Omitted Liver Disease Screening in Acute Coronary Syndrome (ACS) Hides Prevalence of High Levels of Severe Liver Fibrosis
Received: 03-Aug-2022 / Manuscript No. JOWT-22-71163 / Editor assigned: 04-Aug-2022 / PreQC No. JOWT-22-71163 (PQ) / Reviewed: 18-Aug-2022 / QC No. JOWT-22-71163 / Revised: 23-Aug-2022 / Manuscript No. JOWT-22-71163 (R) / Published Date: 30-Aug-2022
Abstract
Background and Aims: Non-alcoholic fatty liver disease (NAFLD) has important associations with the metabolic syndrome and shares features with cardiovascular diseases, such as acute coronary syndrome (ACS). It is not standard of care to screen ACS patients for liver disease. We sought, to determine what proportion of patients presenting with ACS had full liver functions tests (LFTs) performed, and using available LFTs assessed for liver fibrosis.
Methods: Retrospective study of patients admitted with ACS to St Thomas’ teaching hospital between 01/09/17 and 31/08/18. We determined if full LFTs had been measured to allow calculation of a fibrosis score. As a secondary outcome we audited metabolic parameters for each patient. This study was exempt from IRB review.
Results: 360/521 patients (69%) met inclusion criteria of having an acute admission due to ACS: 272 males and 88 females, aged 34-91. 181(50%) had an ST elevation Myocardial infarction (STEMI), 176(49%) had a Non-ST elevation Myocardial Infarction (NSTEMI), and 3 had an unspecified ACS. 30 (8.3%) had sufficient tests performed to calculate FIB-4; 263(72%) had basic LFTs performed, and 67(19%) had no LFTs measured. Of the 30 with sufficient tests, 47% had a FIB-4 score >3.25, indicating advanced liver fibrosis (bridging fibrosis or cirrhosis). Only 2 had pre-existing known liver disease including NAFLD.
Conclusion: Few patients presenting with ACS are assessed for liver fibrosis. A change in standard of care to performing a full liver screen on patients admitted with ACS would allow for the increased diagnosis of liver fibrosis.
Keywords
Non-Alcoholic fatty liver disease; Acute Coronary syndrome; Liver fibrosis; Metabolic syndrome
Introduction
Non-alcoholic fatty liver disease (NAFLD) is now the most common cause of chronic liver disease in affluent countries and emerging economies with an estimated prevalence worldwide of 20- 30% [1]. NAFLD results from the deposition of triglycerides within hepatocytes, such that the presence of triglycerides exceeding 5% of total liver weight is considered pathognomonic of NAFLD [2]. The deposition of triglycerides occurs early within the disease and as such, NAFLD exists as a spectrum of disease ranging from steatosis through non-alcoholic steatohepatitis to fibrosis, cirrhosis and hepatocellular cancer [3].
The principle mechanism in its development likely involves aspects of the metabolic syndrome (Mets) including insulin resistance, dyslipidaemia, hypertension and obesity [1]. In such patients, the development of insulin resistance leads to uninhibited lipolysis in adipose tissue, resulting in an excessive influx of fatty acids into the liver [4]. NAFLD has often been regarded as the hepatic manifestation of the metabolic syndrome [5]. Given the common pathways, patients with underlying insulin resistance, Mets and cardiovascular disease (CVD), are at higher risk of NAFLD [6]. NAFLD itself may be a risk factor for CVD [ 7 ]. The association between CVD and NAFLD remains relatively poorly understood but studies show not only that NAFLD often co-exists with other aspects of the Mets [1], but that the presence of NAFLD itself influences exacerbates the course of CVD [8-11]. Current standard of care do not however involve assessing the extent of NAFLD, or other liver disease, in patients presenting with severe CVD.
We sought therefore to examine patients with severe CVD and assess biochemically for evidence of liver fibrosis. During the study,we retrospectively audited a population of patients presenting with severe acute coronary syndrome (ACS) to demonstrate if these patients demonstrated higher risk of liver fibrosis. Due to the largely asymptomatic nature of NAFLD and the increasing prevalence of NAFLD, it seems imperative to examine populations, such as those with CVD, who are at higher risk for the presence of NAFLD. The detection of abnormal metabolic parameters in these patients will also allow their early referral for investigations including ultrasound imaging and liver biopsy [12].
Methods
We performed a retrospective cohort analysis of patients presenting with ACS to St Thomas’ Hospital, a major tertiary teaching hospital, in central London over a 12 month period (1st September 2017 to 31st August 2018). The sample list was populated using the coding function of the discharge summaries to detect patients admitted with a principle diagnosis of ACS or the related terms: ‘acute coronary syndrome’, ‘myocardial infarction’, and ‘heart attack’.
The study team then manually identified patients with an appropriate diagnosis of ACS, which included ST-Elevation Myocardial Infarction (STEMI), Non-ST-Elevation Myocardial Infarction NSTEMI (NSTEMI), or unstable angina (UA) to obtain a data set. All data was stored in a password protected Microsoft excel spreadsheet with access provided only to the authors.
Patients who died during the admission, were transferred to St Thomas’s Hospital from another hospital for ACS management, or who had no records visible on the Hospital’s electronic record system were excluded. Of these those with a coding of co-existing liver disease were noted.
For qualifying patients, we proceeded to examine those patient who had liver function tests completed. In the study hospital the default measure of liver function was Alanine Transaminase, ALT (normal range less than 50 IU/L), Bilirubin (normal range 0-21umol/L) and Alkaline phosphatase (normal range 35-129 IU/L).
Additionally, we proceeded to examine those who had an Aspartate Transaminase, AST (normal range 0-50 IU/L) in order to calculate a fibrosis (FIB-4) score. Patient demographics such as age, gender, weight, random blood glucose/ HbA1C, body mass index (BMI) and lipid profiles where applicable were also recorded.
From these results a FIB-4 score (a non-invasive marker of liver fibrosis) was calculated using the following formula: FIB-4 = [age × AST/platelet count (109/L) × √ALT] as per Sterling et al [13].
The FIB-4 scores were calculated using an electronic web based calculator - https://www.hepatitisc.uw.edu/page/clinical-calculators/ fib-4. All statistics analysis was completed using web based / electronic calculators. In our statistical analysis we have used t-test one tail, p value, spearman’s rank coefficient and standard deviation (SD), which unless stated otherwise has been calculated to 1 SD.
Ethics
Data was stored on the shared drive of trust computers, only accessible by staff with permission to the directorate folder. Patient identifiable information such as name and hospital numbers was removed from the data to maintain confidentiality. The data was provided by the Guy’s and St Thomas’s (GSTT) trust.
Results
Within the stated 12 month period there were 521 patients examined, with 360 patients meeting the inclusion of criteria. The inclusion criteria were a defined diagnosis of ACS comprising STEMI, NSTEMI or unspecified ACS (UA). Of these 360 patients n=272 (76%) were male and n=88 (24%) were female. The ages of the patients ranged from 34 to 91 years old with a mean age of 63 years. The majority of patients n=181 (50.3%) had a diagnosis of ST elevation Myocardial infarction (STEMI); n=176 (49%) suffered with a non-ST elevation Myocardial Infarction (NSTEMI), and n=3 patients (0.8%) were diagnosed with unspecified angina.
Of the 360 patients with a diagnosis of ACS, n=30 patients (8.3%) had a full liver function tests (LFTs) performed, defined as having an aspartate transaminase (AST) recorded in addition to alanine aminotransferase (ALT) required to calculate a liver fibrosis index (FIB-4). 263 patients (72%) had an ALT recorded, and 67 (19%) had no LFTs measured.
A breakdown of the data is demonstrated in Table 1.
| Number of patients identified as ACS N = 360 | Number of patients | |
|---|---|---|
| Sex | Male | 272 |
| Female | 88 | |
| STEMI | 181 | |
| NSTEMI | 176 | |
| Unspecified ACS | 3 | |
| LFT’s completed | Partial | 263 |
| Full | 30 | |
| N | 67 | |
| BMI | Y | 333 |
| N | 27 | |
| Pre-existing liver disease | NAFLD | 1 |
| NASH | 1 | |
| HEP B / HCC | 1 | |
| HEP C | 1 | |
| Cholesterol | Yes | 236 |
| No | 124 | |
| Diabetes | Yes | 104 |
| No | 256 | |
| HbA1C | YES | 223 |
| No | 137 | |
Table 1: Patient characteristics of patients with a diagnosis of ACS. It delineates, sex, category of ACS, LFT, BMI, and identifies those with pre-existing liver disease.
Out of 30 patients, with a full LFT results to enable calculation of a FIB-4 score 90% (n=24) were male and 10% (n=3) were female. The average ALT was 46 +/- 45.11 SD, and the average AST was 58 +/- 34.53 SD. In the 30 patients classified as having a full liver function set, 47% had a FIB-4 score >3.25, indicating a very high (65%) positive predictive value for advanced liver fibrosis [6]. No patients in this group have a known pre-existing diagnosis of NAFLD or other liver disease (Table 2).
Number of patients identified as full LFT’s N = 30 |
Number of patients | Percentage | ||
|---|---|---|---|---|
| Gender | Male | 27 | 90% | |
| Female | 3 | 10% | ||
| STEMI | 2 | 6.7% | ||
| NSTEMI | 27 | 90% | ||
| Unspecified ACS | 1 | 3.3% | ||
| LFT’s completed | Full | 30 | 100% | |
| BMI | Y | 30 | 100% | |
| N | 0 | 0% | ||
| Obesity – BMI > 30 | Y | 6 | 20% | |
| N | 24 | 80% | ||
| Overweight BMI > 25 | Y | 16 | 53% | |
| N | 14 | 47% | ||
| Average BMI with standard deviation. | Average | 29 *** removed one patient because likely erroneous result SD: 4.77 |
Average BMI: 26.61 | |
| Pre-existing liver disease | NAFLD | N | 0% | |
| NASH | N | 0% | ||
| HEP B / HCC | N | 0% | ||
| HEP C | N | 0% | ||
| Average (mean) liver function tests | ALT | 46 | 45.11 SD | |
| AST | 58 | 34.53 SD | ||
| Cholesterol | Yes | 20 | 67% | |
| No | 10 | 33% | ||
| Diabetes | Yes | 9 | 30% | |
| No | 21 | 70% | ||
| HBA1C | YES | 21 | 70% | |
| No | 9 | 30% | ||
Table 2: Examining those with a full LFTs.
In the 30 patients with sufficient data available to calculate a FIB-4 score, 23.3% had a FIB-4 score suggestive of mild disease, 23.3% had a FIB-4 score suggestive of moderate disease and 53.3% indicative of severe disease. The mean average FIB-4 score for these patients was 3.33 SD +/- 1.86, with a maximum score of 9.30 and a minimum score of 0.81.
Using the previous study of Sterling et al [13], we identified a value of 1.45 to denote a FIB-4 score suggestive of no disease. In that study, a fibrosis score of <1.45 had a negative predictive value of 90% with a sensitivity of 70%. The calculated single T-tests, one tail hypothesis of 6.34. That resulted in a p-value of <0.0001. Therefore the mean score of 3.33 +/- 1.86 in our population group is statistically significant from a population group whereby you would expect no evidence of fibrosis. This would suggest that the patients presenting with ACS were at a higher risk of fibrosis. It is also noteworthy that using the FIB-4 score of 3.25 used by Sterling et al [13] to denote a score suggestive of severe liver disease shows that our mean score of 3.33 is potentially suggestive of likely disease. This score had a positive predictive value of 65% and a specificity of 97% (Table 3).
| FIB4 Score | Approximate Fibrosis stage |
|---|---|
| <1.45 | 0-1 |
| 1.45-3.25 | 2-3 |
| >3.25 | 4-6 |
Table 3: FIB-4 Score and approximate fibrosis staging.
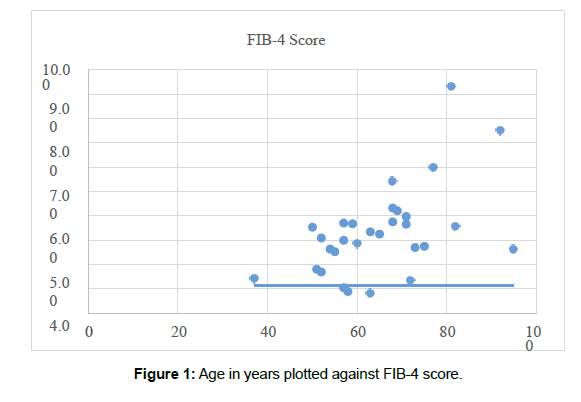
In Figure 1 we have compared a patient’s age with their corresponding FIB-4 score. This demonstrated a positive correlation between age and FIB-4 scores. The Pearson’s correlation for age in relation to their corresponding FIB-4 score was 0.54 which depicts a good degree of correlation which mirrors the work of Sterling et al who identified age as a factor associated with fibrosis.
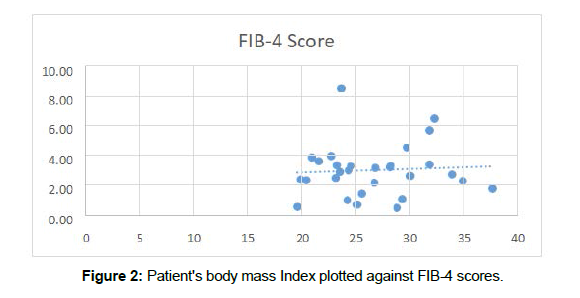
Furthermore, we plotted patient BMI against their corresponding FIB-4 scores (Figure 2). From our data, noticeably, the higher recorded FIB-4 scores appeared in those with higher BMI’s and no patients who were clinically obese registered as having a FIB-4 score <1.45. Moreover, we found a Pearson correlation of 0.06, suggesting that there is a positive correlation between BMI and FIB-4 scores in our identified population. This is to be as expected as obesity has been associated with increased infiltration of hepatocytes by triglycerides [4]. Moreover, many studies have identified obesity as a risk factor for NAFLD [1].
Discussion
With the increasing prevalence of NAFLD which is associated with increasing morbidity and mortality, the early identification of those with the potential to develop significant liver disease is essential. An essential concept for patients with NAFLD is that most are asymptomatic therefore; increasingly innovative and proactive measures are required to identify those who are at risk [1]. By assessing patients attending hospital with acute coronary syndrome as a surrogate for NAFLD, we have been able to demonstrate that this group of patients are at higher risk of disease using a non-invasive measure of liver fibrosis. Our paper highlights that further investigation is required to further elucidate this association.
We have demonstrated that patients with cardiovascular disease appear to have a higher average FIB-4 score (3.33 on average), which is statistically significant from the 1.45 value used by Sterling et al to indicate a low probability of fibrosis [13]. Moreover, we have demonstrated that factors that are associated with a higher FIB-4 score include age and BMI. It is believed that NAFLD may be representative of the hepatic manifestation of the metabolic syndrome which is a collection of cardiovascular risk factors including impaired glucose tolerance, hypertension and obesity [14]. In our study we have also demonstrated a positive correlation between firstly BMI and FIB-4 score and secondly FIB-4 score which appears to be increasing with age as a variable. Considering that in our study the average age was 63, this population was already at risk of having a higher fibrosis score.
Given the reliance of serological markers for the FIB-4 score, one limitation of our study is that severe illness could also alter the FIB-4 score. This includes those with severe ACS which can result in more significant transaminitis as a result of liver hypo-perfusion therein transiently increasing the FIB-4 score. In our data, they may be responsible for possible outlier’s therefore repeat serology several days after a severe illness may yield a more accurate fibrosis score.
Consideration of imaging such as ultrasound or liver biopsy for those with ACS found to have significant fibrosis scores may aid diagnosis confirmation.
The gold standard for the diagnosis of NAFLD is a liver biopsy; however this is an expensive and invasive method which does carry significant risk of complications [13]. The FIB-4 score provides a well validated non-invasive alternative. Although the FIB-4 score is a recognised risk stratification tool to indicate fibrosis, it cannot distinguish between steatosis in isolation and steatohepatitis with fibrosis and therefore cannot independently diagnose NAFLD. Despite this, it remains a useful screening marker to be used in the first instance and identify those who warrant further investigation.
It has been noted that a significant proportion of those with NAFLD may have normal liver function tests. In view of this it is likely that the prevalence of NAFLD in populations may be vastly underestimated making it more important to risk stratify those most at risk [15]. Unfortunately, a second limitation of our study is that patients did not have serological non-invasive liver screens to exclude other causes of liver enzyme abnormalities such as autoimmune hepatitis and viral hepatitis. Moreover, we did not assess alcohol history which could also contribute to liver enzyme abnormality.
Within the context of these limitations, we are still one of the few studies looking specifically at patients with ischaemic cardiovascular disease and the potential for corresponding NAFLD. In this paper we have demonstrated that patients with cardiovascular disease are at higher risk of liver fibrosis. Unfortunately, only 8% of patients presenting with ACS had a full liver function panel in our study. Our data has shown that omitted liver disease screening in patients with acute coronary syndrome risks obscuring the prevalence of high levels of fibrosis. This is suggested by the 47% of the patients included in our study having a FIB-4 score of >3.25 suggestive of fibrosis. It has not been entirely possible to determine the correlation between NAFLD and the burden of cardiovascular disease likely to be present. A change in practice by performing a full liver screen in patients admitted with ACS would allow for the increased diagnosis of liver fibrosis, and would enable earlier management to reduce morbidity and mortality. However crucially, it may highlight those patients in which further investigations such as fibro-scanning may be more appropriate.
We have illustrated the potential significance of understanding and examining patients with cardiovascular disease as a cohort likely to be at risk of NAFLD. Further investigation of NAFLD in relation to cardiovascular risk factors may determine first ‘’where’’ and secondly in ‘’which’’ patients early intervention may be appropriate, as well as further elucidate the disease pathophysiology. Further study is required on a larger scale to identify the burden of liver disease in those presenting with cardiovascular disease [16,17].
NAFLD is a rapidly growing cause of chronic liver disease, mirroring the rising incidence of obesity and the metabolic syndrome. Management requires a multidisciplinary approach with clear risk stratification. Unfortunately, there remains to be any universally accepted pharmacological treatment options. Modifying risk factors, such as weight loss my result in improvement of steatosis. Although lifestyle modification such as physical activity and weight loss remain the cornerstone of management we are about to enter a new era of promising pharmacotherapies for NASH and fibrosis [12].
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
No funding was required for this study.
Conflict of Interest
The authors declare that they have no conflict of interest
Informed Consent
No consent was required
References
- Kneeman JM, Misdraji J, Corey KE (2012) Secondary causes of nonalcoholic fatty liver disease.Therap Adv Gastroenterol 5(3): 199-207.
- Fabbrini E, Sullivan S, Klein S (2010) Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications.Hepatology 51(2): 679-689.
- Stal P (2015) Liver fibrosis in non-alcoholic fatty liver disease - diagnostic challenge with prognostic significance.World J Gastroenterol 21(39): 11077-11087.
- Arab JP, Arrese M, Trauner M (2018) Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu Rev Pathol 13: 321-350.
- Kim CH, Younossi ZM (2008) Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med 75(10): 721-8.
- Lonardo A, Loria P (2009) NAFLD and cardiovascular risk: direct evidence for the tale of two ages. Am J Gastroenterol. 104(7): 1851-2.
- Sinn DH, Kang D, Chang Y, Ryu S, Gu S, et al. (2017) Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut 66(2): 323-329.
- Duseja A, Chalasani N (2013) Epidemiology and risk factors of nonalcoholic fatty liver disease (NAFLD). Hepatol Int 7 Suppl 2: 755-64.
- Santos RD, Valenti L, Romeo S (2019) Does nonalcoholic fatty liver disease cause cardiovascular disease? Current knowledge and gaps. Atherosclerosis 82: 110-120.
- Lonardo A, Sookoian S, Pirola CJ, Targher G (2016) Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism 65(8): 1136-50.
- Anstee QM, Targher G, Day CP (2013) Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 10(6): 330-44.
- Maurice J, Manousou P (2018) Non-alcoholic fatty liver disease.Clin Med (Lond) 18(3): 245-250.
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, et al. (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43(6): 1317-25.
- Kim CH, Younossi ZM (2008) Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med 75(10): 721-8.
- Angulo P (2007) GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther 25(8): 883-9.
- Dharmalingam M, Yamasandhi PG (2018) Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus.Indian J Endocrinol Metab 22(3): 421-428.
- Huang PL (2009) A comprehensive definition for metabolic syndrome.Dis Model Mech 2(5-6): 231-237.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Saliu D, Veys S, Reynolds RC, Mandour MO, Oben JA (2022) OmittedLiver Disease Screening in Acute Coronary Syndrome (ACS) Hides Prevalence ofHigh Levels of Severe Liver Fibrosis. J Obes Weight Loss Ther 12: 511.
Copyright: © 2022 Saliu D. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2505
- [From(publication date): 0-2022 - Nov 26, 2025]
- Breakdown by view type
- HTML page views: 2115
- PDF downloads: 390