Optical Fluorescence Diagnostic of Wheat Leaf Rust with Laser Scanning Confocal Microscopy
Received: 09-Mar-2018 / Accepted Date: 11-Apr-2018 / Published Date: 17-Apr-2018 DOI: 10.4172/2329-8863.1000355
Abstract
Wheat is the most important grain crop and food source worldwide. The management of diseases and early detection of pathogens is a crucial step in diagnosis programs in wheat. In the primary stage, the symptoms of rust fungus are difficult to identify with visual monitoring and other conventional techniques. In this study, we intended to investigate the early stage leaf rust in wheat crop produced through rust fungus using light fluorescence from laser scanning confocal microscopy (LSCM). The leaf rust and normal samples were analyzed with an excitation of 488 nm wavelength of Ar+ laser without any marker or photosensitizer. The small dark pores instead of stomata appears in leaf due to fungus infection and can be observed after two week of leaf tillering. These spots are orange or brown in the beginning and become black, when plants reach maturity. In recent study, the potential of non-invasive techniques for the detection of plant diseases are demonstrated for the development of a rapid and less complex early stage detection procedure that can be utilized to evaluate the infection structures during fungus infection of wheat. The newly developed rapid procedure will be helpful for early stage detection and management fungal infection before proper development during wheat interaction.
Keywords: Wheat leaf rust, Laser scanning confocal microscopy, Fungus infection, Optical sensors
Introduction
Wheat represents one of the most important food crops worldwide and its yields are continuously under threat from plant diseases often caused by pathogenic fungus [1-3]. The wheat leaf fungus Puccinia triticina is currently one of the most economically important diseases, and causes premature death of wheat leaves, reducing the ability of plants to capture sunlight, which ultimately reduces their grain production [4,5]. Leaf rust caused by Puccinia triticina produces high yield losses up to 20% worldwide [6]. Wheat crop is the richest source of the calories and proteins consumed by humans each day. Wheat production needs to increase dramatically in coming years to meet the needs of a rapidly growing world population, but disease is a continuing threat to current and future yields. Symptoms of leaf rusts include bright yellow, orange or red leaf spots. These leaf spots often produce an abundance of yellow, orange, red, or brown powdery spores that can be easily seen on leaf surface caused by a group of related fungus [7-9].
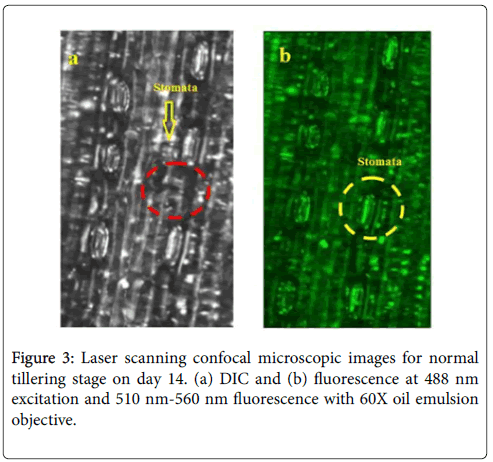
The optical sensors and laser spectroscopy has become essential approach and latest research fields of applied plant sciences. As a rapid, accurate, non-destructive and economical technology, Laser scanning confocal microscopic study of leaf rust at an early stage of disease provided researchers to gain a better understanding on the overall behaviour and health of plants [10,11]. A microscope image of a leaf epidermis can provide a clear view of epidermal cells, stomata and plant leaf veins. Among these elements, stomata play a major role in protecting the plant against water loss and regulating the gas exchange with the external environment [12]. As a result, the behaviour of stomata provides key information on the water stress level, food production rate and the overall health of the plant [13,14]. The rust fungus are destructive plant pathogens looked as small-rounded orange or yellow pores on the upper surface of the leaves and reduces both grain yield and weight. A number of fungicides are label to control the disease but scouting and early detection is crucial to time pesticide applications for effective control [4].
Laser scanning confocal fluorescence imaging in plants is unusually challenging because of the large amounts of photosynthetic pigments contained in green plant tissues. For example, chlorophyll can obstruct the penetration of light and has high levels of auto fluorescence at wavelengths that used for fluorescence imaging. Until now, mostly confocal laser scanning microscopes used to overcome these limitations. The rust fluorescence in viral infected wheat leaf recorded by confocal laser scanning microscopy for early stage disease detection [15,16].
Current research activities are towards the development of such technologies to create a practical tool for disease monitoring under field conditions. The spectroscopic and imaging techniques can provide information on disease detection at early stages to control the spread of plant diseases. In LSCM the light collected from point source and detected at sensitive detectors through dichroic mirrors and lenses [17,18].
Materials and Methods
The wheat leaf collected from National Agriculture Research Council (NARC) Islamabad. The sampling done with general laboratories protocol. Several plants were selected randomly to assess disease prevalence and severity we prepared fresh leaf cross sections using freehand sectioning method for optical and laser scanning confocal microscopy [19].
Laser scanning confocal microscope (LSCM) Carl Zeiss 510, Jena, Germany was used to visualize fungal structures by their light and laser fluorescence imaging. To analyses the growth of fungus structures of leaf rust in wheat leaves, the maximum length of the fungal colonies parallel to the length of the leaf measured. Microscopic chlorophyll fluorescence intensity of the axial leaf surfaces are captured with the CLSM system (40X water emulsion and 60X oil emulsion objective) at 488 nm wavelength of Argon ion laser of 30 mW power. The leaf specimen set on a cover glass and fluorescence image taken using Ar+ laser and He-Ne lasers. The excitation light with the excitation wavelengths of 488 nm passed through 450-490 nm dichroic mirrors down to the sample by 510 nm long pass filter and emitted fluorescence by emission wavelength of 510-565 nm to the PMT tubes. The images plotted by Zen software of Carl Zeiss, Germany [20,21].
Results and Discussion
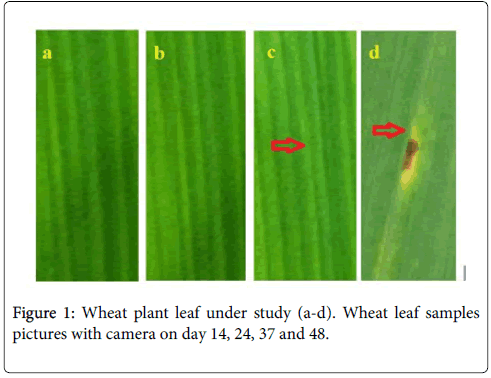
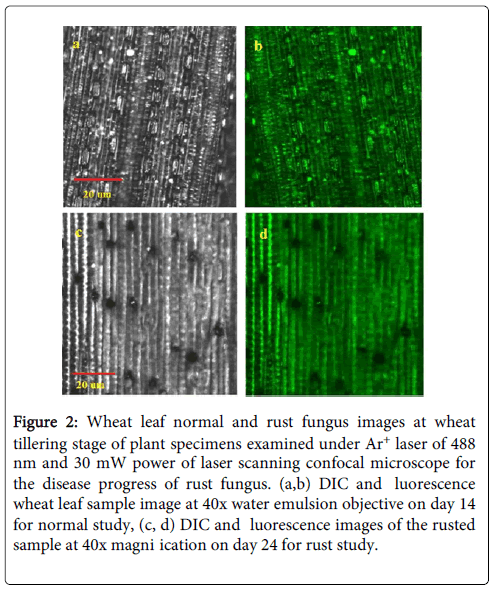
The entrance of microbial species into host plant tissues is a first step toward infection. In plants, the stomata of plants regulate gas exchange and water transpiration in response to changing environmental conditions. The stomata also have play an important role in host defence for infections and close upon detection of potential microbial pathogens to prevent the infection of the leaf interior [22]. The stomata is also important in Photosynthesis process of plants for food production with the help of sunlight, carbon dioxide and water. In case of leaf rust, the pathogen inters through stomata and start destruction of stomata cells, due to which the leaf stops Photosynthesis process, and with time appears brown, yellow or blackish. The immune system of plant initiated by stomata and with the destruction of stomata immune system of the plant stops working [23,24]. The wheat plant field along with fresh leafs of day 14, 24, 37 and 48 are illustrated in Figure 1. It can be seen that by visual observation no symptom of rust on leaves can be observed before day 37. But in case of laser scanning confocal microscopic imaging rust stomata destruction can be observed from day 18 of wheat tillering. In our study we have taken different samples of wheat plant leaf from second weak to onward and observed with laser scanning confocal microscopy setup describe above. When a fresh leaf illuminated with Ar+ laser of 488 nm laser using a laser scanning confocal microscope (LSCM), the chlorophyll fluorescence is collected and appears green under the confocal microscope because chlorophyll absorbs 488 nm laser energy and gives off fluorescence between 510 nm to 550 nm wavelength. In Figure 2a and 2b the differential interference contrast (DIC) and fluorescence images of fresh wheat leafs on day 18 are shown and rust appears after day 18 can be observed in Figure 2c and 2d for DIC and fluorescence.
Figure 2: Wheat leaf normal and rust fungus images at wheat tillering stage of plant specimens examined under Ar+ laser of 488 nm and 30 mW power of laser scanning confocal microscope for the disease progress of rust fungus. (a,b) DIC and luorescence wheat leaf sample image at 40x water emulsion objective on day 14 for normal study, (c, d) DIC and luorescence images of the rusted sample at 40x magni ication on day 24 for rust study.
The microscopic images of the infection in leafs and intracellular movement of the fungus through vascular bundles of xylem and phloem recorded. The confocal micrograph of infected leafs showed the dark pores appears after stomata infection. Once the fungi colonize and infect the tissues, they produce mycotoxins, which spread to the surrounding tissue, and can move to the rest of the plant through xylem and destroy immune system of the stomata to plant defence [25]. The stomata become dark after infection and up till day 48 the rust appears on the surface of wheat leaf. Fungus enters the plant via the stoma and host and fungal membranes allows the transport of nutrients from the host to the fungus. The initial symptoms of rust starts in leaf and small chlorotic spots on the leaves appear from day 18 and cannot seen with necked eye. We can see that in Figure 1, it cannot observed before day 48, but with laser fluorescence it can be seen at day 18 as shown in Figures 2c, 2d, 3 and 4. The dark pores start developed after day 14 and appears on leaf surface from day 35. The rust dark pores on baby plant are observed round and mature leaves most often are shaped irregularly, long and narrow as can be seen in Figures 4a-4d.
Figure 4: Wheat leaf rust images with dark pores plant specimens examined under Ar+ laser of 488 nm and 30 mW power of laser scanning confocal microscope with 30 μm dimension for the disease progress of rust fungus. (a,b) DIC wheat leaf sample image with dark pores (5 μm) at 60x oil emulsion objective on day 24, (c,d) Fluorescence rusted sample at 60x magnification on day 24 for rust study.
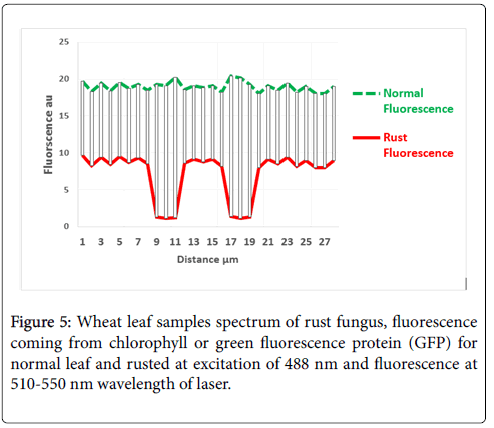
In this study, the biomarker used is the auto fluorescence coming by chlorophyll or green fluorescence protein (GFP) at excitation of 488 nm laser energy and gives off fluorescence between 510 nm to 550 nm. Chlorophyll has an absorption band in the green region of the visible spectrum, which produces a significant amount of fluorescence at wavelengths 510-565 nm when excited with wavelengths of 488 nm of Ar+ laser. The light spectrum of normal leaf at day 14 and rusted wheat can be seen in Figure 5. The fluorescence from normal shows continues spectrum as compared to the rusted, in which fluorescence suddenly drops at dark pores.
The wheat leaf study with ultraviolet radiations and light microscopy was conducted by different scientists in past and the work [26] applies UV radiation to leaves under blue florescence. The plant leaves are then captured using a fluorescent microscope and the resulting images are filtered and segmented to extract stomata and guard cells. Even though this method produces reliable results, it requires a relatively featureless background as well as methods of applying UV radiation to the leaf. In addition, the work presented uses image-processing techniques on microscope images to classify different stomata structure [27]. A watershed technique employed to extract a single stoma from a nearly featureless background. However, the proposed method would not perform well in the presence of multiple stomata and a feature-rich background. In our previous research, we have used different optical methodologies to characterize viral and infectious diseases and in recent study we extended it to an agriculture [28-35].
Conclusion
In plant and animals, the early detection of pathogens is a crucial step in diagnosis and management programs. The failure to adequately identify and detect plant pathogens using conventional, culture based morphological techniques has led to the development of nucleic acidbased molecular approaches. At the microscopic level, confocal fluorescence imaging of leaves infected by pathogens can be easily detected at an early stage to manage the disease effectively. Recent results confirm several previous findings based on traditional techniques but also provide new leaf rust detection mechanism in plants that is much more dynamic and flexible than previously used to study fungal infection process in plants.
In this study, the wheat leaf rust associated with leaf death due to fungus entrances via stomata presented using laser scanning confocal microscope. The fungus pathogen inters through stomata and start destruction of stomata cells, due to which the leaf stops photosynthesis process, and with time appears brown, yellow or blackish. The immune system of plant is initiated by stomata against fungus with the destruction of stomata immune system of the plant stops working. The wheat plant field along with fresh leafs of day 14, 24, 37 and 48 are studied and it can be seen that by visual observation no symptom of rust on leaves can be observed before day 24. But in case of laser scanning confocal microscopic imaging rust stomata destruction can be observed from day 18 of wheat tillering. This technique can developed a rapid and effective way to early detection of wheat leaf tissue infected with the fungal pathogen.
References
- Peter RS, Sandra JH (2015) The contribution of wheat to human diet and health. Food and Energy Security 4: 178–202.
- Sankaran S, Mishra A, Ehsani R, Davis C (2010) A review of advanced techniques for detecting plant diseases. Comput. Electron Agric 72: 1–13.
- Kolmer JA, Ordonez ME, Groth VJ (2009) The Rust Fungi. In: Encyclopedia of Life Sciences. John Wiley & Sons, Chichester.
- Bolton M, Kolmer J, Garvin D (2008) Wheat leaf rust caused by Puccinia triticina. Mol Plant Patol 9: 563–575.
- Ashourloo D, Mobasheri MR, Huete A (2014) Evaluating the effect of different wheat rust disease symptoms on vegetation indices using hyperspectral measurements. Remote Sens 6: 5107-5123.
- Topping D (2007) Cereal complex carbohydrates and their contribution to human health. J Cereal Sci 46: 220–229.
- Kolmer JA, Ordonez ME, Groth JV (2009) The rust fungi. Encyclopedia of Life Sciences. John Wiley & Sons, Ltd.
- Rafiqi M, Ellis JG, Ludowici VA, Hardham AR, Dodds PN (2012) Challenges and progress towards understanding the role ofeffectors in plant-fungal interactions. Curr Opin Plant Biol 15: 477–482.
- Song X, Rampitsch C, Soltani B, Mauthe W, Linning R, et al. (2011) Bakkeren G Proteome analysis of wheat leaf rust fungus, Puccinia triticina, infection structures enriched for haustoria. Proteomics 11: 944–963.
- Pawley JB (2010) Handbook of Biological Confocal Microscopy, Plenum Press, New York.
- Soleiman NH, Solis I, Sillero JC, Foessel HSA, Ammar K, et al. (2014) Evaluation of macroscopic and microscopic components of partial resistance to leaf rust in durum wheat. J Phytopatholl 62: 359-366.
- Dutech C, Fabreguettes O, Capdevielle X, Robin C (2010) Multiple introductions of divergent genetic lineages in an invasive fungal pathogen, Cryphonectria parasitica, in France. Heredity 105: 220-228.
- Szabo LS, Kolmer JA (2007) Development of simple sequence repeat markers for the plant pathogenic rust fungus Puccinia triticina. Mol Ecol Notes 7: 708-710.
- Tai YS, Bragg J (2007) Dual applications of a virus vector for studies of wheat-fungal interactions. Biotechnology 6: 288-291.
- Qiong Z, Wenjiang H, Ximin C, Yue S, Linyi L (2018) New Spectral Index for Detecting Wheat Yellow Rust Using Sentinel-2 Multispectral Imagery. Sensors 18: 868-872.
- Burling K, Hunsche M, Noga G (2012) Presymptomatic detection of powdery mildew infection in winter wheat cultivars by laser-induced fluorescence. Appl Spectrosc 66: 1411–1419.
- Burling K, Hunsche M, Noga G (2011) UV-induced fluorescence spectra and lifetime determination for detection of leaf rust (Puccinia triticina) in susceptible and resistant wheat (Triticum aestivum) cultivars. Funct Plant Biol 38: 337–345.
- Lenk S, Chaerle L, Pfundel EE, Langsdorf G, Hagenbeek D et al. (2007) Multispectral fluorescence and reflectance imaging at the leaf level and its possible applications. J Exp Bot 58: 807–814.
- Pathan AK, Bond J, Gaskin RE (2010) Sample preparation for SEM of plant surfaces. Materialstoday 12: 32-43.
- Pretorius Z, Szabo L, Boshoff W, Herselman L, Visser B (2012) First report of a new TTKSF race of wheat stem rust (Puccinia graminis f. sp. tritici) in South Africa and Zimbabwe. Plant Dis 96: 590-593.
- Jozef S, George K, Dominik N, Miroslav O, Olga S (2018) Advances in Imaging Plant Cell Dynamics. Plant Physiol 176: 80-93.
- Ashourloo D, Mobasheri M, Huete A (2014) Developing two spectral disease indices for detection of wheat leaf rust (Pucciniatriticina). Remote Sens 6: 4723-4740.
- Mahlein A, Rumpf T, Welke P, Dehne H, Plumer L, et al. (2013) Development of spectral indices for detecting and identifying plant diseases. Remote Sens Environ 128: 21-30.
- Bajwa S, Rupe J, Mason J (2017) Soybean disease monitoring with leaf reflectance. Remote Sens 9: 127-131.
- Maeli M, William U, Sheng YH (2008) Role of Stomata in Plant Innate Immunity and Foliar Bacterial Diseases. Annu Rev Phytopathol 46: 101-122.
- Franke J, Menz G (2007) Multi-temporal wheat disease detection by multi-spectral remote sensing. Precis Agric 8: 161-172.
- Huang W, Guan Q, Luo J, Zhang J, Zhao J, et al. (2014) New optimized spectral indices for identifying and monitoring winter wheat diseases. IEEE J Sel Top Appl Earth Obs Remote Sens 7: 2516-2524.
- Tariq S, Bilal M, Shahzad S, Firdous S, Aziz U, et al. (2017) Diagnosis of thalassemia and iron deficiency anemia using confocal and atomic force microscopy. Laser Physics Letters 14: 115703.
- Firdous S, Anwar S (2016) Optical diagnosis of dengue virus infected human blood using Mueller matrix polarimetry. Optics and Spectroscopy 121: 322-325.
- Firdous S, Anwar S (2016) Noninvasive optical diagnostic of breast cancer using depolarization of light. International Journal for Light and Electron Optics 127: 3035-3038.
- Anwar S, Firdous S (2015) Optical diagnostic of Hepatitis B(HBV) and C (HCV) from Human blood serum using Raman Spectroscopy. Laser Physics Letter 12: 076001.
- Firdous S, Anwar S (2015) Dengue viral infection monitoring from diagnostic to recovery using Raman spectroscopy. Laser Physics Letter 12: 085601.
- Anwar S, Firdous S, Rehman A, Nawaz M, Raman R (2015) Optical Diagnostic of Breast Cancer using Raman, Polarimetric Fluorescence Spectroscopy. Laser physics Letter 12: 045601.
- Firdous S, Nawaz M, Ahmed M, Anwar S, Mahmood A (2012) Measurement of Diabetic Sugar Concentration in Human Blood Using Raman Spectroscopy. Laser Physics 22: 1090-1094.
- Firdous S, Ahmed M, Rehman A, Nawaz M, Anwar S, et al. (2012) Transmission Spectroscopy of Dengue Viral Infection. Laser Physics Latter 9: 317-321.
Citation: Firdous S (2018) Optical Fluorescence Diagnostic of Wheat Leaf Rust with Laser Scanning Confocal Microscopy. Adv Crop Sci Tech 6: 355. DOI: 10.4172/2329-8863.1000355
Copyright: © 2018 Firdous S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5239
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 4389
- PDF downloads: 850