Editorial Open Access
One Year Passed Since A Bile Acid Transporter (Sodium Taurocholate Cotranspoting Peptide [NTCP] Was Nominated as A Hepatitis B Virus (HBV) Entry Receptor; Has the NTCP Has Been as A Real HBV Receptor?
| Keiji Ueda* | ||
| Division of Virology, Department of Microbiology and Immunology, Osaka University Graduate School of Medicine, 2-2 Yamada-oka, Suita, Osaka 565-0871, Japan. | ||
| Corresponding Author : | Keiji Ueda Division of Virology, Department of Microbiology and Immunology Osaka University Graduate School of Medicine 2-2 Yamada-oka, Suita, Osaka 565-0871, Japan Tel: +81-6-6879-3783 or 3780 Fax: +81-6-6879-3789 Email: kueda@virus.med.osaka-u.ac.jp |
|
| Received November 29, 2012; Accepted December 02, 2012; Published December 10, 2012 | ||
| Citation: Ueda K (2013) One Year Passed Since A Bile Acid Transporter (Sodium Taurocholate Cotranspoting Peptide [NTCP] Was Nominated as A Hepatitis B Virus (HBV) Entry Receptor; Has the NTCP Has Been as A Real HBV Receptor? J Infect Dis Ther 1:e102. doi: 10.4172/2332-0877.1000e102 | ||
| Copyright: © 2013 Ueda K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
About one year ago, one important report in the virology field was published. It was a report by Yan et al. that an entry receptor for hepatitis B virus (HBV) was isolated and identified. It was also verified that this molecule termed sodium taurocholate cotransporting peptide (NTCP) was functionally active as an HBV receptor when it was introduced into hepatocyte originated hepatocellular carcinoma cell lines such as HepG2 and Huh7 cells. There has been, however, only one report published from the same group after this exciting report and the others are several short commentaries and no report to show the reproducibility. Recently, Meier et al. reported that the ligand region of the HBV membrane protein for NTCP, myristoylated preS1: 2-48 aa (myr2-48) could bind mouse hepatocyte but it was functionally inactive. Yan et al. reported that a functional determinant was the short amino acid sequence from 84 to 87 aa of NTCP. If so, what does this difference affect the function of NTCP as a HBV receptor. Thus, there are a lot of to do in order to understand how the HBV receptor works though we need to prove its reproducibility.
| Isolation and identification of HBV entry receptors seemed to be one of the biggest questions in the virology field. This molecule must be important to understand the real life cycle of HBV and to explore new drugs preventive against HBV infection. | |
| We know that HBV infects parenchymal hepatocytes but HBV infection was not reproduced in vitro by using hepatocyte originated hepatocellular carcinoma cell lines and a functional HBV receptor had not been identified for a long time, although these hepatocellular carcinoma cells could be totally deviated from normal well-differentiated hepatocytes. Yan et al. solved this question finally [1]. They identified that NTCP was a functional HBV entry receptor last year. They used a photoactivated myristoylated preS1 peptide which is agreed to be a viral ligand interacting with a cellular receptor on the surface to clone an HBV receptor [2]. This kind of approach, including the same region expressed in E. coli and purified, could have been performed by other researchers. It might be a wonder and of course a good news for them to successfully identify the HBV receptor. | |
| There should be now a glorious road to be open for HBV research by establishing convenient and simple infection system of HBV in vitro and in vivo. Currently, however, reports proving that NTCP is reproducibly working as an HBV entry receptor have not been published after the report by Yan et al. except several reports from the same group and a few short commentaries or reviews [3-7]. | |
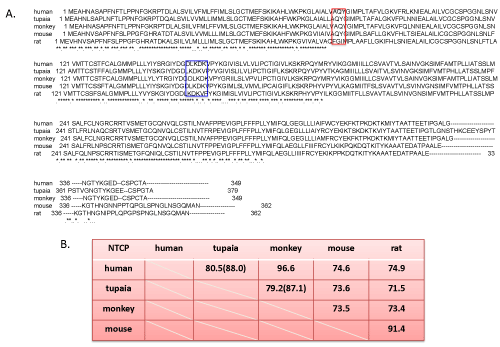
| Homology between human vs mouse and human vs monkey NTCP is about 75% and 97% (Figure 1A and 1B), respectively, but functionally distinctive in terms of HBV infection. Monkey NTCP (mkNTCP) seems to be actually identical but mkNTCP does not allow HBV to attach [1]. Well-differentiated hepatocytes, either human or mouse, express NTCP on the cell surface but not human hepatocellular carcinoma originated cell lines such as HepG2 cells. HepaRG cells, which become susceptible to HBV infection after differentiation with 2% dimethyl sulfoxide (DMSO) for several weeks, does not express NTCP without differentiation [8]. Both human and mouse can bind with HBV preS1 but only human NTCP (hNTCP) expressed on hepatocyte is competent for HBV entry [3,9]. Yan et al. reported that his functional discrimination was attributed to differences of short amino acid sequence from 84 to 87 and 157 to 165 [1,3]. Especially, the former could be important for HBV uptake and entry irrespective of the binding and taurocholate transportation [3]. Some replacement mutants of human NTCP with mouse one lose binding activity with HBV. To the contrary, replacement mutants of mouse NTCP (mNTCP) with hNTCP at the same region keep binding activity with HBV and infection [3]. | |
| Thus, the binding activity of NTCP with HBV should be agreed, but it needs further investigation to understand how NTCP works at the HBV attachment and entry step. Nevertheless, there should be reports showing that NTCP is reproducibly a key factor for HBV attachment and entry into cells. Otherwise, there could be missing some other cofactors required for HBV infection like coreceptors for human immunodeficiency virus-1 (HIV-1). | |
References
- Yan H, Zhong G, Xu G, He W, Jing Z et. al (2012) Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1:e00049
- Glebe D (2007) Recent advances in hepatitis B virus research: a German point of view. World J Gastroenterol 13: 8-13.
- Yan H, Peng B, He W, Zhong G, Qi Y et. al (2013) Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J Virol 87: 7977-7991.
- Zhong G, Yan H, Wang H, He W, Jing Z et. al (2013) Sodium taurocholate cotransporting polypeptide mediates woolly monkey hepatitis B virus infection of Tupaia hepatocytes. J Virol 87: 7176-84.
- Warner N, Locarnini S (2013) The new front-line in hepatitis B/D research: identification and blocking of a functional receptor. Hepatology 58: 9-12.
- Anwer MS, Stieger B (2013) Sodium-dependent bile salt transporters of the SLC10A transporter family: more than solute transporters. Pflugers Arch .
- K. Ueda (2013) Start or End? One of the Biggest Mysteries is Finally Solved. J Infect Dis Ther 1.
- Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, et al. (2002) Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A 99: 15655-15660.
- Meier A, Mehrle S, Weiss TS, Mier W, Urban S (2013) Myristoylated PreS1-domain of the hepatitis B virus L-protein mediates specific binding to differentiated hepatocytes.Hepatology 58: 31-42.
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 15022
- [From(publication date):
December-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10358
- PDF downloads : 4664
