Research Article Open Access
Optimization of Alcalase hydrolysis conditions for production of Dagaa (Rastrineobola argentea) Protein hydrolysate with antioxidative properties
Ogonda Lydia Awuor1*, Muge Edward Kirwa1, Mulaa Francis Jackim3 and Mbatia Betty21Department of Biochemistry, School of Medicine, College of Health Sciences, P.O BOX30197, Nairobi, Kenya
2Department of Biochemistry and Biotechnology, Technical University of Kenya, P.O BOX52428-00200, Nairobi, Kenya
3Principal, Garissa University College, Moi University, P.O BOX63056-00200, Nairobi, Kenya
- *Corresponding Author:
- Ogonda Lydia Awuor
Department of Biochemistry
School of Medicine, College of Health Sciences
P.O BOX30197, Nairobi, Kenya
E-mail: Lydia.Ogonda@gmail.com
Received date: January 25, 2017; Accepted date: January 27, 2017; Published date: January 30, 2017
Citation: Awuor OL, Kirwa ME, Jackim MF, Betty M (2017) Optimization of Alcalase Hydrolysis Conditions for Production of Dagaa (Rastrineobola argentea) Hydrolysate with Antioxidative Properties. Ind Chem 3:122. doi:10.4172/2469-9764.1000122
Copyright: © 2017 Awuor OL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Industrial Chemistry
Abstract
Enzymatic conditions; stirring speed, pH, temperature, enzyme substrate (ES) ratio, solvent ratio, reaction time; for Dagaa (Rastrineobola argentea) hydrolysis by Alcalase 2.4L® were optimized. This was done to guarantee maximum enzyme activity, minimized losses and subsequent maximized product yield.
This study showed optimized hydrolysis conditions to be fixed at minimum stirring speed (overhead stirrer, Stuart, UK), pH 7, 56°C, ES ratio of 2% (v/w) and solvent ratio of 0.5% (v/w) for 6 h. At these combined optimal conditions, Alcalase 2.4L®Dagaa (Rastrineobola argentea) hydrolysis resulted in 71% protein recovery with 83% degree of hydrolysis.
These findings substantiated the use of Dagaa which proved to be a relatively good substrate for protein hydrolysate production.
Keywords
Dagaa; Optimization; hydrolysis; Alcalase
Introduction
Final product yield of enzymatic processes depends on several factors. These include; the type of enzyme and substrate, hydrolysis conditions; pH, temperature, time and enzyme/substrate ratio, solvent ratio and stirring speeds [1-4].
In addition to effect on yield, enzyme type also affects the bioactivity of the obtained hydrolysate. Therefore, appropriate selection of suitable enzyme and substrate as well as hydrolysis conditions such as; enzyme to substrate ratio, hydrolysis time, pH and temperature are crucial in obtaining protein hydrolysates with desirable functional and biological properties [5]. Moreover, from an economical point of view, the amount of enzyme used should be optimized to prevent enzyme waste and manage its costs [6].
Enzymatic hydrolysis can be done using proteolytic enzymes already present in the fish viscera and muscle (endogenous proteases); a process known as autolysis, or by exogenous enzymes. Autolysis by endogenous enzymes is difficult to control. This is attributed to several factors including the fish species, seasonality, as well as the type and amount of enzymes [7]. In addition, gender and age dependent changes may lead to variability in endogenous enzymes present in fish and other marine sources making it difficult to produce protein hydrolysates of same quality and properties [8,9]. The method also adversely affects the functional and organoleptic properties of the fish protein hydrolysates (FPH) and may produce toxic by-products.
These numerous shortcomings of autolysis have been overcome by exogenous enzymes. The addition of exogenous enzymes to hydrolyse food proteins is a process of considerable importance used to improve the physicochemical, organoleptic and functional properties of the initial protein substrates. There are a number of different proteolytic enzymes that can be used for the production of hydrolysates [10-12]. However, the preferred enzymes are large spectra proteases such as Alcalase [13], Neutrase [14], Protamex [14] and Kojizyme [15]. Similarly, lot of fish by-products have been hydrolyzed such as salmon head [16], salmon frame [17], sole frame [18], sardine viscera and head [19], cod [20,21], pacific hake [5] and shark [23] using exogenous enzymes.
However, Dagaa protein hydrolysates are yet to be produced. This study aimed at optimizing hydrolysis conditions of Dagaa (Rastrineobola argentea) by Alcalase 2.4L® for protein hydrolysate production. Optimized hydrolysis conditions, as well as effects of each factor have been reported.
Materials and Methods
Materials
The enzyme used was food-grade quality Alcalase® 2.4 L with a 2.4 AU/g activity and a density of 1.18 g/ml. Fresh Dagaa (1 kg each) was obtained from various landing sites: Dunga, Nduru, Paga, Rota and Usari from four fishermen around the shores of Lake Victoria resulting in a total sample weight of 20 kgs. This was well mixed for a representative sample, apportioned and stored at -20°C for further use.
Enzymatic hydrolysis of Dagaa protein
A 1 Kg portion of Dagaa representative sample was thawed at 4°C overnight and homogenized using a Multi grind (Sumeet research and Holdings PVT limited, Tamil Nadu, India ) for about 2 min. The homogenate was put in a reactor and mixed with a buffer (pH 7) at a ratio of 2:1 (w/v). The contents were allowed to attain a temperature of 56°C, with stirring at minimum rpm (Stuart stirrer, UK). A predetermined optimized ES (enzyme substrate) ratio 2% (v/w) of Alcalase was added and hydrolysis allowed to proceed for 6 h. The enzyme activity was then terminated by placing the hydrolysate in a water bath at 100°C for 20 min. The hydrolyzed Dagaa was centrifuged at 10000 × g for 20 min. After decantation and removal of sludge, the soluble fraction was freeze-dried and stored in airtight plastic container at -20°C for further use.
The effect of stirring, solvent ratios, ES ratio, pH, temperature, and time was carried out for 120 min, which was also based on the preliminary optimization study. The progress of the enzymatic hydrolysis was monitored based on the percent nitrogen recovery (% NR).
Optimising stirring speeds
Stirring was done with an overhead stirrer (Stuart, UK). Enzymatic hydrolysis was carried out at speeds 0-2000 rpm for 2 h.
Optimising solvent ratio
Solvent ratios % (v/w) were optimised between 0-3% for 2 h. The optimum solvent ratio was then set at the solvent ratio at which the highest % Nitrogen Recovery (% NR) was obtained. All assays were done in triplicate.
Optimising hydrolysis pH
The pH range of interest was alkaline since Alcalase is an alkali enzyme. The effect of pH was investigated according to Normah et al. [4]. Dagaa was added to buffer ranges (pH 7-11) and the temperature allowed to equilibrate to 50°C. The pH of the mixture was held constant by the addition of 4N NaOH or 4N HCl. The hydrolytic process was then terminated by heating the mixture at 100°C in a water bath for 20 min to inactivate the enzyme. The mixture was then cooled to room temperature followed by centrifugation at 10000 g for 20 min at 4°C using a refrigerated centrifuge to obtain the supernatant. The supernatant was then analyzed for % nitrogen recovery. The optimum pH was the pH with the highest % nitrogen recovery. All assays were done in triplicate.
Optimization of hydrolysis temperature
Temperature optimization was carried out in temperature range of 50°C–58°C for 2 h. The optimum temperature was the temperature at which the highest % nitrogen recovery (% NR) was obtained. All assays were done in triplicate.
Optimization of enzyme substrate (ES) ratio
The use of ES ratios between 1 and 2 % (v/w) has been proposed for industrial purposes in order to achieve nitrogen recovery as high as 60-70 % [24]. Enzyme substrate (ES) ratios within the range of 0–3 % (v/w) were tested for 2 h. The results obtained were used to fix the ES ratio at the point with maximum nitrogen recovery [4]. All assays were done in triplicate.
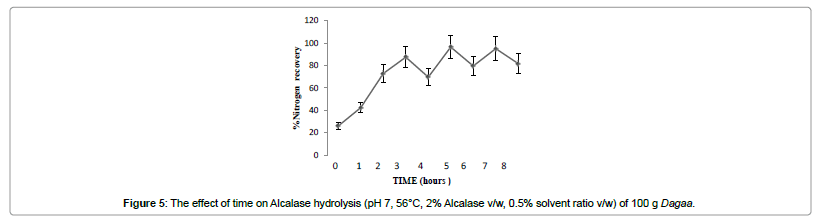
Optimization of hydrolysis time
The hydrolysis was run for 480 min at the optimum conditions: minimum stirring speed, pH 7 and solvent ratio 0.5 % (v/w), ES ratio 2 % (v/w) and temperature 0f 56°C obtained. Aliquots of hydrolysis mixture were then collected every 30 min and placed into a water bath at 100°C for 20 min for enzyme inactivation. The hydrolysis mixture was then cooled to room temperature and centrifuged at 10000 × g for 20 min at 4°C to obtain the supernatant. The pH of solution was checked every 30 min. An alkali/acidic solution (4N NaOH)/ 4N HCl was added to maintain a constant pH throughout the hydrolysis period. The time with the highest % NR was considered as optimal time. All assays were done in triplicate.
Determination of % nitrogen recovery
Total nitrogen in the supernatant was determined using the Biuret method. Nitrogen recovery was calculated as the percent of total nitrogen in the supernatant relative to total nitrogen present in the substrate [25]. All assays were done in triplicate.
Determination of % degree of hydrolysis
Degree of hydrolysis (DH) was calculated according to percent of trichloro acetic acid (TCA) ratio method as described by [13]. After hydrolysis, 20 ml of protein hydrolysate was added to 20 ml of 20% (w/v) TCA to produce 10% TCA soluble material. The mixtures were then left to stand for 30 min to allow precipitation, followed by centrifugation (7800 g for 15 min). The supernatant was then analyzed for protein content using Kjeldahl method [26]. All assays were done in triplicate.
The degree of hydrolysis (DH) was then computed as shown in the formula below:
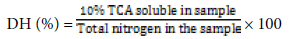
Statistical analysis
Statistical analyses were performed using the statistical program. Data was presented as mean ± standard deviation.
Results and Discussion
Stirring
For this study, optimised stirring for Dagaa (Rastrineobola argentea) was fixed at minimum stirring speed for an overhead stirrer (Stuart, UK). This was the point at which the substrate was seen to move as one (without a broken meniscus) reflecting optimum mixing. On the other hand, Speeds (500-2000 rpm) had spattering indicative of poor mixing.
Optimization for solvent ratio
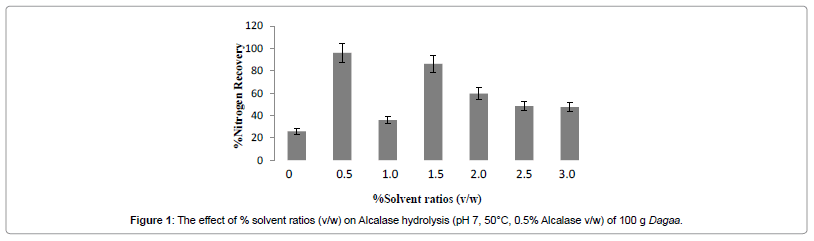
Solvent plays an important role in enzyme processes. This is important for the type of solvent as well as the amount of solvent. This is because the solvent components have an effect on hydrolysate components and enzyme stability.
In this study, there was a negative correlation between solvent ratios and percent nitrogen recovery. Hence an increase in percent solvent ratio (%v/w) led to decrease in percent nitrogen recovery (Figure 1). The maximum percent nitrogen recovery was obtained at solvent ratio 0.5% (v/w). This was considered to be the optimum solvent ratio.
This could be explained by the dilution. High solvent ratio dilutes the product, whereas low solvent ratios result in concentration of the substrate thus low activity demonstrated by minimised low percent nitrogen recovery. A decrease or increase in solvent ratio above the optimum reduces the yield (%NR). Similar results have been obtained for lipase where increasing solvent ratio resulted in decreased product yields [27].
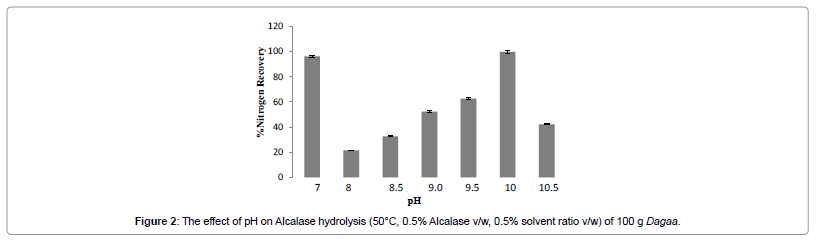
Optimization for pH
Previous studies on other substrates have shown Alcalase to have activity within the alkaline pH range. The pH profile for the hydrolysis of Dagaa by Alcalase is shown in Figure 2. There was a significant (p<0.05) difference in % NR obtained at the different pH values. However, There was no significant (p>0.05), increase in % NR with increase in pH.
The maximum % NR was obtained at pH 10.0. This is similar to previous studies on Pacific whiting (Merluccius productus) solid waste that showed maximum % NR at pH 9.5 [25]. However, at this pH 10.0, the hydrolysis solution showed a colour change from brown to black with the smell of urine. Urea is the main nitrogen containing substance in urine. This was indicative of very extensive protein degradation to a level of amino acid breakdown. Previous studies have however shown that amino acids do not exhibit very good antioxidative properties thus for this study that involved antioxidative function analysis, it was a requirement to stop the proteolysis at least at a dipeptide [28-31]. Consequently, the pH with the next highest % NR was selected. The hydrolysis was fixed to pH 7.
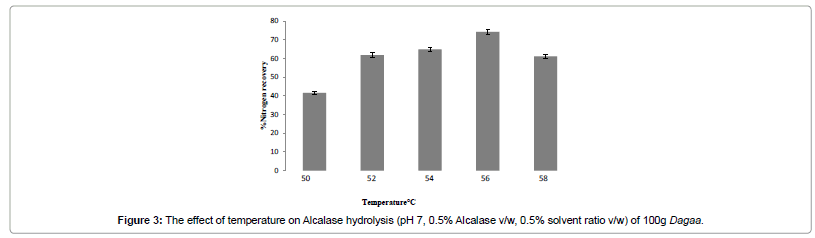
Optimization for temperature
There was a significant (p<0.01) increase in % NR with increase in temperature (Figure 3). Alcalase enzyme showed an optimum activity at 56°C. This was similar to studies on Alcalase hydrolysate of harp seal (Phoca groenlandica) that showed maximum yield at 55°C [6]. Alcalase reported a maximum yield at 60°C in Pacific whiting solid wastes and Threadfin Bream (Nemipterus japonicus) respectively [4,25].
A gradual increase in temperature led to the breakdown of substrate from an insoluble into a soluble form increasing the % NR [32]. However, a further increase in temperature to 58°C led to a decrease in % NR by 13%. This confirmed findings that a decrease of about 5–6% NR followed thereafter when the temperature was raised above the optimum for Alcalase. This could be attributed to enzyme destabilization resulting in reduction in total activity [4].
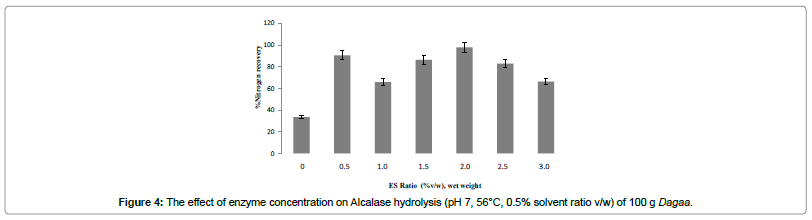
Optimization for enzyme/substrate ratio
Apart from pH and temperature, enzyme substrate ratio is one of the factors, which shows a marked influence on peptide bond cleavage of the protein substrate [32]. The profile of % NR during the 2 h hydrolysis of Dagaa is shown in Figures 4. Maximum %NR was obtained at ES ratio of 2% (v/w).
Approximately 20% of the total nitrogen remained insoluble at the end of a hydrolysis process even if more enzymes were added during the stationary phase of hydrolysis [35]. This insoluble residue contains peptides enriched with hydrophobic amino acids that are highly resistant to further degradation by the enzyme [33]. Furthermore, the increase in peptide concentration in the hydrolysis mixture and the total cleavage of all the susceptible peptide bonds inhibits further increase of hydrolysis rate and soluble nitrogen production [6].
Nitrogen recovery (71%) obtained in this study was higher than those obtained in the hydrolysis of sardine (Sardine pilchardus) and black tilapia (Oreochromis mossambicus) using Alcalase at a 2% ES ratio in which only 65 and 40% soluble nitrogen, respectively, were recovered [25]. The use of ES ratios between 1 and 2% has been proposed for industrial purposes in order to achieve nitrogen recovery as high as 60–70% [24] (Figure 5).
In this study, an ES ratio of 2% (v/w) was capable of producing a hydrolysate containing 71% nitrogen, higher than some of the earlier reports. Hence, Dagaa is a comparatively a good substrate for fish protein hydrolysate production.
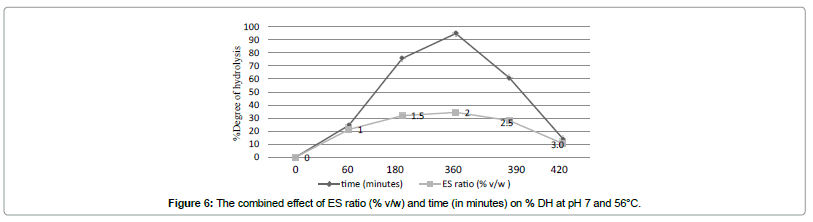
Effect of hydrolysis time and ES ratio on % DH
Efficiency of hydrolysis was determined by % DH. In addition to hydrolysis time, ES ratio has also been shown to affect the % DH [6,24].
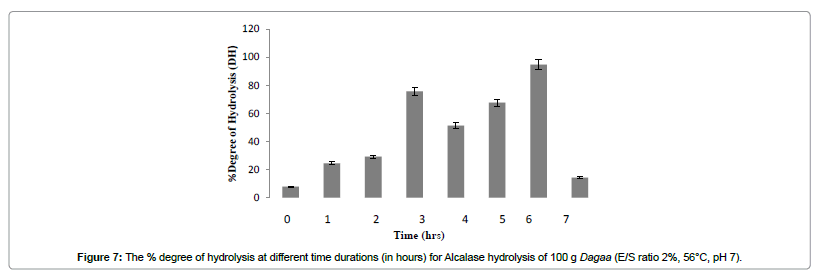
There was a significant (p<0.05) increase in % DH with increase in both hydrolysis reaction time and enzyme substrate ratio. An asymptotic hydrolysis curve was obtained as shown in Figure 6. Degree of hydrolysis increased as ES ratio increased (Figure 6). At 2 h and above, degree of hydrolysis increased significantly as ES ratio increased from 1 to 2%, however, no significant increase was observed above an ES of 2% (Figure 6). At low ES ratio, the enzyme initially attacks the most susceptible peptide bonds continuously hydrolysing the bonds during the entire hydrolysis period because of the availability of the substrate [34]. As ES ratio increases, hydrolysis becomes more rapid because of a relative increase in enzyme concentration and rapid cleavage of the susceptible peptide bonds of the substrate during the initial stage of hydrolysis followed by a slower cleavage of the less susceptible bonds at the later stage [34]. When ES ratio increased to above 2%, hydrolysis decreased, indicative of possible product inhibition in which the peptides compete with the remaining substrates for the binding sites of the enzymes during the later stage [32]. This was in spite of increase in % NR (Figure 4) indicating the possibility that the enzyme hydrolyzes itself [22]. As well as that the heat inactivation of endogenous enzymes was not efficient; also illustrated by % DH recorded at the starting time (time 0) before addition of enzyme. However, a gradual plateauing of the curve occurred when the substrate was further hydrolysed indicating no significant increase in % DH. At the end of 6 h hydrolysis, the substrate was hydrolysed to at least 94% (Figure 7).
A similar profile has also been reported for Alcalase-assisted hydrolysis of other seafood proteins such as Pacific Whiting solid waste and sardine [24,25].
Conclusion
In this study, optimized hydrolysis conditions were fixed at minimum stirring speed (overhead Stuart stirrer, UK), 56°C, pH 7, ES ratio of 2% (v/w) and solvent ratio of 0.5 (v/w) for 6 h. At these optimum conditions, 83 % degree of hydrolysis was achieved and 71% protein was recovered.
Acknowledgements
The author wishes to show utmost appreciation to colleagues, the laboratory technical staff, supervisors, at Biochemistry Department for the unwavering support and guidance, the University of Nairobi for the laboratory facilities, and the National Council for Science and Technology-Kenya (NCST) for their financial support.
References
- Sterbacek Z,Tausk P (1965) Mixing in the Chemical Industry, Pergamon, London.
- Baldyga J, Bourne JR (1999) Turbulent Mixing and Chemical Reactions, Wiley, New York.
- Paul EL, AtiemoObeng VA, KrestaSM (2004) Handbook of Industrial Mixing, Wiley Inter Science, New York.
- Normah I, Jamilah N,Saariand B,YaakobChe man (2005) Optimization of hydrolysis conditions for the Production of threadfin bream (NemipterusJaponicus )hydrolysate by alcalase. J Muscle Foods16: 87-102.
- Samaranayaka AGP (2010)Pacific Hake(Merlucciusproductus) fish protein hydrolysates with antioxidative properties P.H.D University OfBritish Columbia
- Shahidi F, Han XQ, Synowiecki J (1995) Production and characteristics of protein hydrolysates from capelin (Mallotusvillosus). Food Chem 53: 285-293.
- Sikorski ZE,Naczk M (1981) Modification of technological properties of fish protein concentrate. CRC Crit Rev Food SciNutrpp:201-230.
- Kristinsson HG, Rasco BA (2000) Biochemical and functional properties of Atlantic salmon (Salmosalar) muscle proteins hydrolyzed with various alkaline proteases. J Agric Food Chem 48: 657–666.
- GuérardF(2007) Enzymatic methods for marine by-products recovery. In: Shahidi F, editor. Maximizing the Value of Marine By-products. Cambridge, England: Woodward.
- Venugopal V (1994) Production of fish protein hydrolysates by microorganisms. Ch.10 In Fisheries Processing: Biotechnological Applications. Martin AM (Ed) Pp: 223-231. Chapman and Hall, London, Engl.
- Hall GM, Ahmad NH(1992) Functional properties of fish protein hydrolysates.Ch.11 InFish Processing Technology.Hall GM (Ed) pp: 249-265. Blackie Academicand Professional N.Y, U.S.A
- Baca DR, Pena-Vera MT, Diaz-Castaneda M (1991) Production of fish protein hydrolysates with bacterial proteases; yields and nutritional value. J Food Sci 56: 309-314.
- Hoyle NT, Merritt JH (1994) Quality of fish protein hydrolysatesfrom herring (Clupeaharengus). J Food Sci 59: 76–79.
- Dumay J, Allery M, Donnay-Moreno C, Barnathan G, Jaouen P, et al. (2009)Optimization of hydrolysis of sardine (Sardinapilchardus) heads with Protamex: Enhancement of lipid and phospholipid extraction. J Sci Food Agric 89:1599–1606.
- Liaset B, Lied E,Espe M (2000) Enzymatic hydrolysis of by-products from the fish-filleting industry: Chemical characterization and nutritional evaluation. J Sci Food Agric 80:581–589.
- Gbogouri GA, Linder M, Fanni J,Parmentier M(2006) Analysis of lipids extracted from salmon (Salmosalar) heads by commercial proteolytic enzymes, Eur J Lipid SciTechnol 108:766–775.
- Liaset B, Nortvedt R, Lied E,Espe M (2002) Studies on the nitrogen recovery in enzymic hydrolysis of Atlantic salmon (Salmosalar, L.) frames by ProtamexTM protease.Process Biochem 37:1263–1269.
- Jun SY, Park PJ, Jung WK, Kim SK (2004) Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limandaaspera)frame protein. Eur Food Res Technol 219: 20-26.
- Souissi N, Bougatef A, Ellouz YT, NasriM(2007) Biochemical and functional properties of sardinella (Sardinellaaurita)by-product hydrolysates, Food TechnolBiotechnol 45:187–194.
- Aspmo SI, Horn SJ,VGH (2005) Eijsink Enzymatic hydrolysisof Atlantic cod (Gadusmorhua L.) viscera. Process Biochem 40: 1957–1966.
- Slizyte R, Mozuraityte R, Martınez-Alvarez O, Falch E, Fouchereau-Peron M, et al. (2009) Functional, bioactive and antioxidative properties of hydrolysates obtained from cod (Gadusmorhua) backbones. Process Biochemistry 44: 668-677.
- Diniz FM, Martin AM (1996) Use of response surface methodology to describe the combined effects of pH, temperature, and E/S ratio on the hydrolysis of dogfish (Squalusacanthias) muscle. Int J Food SciTechnol 31: 419-426.
- Diniz FM, Martin AM (1997) Effects of the extent of enzymatic hydrolysis on functional properties of shark protein hydrolysate. Food Sci and Technol-Lebensmittel-Wissenschaft&Technologie 30: 266-272.
- Quaglia GB &Orban E (1987) Enzymatic solubilisation of proteins of sardine (Sardinapilchardus) by commercial proteases. Journal of the Science of Food and Agriculture 38: 263–269.
- Benjakul S, Morrissey MT (1997) Protein hydrolysates from Pacific whiting solid waste. Journal of Agricultural and Food Chemistry 45: 3423-3430.
- AOAC(1995)Official Methods of Analysis of AOAC International, 16th edition.Methods 950.46, 928.08, 991.36, 920.153 Washington (D.C).
- Mbatia BN, AdlercreutzD, AdlercreutzP, MahadhyA,Mulaa F, et al. (2010) Enzymatic oil extraction and positional analysis of ω-3 fatty acids in Nile perch and salmon heads. Process Biochemistry 45: 815-819
- Chan KM, Decker EA (1994) Endogenous muscle antioxidants. Crit Rev Food SciNutr 34:403-426.
- Ostdal H, Andersen HJ, Davies MJ(1999)Formation of long-lived radicals on proteins by radical transfer from heme enzymes-a common process. Arch BiochemBiophys 362:105-112.
- ZhouS, Decker EA (1999) Ability of amino acids, dipeptides, polyamines, and sulfhydryls to quench hexanal, a saturated aldehydic lipid oxidation product. J Agric Food Chem 47:1932-1936.
- Rival SG, FornaroliS, Boeriu CG, Wichers HJ(2001) Caseins and casein hydrolysates. 1.Lipoxygenase inhibitory properties. J Agric Food Chem 49: 287-294.
- Adler-Nissen J (1986) Enzymatic Hydrolysis of Food Proteins. Elsevier Applied Science Publishers, New York.
- Iacobucci GA, Myers MJ, Emi S, Myers OV (1974) Large scale continuous production of soybean protein hydrolysate in constant flux membrane reactor. Proc IV IntCongrFood SciTechnol Madrid V 83–95.
- Omeara GM, Munro PA (1984)Effects of reaction variables on the hydrolysis of lean beef tissue by Alcalase. Meat Sci 11: 227-238.
- Mackie IM (1982) Fish protein hydrolysates. Process Biochem 17: 26-28, 31.
- SPSS vs16, SPSS Inc, and Chicago, IL.
- Mbatia B, Ogonda LA, Muge EK, Mulaa FJ (2014) Antioxidative and functional properties of Rastrineobolaargentea (Dagaa) fish protein hydrolysate.Discourse Journal of Agriculture and Food Sciences 2: 180-189
- Ogonda LA, Muge EK, Mulaa FJ, MbatiaBN (2014)Proximate composition of Rastrineobolaargentea (Dagaa) of Lake Victoria-Kenya. African J Biochem Rese 8: 1-6.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 6608
- [From(publication date):
March-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 5542
- PDF downloads : 1066