Omics Technologies: A Hope for Translational Research in Bovine Tuberculosis
Received: 05-Feb-2019 / Accepted Date: 15-Feb-2019 / Published Date: 22-Feb-2019 DOI: 10.4172/2332-0877.1000394
Abstract
Bovine tuberculosis diagnosis is one of the main challenges faced by animal and public health systems. The incidence of M. bovis infections remains undefined in developed countries. So it is necessary to carry out an extensive study and surveillance to determine the status of bovine tuberculosis as an urgent need for control eradication program. Furthermore, developed countries, microbiological (bacteriological) and immunological (histochemistry) techniques are still used, making more difficult to homogenize epidemiological knowledge of bTB. Recent reports describing the potential of microarray technology not only to explore subunit vaccine agents (biomarkers), but to pinpoint immunomodulation, and signatures in the journey of pathogen interaction with the host in bovine tuberculosis. Omics and next generation high-throughput technologies have risen as promising tools that will enable translational research (development of prognostic and diagnostic methods with high accuracy and sensibility) and in depth molecular analysis even at single cell level to underpin dynamics in the transcripts regulation of the host response in bTB.
Keywords: Bovine tuberculosis; M. bovis ; RNA seq; Transcritptomic; Genomics; Omics technologies; Printing signatures; Gene expression profiles
Introduction
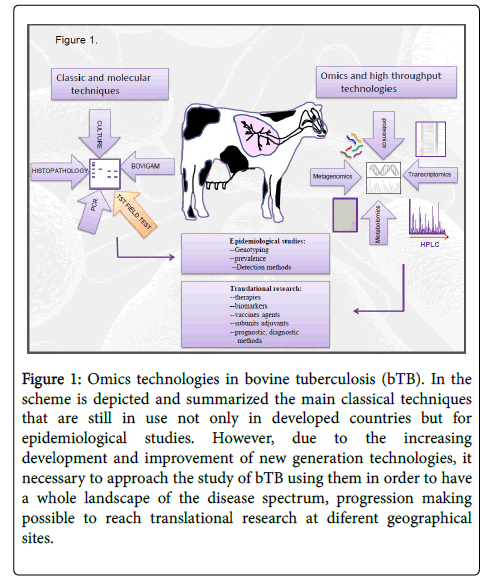
The diagnosis of bovine tuberculosis in Mexico is one of the main challenges faced by animal and human public health systems. Actually, accurate diagnosis with a high degree of sensitivity, specificity is an urgent need that should be pursued worldwide [1-5]. The conventional methods that have been used for the detection of Mycobacterium bovis (M. bovis) as described in the Manual of the World Organization for Animal Health (OIE) on terrestrial animals reside primarily in the delayed hypersensitivity test (tuberculin test), or tuberculin skin test (TST), bacteriological and histopathological exams [5-6]. More recent reports indicate that the molecular detection of M. bovis has been possible thanks to the development of multiplex PCR (primers to several targets) [7-23], allowing identification and differentiation between members of the M. tuberculosis complex. In conjunction with this molecular methods of detection, a hot spot, is the approach of the study of gene expression profile, using microarrays technologies, that is, hybridization of cDNA with bovine DNA, enabling thus, determination and identification of genes and the pathways that involved and touched by the pathogen interaction with the host response [24-26]. More recently, high-throughput sequencing technologies such as RNA seq (massive, 2do generation) and third generation (single cell sequencing) [27,28] have been approximate to the study of the alveolar macrophages gene expression profile, comparative studies of host preference between M. tuberculosis and M. bovis [29-34]. Furthermore, omics technologies as a whole, including, metagenomics, proteomics, metabolomics, transcriptomics arise and could provide a promising tool to define even at single cell level, the spectrum of the sisease (latent versus active) as well as the dynamics of innate and adaptive immune response in either case, that in conjunction might promote translational research in bTB (35-46) (Figure 1).
Figure 1: Omics technologies in bovine tuberculosis (bTB). In the scheme is depicted and summarized the main classical techniques that are still in use not only in developed countries but for epidemiological studies. However, due to the increasing development and improvement of new generation technologies, it necessary to approach the study of bTB using them in order to have a whole landscape of the disease spectrum, progression making possible to reach translational research at diferent geographical sites.
What We have in Terms of Detection and Identification of M. bovis
In the last decades, a huge of group have been focused in the development of molecular detection of M. bovis which in general terms started with isolation from tissue homogenate with lesion, seeding in Middlebrook brook solid medium supplemented with OADC and THF followed by DNA extraction, nested PCR and multiplex PCR amplification of specific regions of the M. bovis genome [8-14], a screening test used to prevent infection and introduction of disease in healthy herds. The application of the PCR technology, have been seen as a reliable and accurate diagnostic development [8-14]. Moreover, real-time Multiplex PCR was standardized with reference to Mycobacterium strains and was subsequently tested with 66 clinical isolates [15-17]. The sensitivity and specificity of the designed primers were for each one as follows: 100% for MTC, M. abscessus , M.fortuitum , M. aviumcomplex , M. kansasii , and M. gordonae . While the sensitivity and specificity of the primers designed for the genus Mycobacterium were between 96 and 100% [15-17]. By other hand, epidemiological analysis using techniques such as spoligotyping, VNTRs, RFLPs, for typification of M. bovis substrains and for simultaneous differentiation of other members of the Mycobacterium complex with ther mycobacterial species not included in the complex. Non-tuberculous mycobacterial species (NTM) that may have a clinical significance and interference with the detection and identification of M. tuberculosis [18-20] were analysed. Thus, MTBC and NTM were simultaneously evaluated in respiratory specimens using real-time PCR multiplex and RFLPs. and the Geno Blot Advan Sure Mycobacteria trial (LG Life Sciences). The data obtained using this approach, is that species commonly detected in mixed cultures were M. intracellulare (29.0%) and M. abscessus (29.0%) [18-20] to carry out a rapid and simultaneous detection of the M. tuberculosis complex (MTC), as well as of differentiation with M. bovis ; a multiplex assay based on microspheres was developed using xMAP technology [21]. Briefly, these methods detects 4 target sequences, including the insertion-specific elements IS6110 and IS1081 of the MTC, a specific fragment of 12.7-Kb for M. tuberculosis , and an uninterrupted sequence of 229 sub specific for M. bovis [17,22]. The specificity of the assay was validated by testing 13 reference strains of mycobacteria; 22 isolates of M. tuberculosis and M. bovis , and 25 species of nonmycobacterial microorganisms. The assay can be completed within 2 h with a limit of detection per assay above 6 to 10 bacteria per reaction. The sensitivity of the temperate cloned DNA was 0.37 to 0.74 fg of DNA per reaction. The trial also allowed to distinguish between MTC and M. bovis with a high percentage, 98.9% (89/90) and 91.9% (34/37) as MTC and/or M. bovis in human sputum samples and/or tissue from bovine [17,18,22] (Figure 1).
Why OMICS has Arisen as a Hope for Translational Research in Bovine Tuberculosis?
The increasing necessity not only to detect the pathogen but with the firm aim to control disease and if possible to eradicate due to arise of multi-drug resistant strains of the M. tuberculosis complex, which includes M. bovis [1]. In addition to other social factors in developed countries. Moreover, while in human TB is very clear and it is very well established [26,27], the spectrum and stages of infection (latent virus active disease, in cattle, is still undefined. Studies highlighted from the literature have evidenced that biological systems based on the integration of data generated by -omics studies are a very useful approach to identify biomarkers with therapeutic potential for human Tb [26] and/or bovine tuberculosis [28,31], to determine gene signatures that can predict and / or correlate with protection following vaccination (either in cows and/or in humans humans) [30,31]. At the beginning, DNA microarrays were used for the rapid and direct detection of M. tuberculosis and M. bovis in bovine milk has also been reported [20], based on the mtp40 and pncA sequences. The limit of detection for mycobacterial DNA using the DNA microarray was 50 fg (5 tubercle bacilli). M. tuberculosis and/or M. bovis were detected in 7.1% (24/336) of cow specimens using the DNA microarray compared to 6.0% (20/336) using culture methods [17]. Mixed infections were detected in 3 animals using the DNA microarray method, while mixed infections were detected in 2 animals using either culture or PCR methods. Thus, in vitro detection methods (PCR) together with DNA microarrays increased the detection of cows infected with M. tuberculosis and/or M. bovis and reduced the number of false positive animals that could be eliminated. [17,19]. Then microarray technologies was used for the development of molecular diagnostic highly sensible and specific [23-31]. In one these reports it has been described the unknown role of type I IFNs in the pathogenicity of M. tuberculosis [26] while in the second report, it was revealed the IL-22 role in the protection against M. bovis [29]. In order to identify biomarkers profile it is essential to use bioinformatics tools in conjunction with new generation high throughput technologies (massive and RNA seq) to determine a more whole integrated physiologic and immunological (innate and adaptive) response of host to pathogen [28-30]. A wealth of studies have been conducted with bovine peripheral blood mononuclear cells (PBMCs), as well as with alveolar macrophages (are the first cells encountered with M. bovis ) [32,33]. Indeed, bovine monocytes derived macrophages have been challenged with M. bovis and the gene expression signature has been determined [34,35]. Gene signature profile in these cells has been viewed one, as a various tool to study the dynamics of mRNA transcripts in bTB [32,36,37], in particular, innate cytokine pattern induced after infection of alveolar macrophage with M. bovis or M. tuberculosis [38]. By another hand, RNA seq was also approached in conjunction with microarray technologies, to have a higher accurate and dynamic determination of gene expression transcripts and differential gene expression [39] as Nalpas et al., 2013 [40] showed in an in vitro analysis of the whole transcriptome of M. bovis infected macrophages by microarray technologies and later on, using RNA seq, revealed that alveolar macrophage infected with tubercle bacilli have a complex pattern of host immunomodulatory response [40,41], it means to approach dynamics of mRNA. miRNA, sRNA, splicing patterns, expression level, identification of novel transcript, and analyses of global gene expression even at single cell level, any change due to the host-pathogen interaction in bTB by transcriptomics [42] Furthermore, in recent years, microbiome in the different systemic and mucosal compartments have been approached using metagenomics. Thus, in bTB, metagenomics for genetic markers (susceptibility or resistance) using comparative genomic sequence and high-throughput sequencing (NGS) of PBMCs from herds of 1 to 2 months of infection is possible to achieve a more precise accurate and diagnostic of bTB in the early stages of disease progression [43]. Genomic profiles signatures (in terms of innate and adaptive immune response) have recently report to distinguish between active and latent tuberculosis, suggesting that is the genomic data either at nucleotide that information should be flow through to pin point not only to search for gold biomarkers but a set of a more integrated or whole biomarkers tools [44,45]. Malone et al., aimed to compare whether or not are differences in the response to infection by either M. tuberculosis or M. bovis by comparative omics technologies, they found that depending of the species, the macrophage gene expression program is different even both pathogens share 99.5% homology, they still have some percentages of different routes depending of the host human or bovine.
Proteomics
Proteomics is also a powerful tool that should be integrated to the study of bTB [45], to deep insight in protein-protein interactions, to characterize proteins that suffer post-transductional modifications, to study stability, abundance of key role of proteins, glycoprotein, when and how are expressed and migrate, protein patterns and if the proteome overall at the level of cells (macrophages, dendritic or lymphocytes cells) or tissues are affected in response to M. bovis infection. All these issues can be studied, by spectrometric mass (SELDITOFF) [27,45]. Moreover, recent research in this aspect indicate that the knowledge of the antigenic targets of T cells in bTB as well as the increasing knowledge of the subset of T cells and their interactions with infected macrophages with M. bovis can help for the development of better methods of control of disease. In biologic systems based in the integration of data generated by omics studies are a potential approach that can be used to identifiy transcriptional gene signatures to predict or to correlate parameters of protection in vaccinated calves versus unvaccinated, and also 188 to predict vaccination protocol effectiveness, until now mostly applied to human tuberculosis [26,27,31,45,46] (Figure 1).
Conclusion
Despite of the development and improvement of the DNA technologies for diagnostic and prognostic test, in the last decade there have been a raise in the technologies of the new generation which certainly are giving an enormous advance either to epidemiological molecular studies as well as in the knowledge of the epigenetics and deep insight in the knowledge of mutations, genetic markers (SNP), biomarkers, definition of spectrum of disease. Omics technologies and third next generation high-throughput technologies have emerged as a potent technologies that cover the totality of the genome wide studies and importantly the functionality and dynamic of the genomes, transcriptomes, and proteomes that will enable to integrate the complete and define as it was possible to determine for humans, the landscape in the spectrum of the infectious disease, the progression and/or the genetic predisposition to mycobacterial diseases for (Figure 1) and make feasible translational research.
Conflict of interest
None.
Acknowledgement
The author are grateful to the financial support of SAGARPAPIDETEC, PIFI and other federal institutions (PERFIL PRODEP, SNICONACYT).
References
- Abalos P, Retamal P (2005) Tuberculosis: a re-emerging zoonosis. Rev Sci Tech 23: 583-594.
- Neill SD, Bryson DG, Pollock JM (2001) Pathogenesis of tuberculosis in cattle. Tuberculosis (Edinb). 81: 79-86.
- Biet F, Boschiroli M, Thorel M, Guilloteau L (2005) Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC). Vet Res 36: 411-436.
- Menzies FD, Neil SD (2000) Cattle to cattle transmission of bovine tuberculosis. Vet J 160: 92-106.
- Domingo M, Vidal E, Marco A (2014) Pathology of bovine tuberculosis. Res Vet Sci 99: 520-529.
- Milian-Suazo F, Salman MD, Ramirez C, Payeur JB, Rhyan JC, et al. (2000) Identification of tuberculosis in cattle slaughtered in Mexico. Am J Vet Res 61: 86-89.
- Cobos-Marin L, Montes-Vargas J, Rivera-Gutierrez S, Licea-Navarro A, Gonzalezy-Merchand JA et al. (2003) A novel multiplex-PCR for the rapid identification of M. bovis in clinical isolates of both veterinary and human origin. Epidemiol Infect 130: 485-490.
- Zumárraga MJ, Meikle V, Bernardelli A, Abdala A, Tarabla H, et al. (2005) Use of touch-down polymerase chain reaction to enhance the sensitivity of Mycobacterium bovis detection. J Vet Diag Invest 17: 232-238.
- Taylor GM, Worth DR, Palmer S, Jahans K, Hewinson RG (2007) Rapid detection of Mycobacterium bovis DNA in cattle lymph nodes with visible lesion using PCR. BMC Vet Res 3: 1-11.
- Serranoâ€Moreno BA, Romero TA, Arriaga C, Torres RA, Pereiraâ€Suárez AL, et al. (2008) High frequency of Mycobacteriumbovis DNA in colostra from tuberculous cattle detected by nested PCR. Zoonoses Public Health 55: 258-266.
- Duarte da Silva R, Lucena N, Barbosa A F, Borges J, Fernández PR, et al. (2016) Molecular detection of Mycobacterium bovis in cattle herds of the state of Pernamburco, Brazil. BMC Vet Res 12: 1-6.
- Quan Z, Haiming T, Xiaoyao C, Weifeng Y, Hong J, et al. (2017) Development of one-tube multiplex polymerase chain reaction (PCR) for detecting Mycobacterium bovis. J Vet Med Sci 78: 1873-1876.
- de Souza EE, Carvalho RC, Silvestre FG, Lilenbaum W, Fonseca L S, et al. (2010) Detection of Mycobacterium bovis DNA in nasal swabs from tubercles cattle by a multiplex PCR. Brazil J Microbiol 41: 386-390.
- Nasr EB, Rezael YH, Moghim S, GhasemianSafaei H, ZarkeshEsfahani H (2012) Rapid and accurate identification of Mycobacterium tuberculosis complex and common non-tuberculous mycobacteria by multiplex real-time PCR targeting different housekeeping genes. Curr Microbiol 65: 493-499
- Hwang SM,  Lim MS, HongYJ, Kim TS, Park KU, et al. (2013) Simultaneous detection of Mycobacterium tuberculosis complex and non tuberculous mycobacteria in respiratory specimens. Tuberculosis 93: 642-646.
- Sales ML, Fonseca AA, Orzil L, Alencar AP, Hodon MA, et al. (2014) Validation of two real-time PCRs targeting the PE-PGRS 20 gene and the region of difference 4 for the characterization of M. bovis isolates. Genet Mol Res 13: 4607-4616.
- Gormley E, Corner L A, Costello E, Rodriguez-Campos S (2014) Bacteriological diagnosis and molecular strain typing of Mycobacterium bovis and Mycobacterium caprae. Res Vet Sc 97: S30-S43
- Alvarez AH, Estrada-Chávez C, Flores-Valdez MA (2009) Molecular findings and approaches spotlighting Mycobacterium bovis persistence in cattle. Vet Res 40: 1-16.
- Zendejas H, Milián SF, Cuador GQ, Cruz BG, Anaya EAM, et al. (2007) Spatial epidemiology of bovine tuberculosis in México. Vet Ital 43: 629-634.
- Chen R, Bi Y, Yang G, Liu Z, Liu Z, et al. (2010) Development of a fluorescent microsphere-based multiplex assay for simultaneous rapid detection of Mycobacterium tuberculosis complex and differentiation of M. tuberculosis and M. bovis in clinical samples. Diagn Mol Pathol 19: 172-179.
- Viana C, Rosales CA, Bigi F, Santos M, Ferreira J S, et al. (2006) Identification of an IS6110 insertion site in plcD, the unique phospholipase C gene of Mycobacterium bovis. J Med Microbiol 55: 451-457.
- Jia K, Yu M, Zhang GH, Zhang J, Lin ZX, et al. (2012) Detection and identification of Mycobacterium tuberculosis and Mycobacterium bovis from clinical species using DNA microarrays. J Vet Diag Invest 24: 156-160
- De Ketalacre A, Grossens K, Peelman I, Burvenich C (2006) Technical note: Validation of internal control genes for gene expression analysis to bovine polymorphonuclear leucocytes. J Dairy Sci 89: 4066-4069.
- Huang DW, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene list. Nucleic Acids Res 37: 01-13.
- Berry PRM, Graham MCh, McNab WF, Xu Z, Bloch AAS, et al. (2010) An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Lett Nature 466: 973-979.
- Nakaya HI, Li S, Pulendran B (2011) Systems vaccinology: learning to compute the behavior of vaccine induced immunity. Wiley Interdiscip Rev Syst Biol Med 4: 193-205.
- Malone JH, Oliver B (2011) Microarrays, deep sequencing and the true measure of transcriptome. BMC Biology 9: 34.
- Aranday-Cortes E, Hogarth PJ, Kaveh DA, Whelan A.O, Villarreal-Ramos B, et al. (2012) Transcriptional profiling of disease-induced host responses          in bovine tuberculosis and the identification of potential diagnostic biomarkers. Plos one 7: e30626.
- Mortazavi A, Williams BA, McCue K, Schaffer I, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat Methods 5: 621-628.
- Bhuju S,   Aranday-Cortes E, Villarreal-Ramos B, Xing Z, Singh M et al. (2012) Global gene transcriptome analysis in vaccinated cattle revealed a dominant role of IL-22 for protection against bovine tuberculosis. PLoS Pathog 8: e1003077.
- Meade KG, Gormley E, Park SD, Fitzsimons T, Rosa GJ, et al. (2006) Gene expression profiling of peripheral blood mononuclear cells (PBMC) from Mycobacterium bovis infected cattle after in vitro antigenic stimulation with purified protein derivative of tuberculin (PPD). Vet Immunol Immunopathol 113: 73-89.
- Meade KG, Gormley E, O’Farrelly C, Park DS, Costello E, et al. (2008) Antigen stimulation of peripherla blood mononuclear cells from Mycobacterium bovis infected cattle yields evidence for a novel gene expression program. BMC Genomics 9: 447
- Shukla SK, Shukla S, Chauhan A, Sarvjeet, Khan R, et al. (2017) Differential gene expression in Mycobacterium bovis challenged monocyte-derived macrophages of cattle. Microb Pathog 113: 480-489.
- Shukla SK, Shukla S, Khan R, Ahuja A, Singh LV, et al. (2018) Pathway analysis of differentially expressed genes in Mycobacterium bovis challenged bovine macrophages. Microb Pathog 115: 343-352.
- MacHugh DE, Gormley E, Park SD, Browne JA, Taraktsoglou M, et al. (2009) Gene expression profiling of the host response to Mycobacterium bovis infection in cattle. Transbound Emerg Dis 56: 204-214.
- Blanco FC, Soria M, Bianco MV, Bigi F (2012) Transcriptional response of peripheral blood mononuclear cells from cattle infected with Mycobacterium bovis. Plos one 7:e41066.
- Magee DA, Conlon KM, Nalpas NC, Browne JA, Pirson C, et al. (2014) Innate cytokine profiling of bovine alveolar macrophages reveals commonalities and divergence in the response to Mycobacterium bovis and Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 94: 441-450.
- McLoughlin KE, Nalpas NC, Rue-Albrecht K, Browne AJ, Magee DA, et al. (2014) RNA-seq transcriptional profiling of peripheral blood leukocytes from cattle infected with M. bovis. Front Immunol 5: 396.
- Nalpas NC, Park DDE, Magee DA, Taraktsuglou M, Browne JA, et al. (2013) Whole-transcriptome high-throughput RNA sequence analysis of the bovine macrophage response to M. bovis infection in vitro. BMC Genomics 14: 230.
- Nalpas NC, Magee DA, Conlan KM, Browne JA, Healy C, et al. (2015) RNA sequencing provides exquisite insight into the manipulation of the alveolar macrophages by tubercle bacilli. Sci report 5: 13269.
- Lin J, Zhao D, Wang J, Wang Y, Li H, et al. (2014) Transcriptome changes upon in vitro challenge with Mycobacterium bovis in monocyte-derived macrophages from bovine tuberculosis-infected and healthy cows. Vet Immunol Immunopathol 163: 146-156.
- Churbanov A and Milligan B (2012) Accurate diagnostic for bovine tuberculosis based on High-throughput sequencing. Plos one 7: e50147.
- Pan L, Wei N, Jia H, Gao M, Chen X, et al. (2017) Genome-wide transcriptional profiling identifies potential signatures in discriminating active tuberculosis from  latent infection. Oncotarget 8: 112907-112916.
- Malone KM, Rue-Albrecht K, Magee DA, Conlon K, Schubert TO, et al. (2018) Comparative omics analyses differentiate M. tuberculosis and M. bovis and reveal distinct macrophage responses to infection with the human and bovine tubercle bacilli. Microbial Genomics 4: e000163.
- Mayorquin-Luna GA and Guerrero GG (2018) An study of the in vivo gene profile in M. bovis infected cattles from Mexico. Curr Analytical Biotech (inpress).
Citation: Guerrero GG (2019) Omics Technologies: A Hope for Translational Research in Bovine Tuberculsosis. J Infect Dis Ther 7: 394. DOI: 10.4172/2332-0877.1000394
Copyright: © 2019 Guerrero GG. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4482
- [From(publication date): 0-2019 - Nov 22, 2025]
- Breakdown by view type
- HTML page views: 3573
- PDF downloads: 909