T: Student’s test; P: corresponding level of significance; R square 0.9400 and adjusted R square 0.8680.
Figure 1: Pareto chart illustrates the order of significance of the variables affecting D-amino acid oxidase production by Trigonopsis variabilis (A, B, D, E had a positive effects; C and F negative effets; tvalue ranging from 1.24 to 6.02); A represent GSC concentration; B represent DL-alanine concentration; C represent pH; D represent incubation time; E represent temperature; and F represent inoculum concentration.
Optimization by face-centered central composite design
Face-centered central composite design was employed to study the optimal levels and the interactions among the selected significant factors. The others factors were maintained at a constant level which gave maximal yield in the Plackett-Burman experiments. In this study, a total of 20 experiments with different combination of GSC concentration (A), DL-alanine concentration (B), and incubation time (C) were performed and the results of experiments for studying the effect of three independent variables on DAAO activity are presented along with predicted response and residuals (Table 5). The minimum DAAO activity (46,799 U/g YCDW) were observed in run number 6, while the maximum DAAO activity (193.880 U/g YCDW) were achieved in run number 8.
| Run no. | A | B | C | DAAO activity (U/g yeast cell dry weight) | |
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| 1 | 15 | 0.7 | 48 | 115.280 | 113.984 |
| 2 | 10 | 0.5 | 60 | 155.235 | 158.368 |
| 3 | 10 | 0.7 | 48 | 132.521 | 129.619 |
| 4 | 12.5 | 0.5 | 48 | 141.311 | 147.529 |
| 5 | 10 | 0.3 | 72 | 94.320 | 93.662 |
| 6 | 15 | 0.7 | 72 | 46.799 | 44.664 |
| 7 | 10 | 0.7 | 72 | 77.155 | 77.401 |
| 8 | 12.5 | 0.5 | 60 | 193.880 | 173.939 |
| 9 | 15 | 0.5 | 60 | 129.079 | 133.762 |
| 10 | 12.5 | 0.5 | 60 | 176.962 | 173.939 |
| 11 | 12.5 | 0.5 | 72 | 85.828 | 87.425 |
| 12 | 12.5 | 0.5 | 60 | 168.451 | 173.939 |
| 13 | 12.5 | 0.3 | 60 | 189.201 | 190.930 |
| 14 | 15 | 0.3 | 48 | 130.277 | 128.077 |
| 15 | 12.5 | 0.5 | 60 | 163.189 | 173.939 |
| 16 | 15 | 0.3 | 72 | 59.137 | 60.085 |
| 17 | 12.5 | 0.5 | 60 | 188.640 | 173.939 |
| 18 | 10 | 0.3 | 48 | 144.371 | 144.552 |
| 19 | 12.5 | 0.5 | 60 | 168.142 | 173.939 |
| 20 | 12.5 | 0.7 | 60 | 169.668 | 175.754 |
Table 5: Face-centered central composite design represents the response of DAAO production as influenced by GSC (A), DL-alanine (B), and incubation time (C) along with the predicted DAAO production. A: the coded value of GSC, B: the coded value of DL-alanine, and C: the coded value of incubation time.
Analysis of variance (ANOVA) was used to analyze the data (Table 6). The goodness of fit of the model was checked by the coefficient of determination (R2), which found to be 0.9782. the present R2-value reflected a very good fit between the observed and predicted responses and implied that the model is reliable for DAAO production in the present study. The P-values denote the significance of the coefficients and are also important in understanding the pattern of the mutual interactions between the variables. Interactions between two factors could appear as an antagonistic effect (negative coefficient) or a synergistic effect (positive coefficient).
| Variables | Coefficient | SE coefficient | T | P | Source | Sum of Squares | df | Mean Square | F Value | p-value Prob>F |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 114.92 | 24.589 | 4.673 | 0.001 | Model | 36220.1 | 9 | 4024.46 | 42.99 | <0.0001* |
| B | -269.97 | 190.093 | -1.42 | 0.186 | A-GSC | 1513.64 | 1 | 1513.64 | 16.17 | 0.0024* |
| C | 46.4 | 5.123 | 9.057 | 0 | B-DL-alanine | 575.85 | 1 | 575.85 | 6.15 | 0.0325* |
| A × A | -4.46 | 0.933 | -4.778 | 0.001 | C-Incubation time | 9031.39 | 1 | 9031.39 | 96.48 | <0.0001* |
| B × B | 235.08 | 145.859 | 1.612 | 0.138 | AB | 0.35 | 1 | 0.35 | 0 | 0.9523 |
| C × C | -0.39 | 0.041 | -9.678 | 0 | AC | 146.24 | 1 | 146.24 | 1.56 | 0.2398 |
| A × B | 0.42 | 6.841 | 0.061 | 0.952 | BC | 0.88 | 1 | 0.88 | 0.01 | 0.9246 |
| A × C | -0.14 | 0.114 | -1.25 | 0.24 | A2 | 2136.71 | 1 | 2136.71 | 22.83 | 0.0007* |
| B × C | -0.14 | 1.425 | -0.097 | 0.925 | B2 | 243.16 | 1 | 243.16 | 2.6 | 0.1381 |
| C2 | 8766.89 | 1 | 8766.89 | 93.65 | <0.0001* | |||||
| Residual | 936.11 | 10 | 93.61 | |||||||
| Lack of Fit | 174.63 | 5 | 34.93 | 0.23 | 0.9341 | |||||
| Pure Error | 761.48 | 5 | 152.3 | |||||||
| Cor Total | 37156.21 | 19 |
Table 6: Statistical analysis of face-centered central composite design showing coefficient values, standard error (SE) coefficient, t-test (T), P-values and analysis of variance. A: the coded value of GSC, B: the coded value of DL-alanine, and C: the coded value of incubation time; T : Student’s test; P : corresponding level of significance; F : Fisher’s function R square 0.9748 and adjusted R square 0.9521.
Table 6 shown that the GSC (A), DL-alanine, incubation time (C), quadratic effect of cassava glucose syrup (A) and incubation time (C) are significant.
All the interactions (A × B; A × C; and B × C) are not significant, indication that there is no significant correlation between each two variables and they did not help much in increasing the production of DAAO. In order to evaluate the relationship between dependent and independent variables and to determine the maximum DAAO production were proposed to calculate the optimum levels of the variables. By applying the multiple regression analysis on experimental data, the equation that defines predicted response (Y) can be shown as follows:
Y(DAAO activity)=173.94–12.30 × A–7.59 × B–30.05 × C+0.21 × A × B–4.28 × A × C–0.33 × B × C–27.87 × A2+9.40 × B2–56.46 × C2 (1)
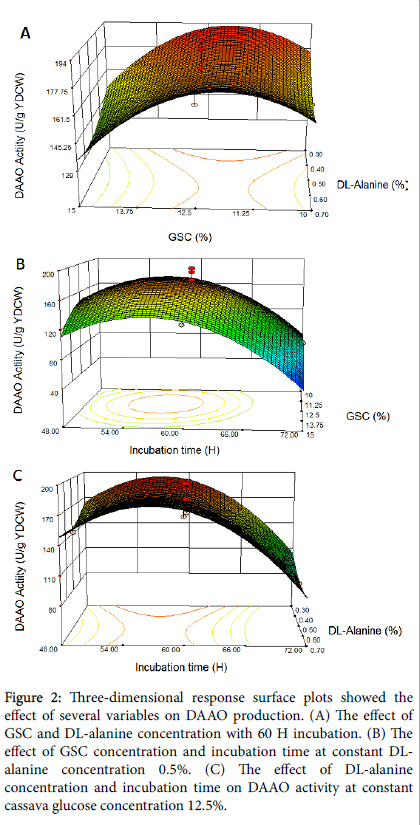
The interaction effects and optimal levels of the variables were determined by plotting the three-dimensional response surface curves (Figures 2a-2c) when one of the variables is fixed at optimum value and the other two are allowed to vary.
Figure 2: Three-dimensional response surface plots showed the effect of several variables on DAAO production. (A) The effect of GSC and DL-alanine concentration with 60 H incubation. (B) The effect of GSC concentration and incubation time at constant DLalanine concentration 0.5%. (C) The effect of DL-alanine concentration and incubation time on DAAO activity at constant cassava glucose concentration 12.5%.
Figure 2a represents the effect of varying GSC and DL-alanin concentration on DAAO production at constant incubation time 60 h. The increase of DAAO yield occurred with an increase of GSC concentration at DL-alanin concentration from 0.3% to 0.4%. Further increase of DL-alanin concentration would decrease the DAAO yield. According to this interaction effects, the maximum yield of DAAO activity were ≈ 193.88 U/g at GSC ≈ 12.5% and DL-alanin concentration ≈ 0.3%.
Figure 2b shows the cooperative effect of GSC concentration and incubation time at constant DL-alanin concentration 0.5%. As shown in Figure 2(b), at low and high value of GSC concentration and incubation time, the DAAO activity decreases. The maximum DAAO activity ≈ 193.88 U/g were obtained at GSC concentration ≈ 12.5% and incubation time ≈ 56 h.
Figure 2c shows the effect of both DL-alanin concentration and incubation time on DAAO activity at constant cassava glucose concentration 12.5%. It is obvious that with the increase of DL-alanin concentration above 0.3%, the DAAO activity decreases. The maximum DAAO activity ≈ 193.88 U/g obtained at lowest DL-alanin concentration ≈ 0.3% and incubation time ≈ 56 h.
Verification of the model
In order to determine the accuracy of the model and to verify the result, an experiment under the optimal conditions from RSM were performed and compared with the predicted data. The measured DAAO activity obtained were 195.38 U/g, close to the predicted one 196.38 U/g, indicating a high degree of accuracy. The predicted optimal levels of the process variables for DAAO production by Trigonopsis variabilis were 12.3% of GSC, 0.3% of DL-alanine and 56.1 hours of incubation.
Conclusions
The experimental design is useful to enhance the DAAO production. Plackett-Burman design can reduce the factors that not significantly affect to the production. Results of Plackett-Burman design showed that the incubation time, concentration of cassava glucose syrup and DL-alanine was significant factor for DAAO production. Optimization of significant factors was done using Facecentered Central Composite Design (FCCD) and the results were evaluated using RSM. The optimum condition for DAAO production as obtained from RSM were as follows: GSC concentration 12.3%, DLalanine (inducer) concentration 0.3% and 56.1 hours of incubation. At these optimum levels, DAAO activity reaches 195.38 U/g dry weight of yeast. The results of this study could be used to design a suitable medium using GSC as carbon source, for economically and efficiently production of DAAO.
Acknowledgements
The authors are thankful to the Biotechnology Center-BPPT Serpong, Tangerang, Indonesia for providing laboratory and technical support and to Pakuan University for financial supports.
References
- Caligiuri A, D'Arrigo P, Rosini E, Tessaro D, Molla G, et al. (2006) Enzymatic conversion of unnatural amino acids by yeast D-amino acid oxidase. Advanced Synthesis & Catalysis 348: 2183-2190.
- Molla G, Melis R, Pollegioni L (2017) Breaking the mirror: L-amino acid deaminase, a novel stereoselective biocatalyst. Biotechnology Advances 35: 657-668.
- Turner NJ (2004) Enzyme catalysed deracemisation and dynamic kinetic resolution reactions. Current Opinion in Chemical Biology 8: 114-119.
- Pollegioni L, Rosini E, Molla G (2013) Cephalosporin C acylase: dream and(/or) reality. Applied Microbiology and Biotechnology 97: 2341-2355.
- Barber MS, Giesecke U, Reichert A, Minas W (2004) Industrial enzymatic production of cephalosporin-based β-lactams. Advances in Biochemical Engineering/Biotechnology 88: 179-215.
- Gröger H, Pieper M, König B, Bayer T, Schleich H (2017) Industrial landmarks in the development of sustainable production processes for the β-lactam antibiotic key intermediate 7-aminocephalosporanic acid (7-ACA). Sustainable Chemistry and Pharmacy 5: 72-79.
- Findrik Z, Vasić-RaÄki Ä (2007) Biotransformation of D-methionine into L-methionine in the cascade of four enzymes. Biotechnology and Bioengineering 98: 956-967.
- Song Y, Li J, Shin HD, Liu L, Du G, et al. (2016) Biotechnological production of alpha-keto acids: Current status and perspectives. Bioresource Technology 219: 716-724.
- Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G (2007) Physiological functions of D-amino acid oxidases: from yeast to humans. Cellular and Molecular Life Sciences 64: 1373-1394.
- Pontoh J, Low NH (1995) Glucose syrup production from Indonesian palm and cassava starch. Food Research International 28: 379-385.
- Gupta N, Gundampati RK, Debnath M (2012) Screening of novel inducer for D-amino acid oxidase by Trigonopsis variabilis. International Journal of Bioscience, Biochemistry and Bioinformatics 2: 200-202.
- Wibisana A, Sumaryono W, Mirawati ST, Sudarmono P (2015) Optimization of surfactin production by Bacillus amyloliquefaciens MD4-12 using response surface methodology. Microbiology Indonesia 9: 120-128.
- Gupta N, Gundampati RK, Debnath M (2012) Optimization of media composition for D-amino acid oxidase production by Trigonopsis variabilis using biostatistical analysis. Indian Journal of Biochemistry and Biophysics 49: 272-278.
- Vanaja K, Shobha Rani RH (2007) Design of experiments: concept and applications of Plackett Burman design. Clinical Research and Regulatory Affairs 24: 1-23.
- Myers RH, Montgomery DC, Anderson-Cook CM (2009) Response Surface Methodology: Process and Product in Optimization Using Designed Experiments. 3rd edn., John Wiley & Sons, New Jersey, USA, pp: 531-567.
- Salihu A, Bala M, Alam MZ (2016) Lipase production by Aspergillus niger using sheanut cake: an optimization study. Journal of Taibah University for Science 10: 850-859.
- Tari C, Gögus N, Tokatli F (2007) Optimization of biomass, pellet size and polygalacturonase production by Aspergillus sojae ATCC 20235 using response surface methodology. Enzyme and Microbial Technology 40: 1108-1116.
- Kujan P, Prell A, Safár H, Holler P, Plhácková K, et al. (2001) D-amino acid oxidase - an improved production of the enzyme by the yeast Trigonopsis variabilis in a laboratory fermentor. Folia Microbiologica 46: 427-431.
- Wang SJ, Yu CY, Kuan IC (2008) Stabilization of native and double D-amino acid oxidases from Rhodosporidium toruloides and Trigonopsis variabilis by immobilization on streptavidin-coated magnetic beads. Biotechnology Letters 30: 1973-1981.
- Oyejola BA, Nwanya JC (2015) Selecting the right central composite design. International Journal of Statistics and Applications 5: 21-30.
Citation: Rusli Z, Suryadi H, Wibisana A (2018) Optimization of D-Amino Acid Oxidase Production by Trigonopsis variabilis Using Glucose Syrup from Cassava as Carbon Source. J Bioremediat Biodegrad 9: 445. DOI: 10.4172/2155-6199.1000445
Copyright: © 2018 Rusli Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4249
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 3418
- PDF downloads: 831