Olfactory Groove Meningioma: Clinical Presentation and Surgical Outcomes of Subfrontal Approach, Experience of The National Institute of Neurology of Tunisia
Received: 20-Aug-2020 / Accepted Date: 12-Oct-2020 / Published Date: 19-Oct-2020 DOI: 10.4172/2161-119X.1000408
Abstract
Objective: To review the clinical presentation, imaging findings, and surgical management of olfactory groove meningiomas (OGM) in a series of 25 patients. Methods: During the period between January 2010 and December 2016, 25 patients were treated for OGM in the neurosurgery department of the National Neurology Institute of Tunisia. All patients were operated via a sub-frontal approach. The mean follow-up period was 3 years. Results: The mean age was 49 years. The major clinical symptoms were olfactory disorders (60%), headaches (56%), seizures (32%), visual impairment (28%), and psychiatric disorders (20%). The mean tumor size was 57mm. Tumor removal was complete (Simpson grades I and II) in 68% of cases. Mean postoperative complications were meningitis (16%), rhinoliquorrhea (8%), and cerebral abscess (8%). Recurrence occurred in 2 patients. The mortality rate was 8%.
Keywords: Olfactory groove meningiomas; Anosmia; Subfrontal approach; Surgical outcome
Introduction
OGM are rare and usually benign tumors that arise from the midline of the anterior fossa at ethmoidal cribriform plate [1]. They have some particularities comparatively to other intracranial meningiomas. OGM are slow growing tumors. Thus, patients are asymptomatic for a long period before diagnosis. The most common presenting symptoms are headaches, olfactory disorders, personality changes and visual impairment. Surgery, aiming to perform a complete tumor removal, is the main treatment. However, nearness to vascular and neural structures and extension into paranasal sinus rise real challenges. We intend through this series to review clinal presentation, radiological characteristics and surgical outcomes of OGM.
Patients and methods
Between January 2010 and December 2016, 25 patients were admitted in the neurosurgery department of the National Neurology Institute of Tunisia for OGM. Data obtained from each patient’s medical file was reviewed. Computed tomographic (CT) scans ad/ or magnetic resonance imaging (MRI) have assessed the diagnosis. All patients were operated on through a sub-frontal approach. Diagnosis was confirmed by anatomo pathological examination. Seventeen patients (68%) had regular follow up in which clinical and imaging findings were reported. The mean follow-up period was three years.
Results
The mean age of the patients was 49 years (range 23 -74 years old). The female-to-male ratio was 1, 8. The mean consultation period was 10 months. The clinical findings revealed olfactory impairment as the most frequent symptom in 15 patients (60%), followed by headache in 14 patients (56%), seizures in 8 patients (32%), blurred vision in 7 patients (28%) and psychiatric disorders in 5 patients (20%). Progressive decreased visual acuity led to blindness in 2 patients. Physical examination revealed motor paresis in 2 patients (8%). Exophthalmia was noted in one patient (4%). Fundus examination revealed papillary edema in 3 cases (12%) and optic atrophy in 3 cases (Table 1).
| Symptoms | Number of patients | (%) |
|---|---|---|
| Olfactory impairments unilateral anosmia bilateral anosmia hyposmia |
15 11 3 1 |
60% 44% 12% 4% |
| Headache | 14 | 56% |
| Seizures | 8 | 32% |
| Blurred vision Blindness |
7 2 |
28% 8% |
| Psychiatric disorders | 1 | 4% |
Table 1: Clinical presentation of OGM.
The presence of OGM was assessed by CT scans that were performed in 22 cases (88%).
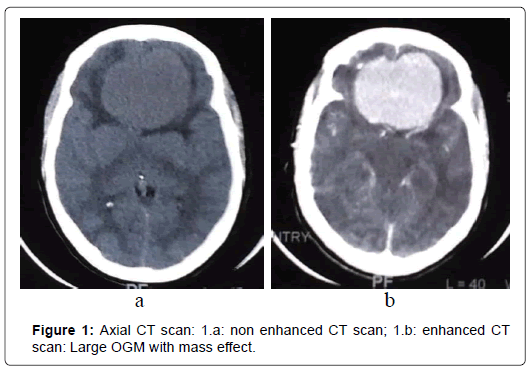
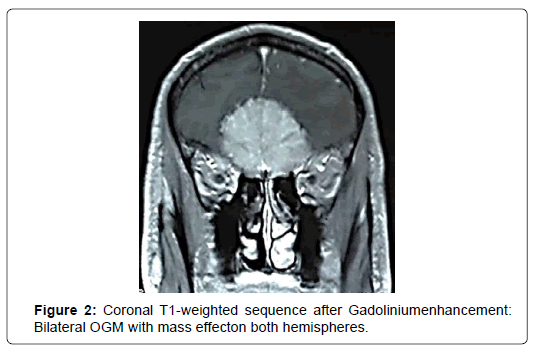
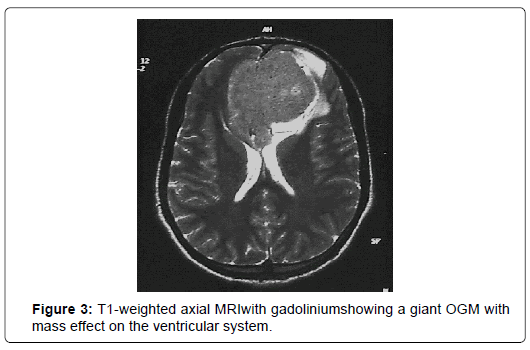
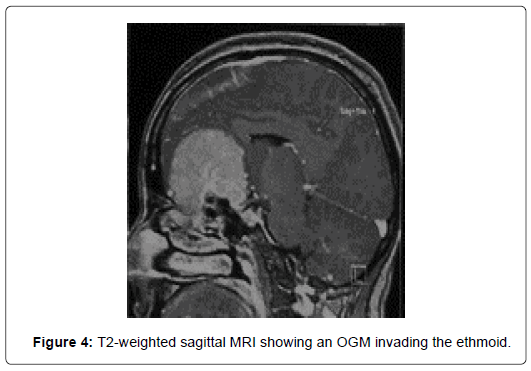
At CT scans, OGM had nearly the same density than brain parenchyma and enhanced homogeneously and brightly after administration of contrast in 76%. In five cases (20%), the tumor was localized in the left side and in 17 cases (68%), the tumor was found bilaterally. CT scans revealed bony hyperostosis in five cases and tumor calcifications in five cases. The average tumor diameter was 57 mm (ranges from 31mm to 80mm). Peritumoral edema was noted in 72% and was associated with mass effect in 68%. MRI was performed in 24 patients (96%). Tumor characteristics are summarized in (Figure 1-4) (Table 2).
| MRI parameters | Characteristics | (%) |
|---|---|---|
| T1-weighted sequence | isointense | 84% |
| T2-weighted sequence | hyperintense | 72% |
| Gadolinium enhancement | homogenous | 96% |
| Tumoral extension | Optic nerve | 48% |
| Sphenoidal sinus | 40% | |
| Ethmoid | 28% | |
| Sella turcica | 8% |
Table 2: OGM characteristics at MRI.
Twenty-three patients (92%) were operated through unilateral subfrontal approach. Bifrontal approach was achieved in two cases (8%). According to Simpson’s grading system, the degree of tumor removal was: grade I in 10 cases (40%), grade II in 7 cases (28%), grade III in 5 cases (20%) and grade IV in 3 cases (12%).
Post-operative period was uneventful for 17 patients (68%). One patient presented intracranial hematoma with resulting surgical evacuation. Cerebrospinal fluid (CSF) leak occurred in 2 patients (8%). Rhinoliquorrhea stopped after several lumbar draining in one patient. The second patient, with persistent rhinorrhea underwent reoperation. One patient (4%) experienced seizures after surgery. Meningitis occurred in 4 patients (16%) and led to death in 2 patients. Two patients had cerebral abscess. They were operated on and treated by antibiotics. Olfactory disorders persisted in 80% of cases. Visual impairments were maintained in 12 patients (48%) and were improved in 3 patients (12%). Residual tumor was found in 4 cases (16%).
According to WHO grading, tumor was classified grade I in 92% of cases, grade II in 4% of cases and grade III in 4% of cases. The meningothelial type was found in 24 cases (96%) and fibroblastic type in one case (4%). The patient who presented a malignant meningioma had radiotherapy after total removal of the tumor.
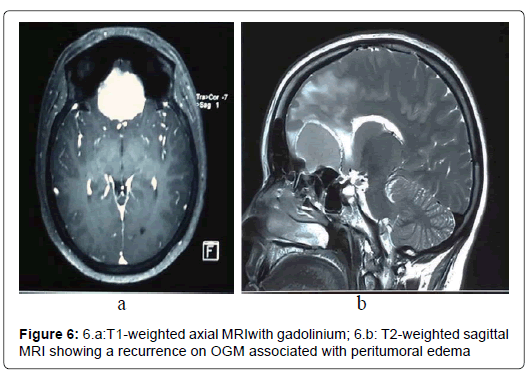
Total remission was found in 9 patients (36%) during follow-up period. Recurrence was noted in 2 patients (8%) within respectively 6 and 12 months. The first patient had an incomplete tumor resection (grade IV). Meningioma recurrence didn’t require reoperation. The second patient had a grade II tumor removal with an OGM extending into the ethmoid. He was symptomatic and tumor size was largest recurrence. He was successfully reoperated (Figure 5 and 6).
Discussion
OGM are rare tumors that represent about10% of intracranial meningiomas [2]. It was first described in 1938 by Cushing and Eisenhardt on the basis of 29 cases [3]. OGM arise in the midline of the anterior fossa over the cribriform plate of the ethmoid bone and the planum sphenoidale [3]. It may be localized in the midline or extend predominantly to one side [4]. OGM have different clinical presentation, surgical approach, post-operative complications and mortality rates from other anterior cranial fossa meningiomas [2].
Clinical presentation
OGM are slow-growing tumor. Thus, patients are asymptomatic for a long period and tumor usually reaches large size before diagnosis [4]. Mean tumor diameter at diagnosis was 41,6mm in the series of Koutourousiou [5], 43mm in the series of Aguiar [6] and 57mm in our series. A high frequency of females is observed in published series [7-9]. In our series, women represent 64% of cases. This might be due to the presence of progesterone and estrogen receptors in meningioma cells. Tumor size may increase during pregnancy and in patients with breast cancer [10].
Headache, anosmia, visual impairment and psychiatric disorders are the most common symptoms in published studies and have been correlated with tumor volume and local extension [5-11]. Anosmia is thought to be the first symptom, but rarely detected by patients [8]. This is due to the fact that olfactory disorders are gradual and usually unilateral. Besides, contralateral compensation maintains an apparent normal olfactory function [12]. Hendrix and al. found that loss of smell occurred when tumor size was over than 4 cm3 [13]. Personality changes such as depression, dementia and akinesia are progressive and are correlated with bilateral frontal lobe compression. They range from 21 to 70% [4]. Visual disorders result from compression of the optic nerves or increased intracranial pressure. They occur in 15,3% to 58,1% [14] (Table 3).
| Authors | Number of cases | Clinical signs | |||
|---|---|---|---|---|---|
| Headache | Dysosmia | Visual disorders | Mental changes | ||
| Hentschel [8] | 13 | 38% | 62% | 46% | 69% |
| Spektor [15] | 80 | 51% | 58% | 27% | 26% |
| Koutourousiou [5] | 50 | 24% | 16% | 30% | 36% |
| Tsikoudas [14] | 13 | 62% | 54% | 31% | 62% |
| Nakamura [11] | 82 | 31% | 58% | 24% | 72% |
| Present series | 25 | 56% | 60% | 28% | 4% |
Table 3: Most frequent clinical signs of OGM in some published series.
Imaging findings
With the introduction of imaging techniques, including CT scans and MRI, detection of OGM became earlier. Imaging findings of OGM are similar to those of other meningiomas. Ninety-five percent of meningiomas are detected with enhanced CT scans [2]. OGM appear is dense or slightly hyper dense than brain parenchyma and enhances homogeneously and brightly after administration of contrast [8]. Calcifications are frequently observed. Bony reactions such as erosion and hyperostosis and dural attachment are well defined which facilitates planning surgical technique. Hyperostosis was proved to be histologically resulting of tumor invasion rather than associated reaction [15,16]. Paranasal sinus extension is demonstrated particularly on coronal sections[8]. On MRI, meningiomas usually appear iso intense on T1-weighted sequences and iso-or hyper intense on T2- weighted sequences. The relationship between tumor and optic nerves, chiasms and anterior cerebral artery (ACA) is well described in MRI [8]. Cerebral edema, when associated reflects the disruption of blood-brain barrier and adherence of the tumor to brain tissue on T2-weighted sequences [2]. Angiography sequences are important to configure the ACA position and tumoral blood suppliencies [2].
Surgical management
Surgical resection of the tumor is challenging since OGM are close to critical anatomic structures such as the orbit, the frontal lobes, the olfactory nerves and the ACA. The first OGM surgery was performed in 1884 by Francesco Durante via a left fronto-temporal craniotomy [17]. Since then, many surgical approaches have been developed. The most commonly used are pterional, bifrontal and unilateral subfrontal approaches [4]. Each approach has its advantages and possible complications. Regardless of surgical technique, the goal of surgery is total removal of the tumor. The rate of total resection varies from 60 to 100% [4]. In our series, a total tumor resection (grade I) was performed in 40%.Subfrontal approach was approved by many authors since it was introduced in 1938 by Tönnis [18]. It may include a bilateral or a unilateral craniotomy. The advantage of this approach is the possibility of direct access to the cranial base and thus easier devascularization of the tumor, which facilitates the dissection of dural attachments according to Al-Mefty and Co [19]. The main disadvantages are longer surgical time and risk of CSF leak [4].
Pterional approach was proposed in 1989 to treat OGM [20]. There are several advantages of this technique. Since basal cisterns may be opened, risk of CSF leak is minimized compared to subfrontal approach [15]. Frontal sinus is preserved as well. Optic nerves, chiasmas and ACA smay be localized and thus, secured [4]. The main drawback of this approach is the narrow working angle. Large tumor couldn’t be controlled. Therefore, significant brain retraction may be required [15].
Other techniques are proposed when the tumor extends into paranasal sinus and orbit even though it is an unusual condition. They include transbasal, subcranial, fronto-orbital approaches, frontal craniotomy combined with orbital and craniofacial resection [15]. When OGM slightly extends into paranasal sinus, transcranial approach is generally sufficient. For large tumor, a combined subfrontal and nasal approach is necessary [21].
In the last years, endonasal endoscopic surgery (EES) has gained ground with constantly improved outcomes and increased indications. EES offers an excellent control of the skull base, a direct visualization of the neurovascular structure and thus, a safe tumor resection. In addition, decompression of bilateral optic canals is feasible. Brain retraction is avoided. The mean advantages of this technique are shorter surgical time, shorter hospitalization and satisfactory cosmetic result [5]. However, total resection of large tumor through EES remains challenging. Main contraindications of EES are lateral extension to the optic canal, presence of significant mass effect on the frontal lobes and potential intra-tumoral calcifications [22]. Post-operative CSF leak is the major inconvenient of EES [5].
Recurrence
Recurrence rate ranged from 0 to 41%in the literature [4]. In our series, two cases (8%) of recurrence were reported. Occurrence of recurrence depends on surgical technique, Simpson grade and duration of follow-up[19]. Extent of surgical removal is thought to be the most relevant factor of both short and long-term cure rates [19- 24]. In our series, tumor recurrence occurred in a patient with an incomplete tumor resection (Simpson IV). According to Adegbite, “Radical excision, whenever possible, remains the only uncontroversial safeguard against recurrence” [25]. The rates of recurrence in Simpson series for grades I through IV were 9, 19, 29, and 40%, respectively at 10 years [24]. Radical resection should include the dural attachment and involved bone [23-26]. Miller showed that extent of resection and mitotic index of the tumor were predictive factors of recurrence [27]. In the series of Obeid, involved bone of cranial base was involved in all of the recurrent cases [19]. Some surgeons, however, adopt conservative technique toward involved bone in order to avoid CSF leak. It consists on resecting visible part of tumor, limited removal of invaded bone and coagulating involved dura [21, 28]. In fact, risk of infection and rhinorrhea is greater than risk of recurrence according to Maiuri [21]. Coagulating or excising dura is necessary but can’t avoid recurrence when performed alone [19]. For some authors, extension to paranasal sinuses is associated with high risk of recurrence [29]. This is due to the relationship between the anatomic origin of OGM and ethmoid and sphenoid sinuses. A long term follow-up is necessary to evaluate effective recurrence rate [19]. Mirimanoff found a recurrence rate of 30% at 5 years and 41% at 10 years [30].
Morbidity and Mortality
Nowadays, mortality rates and complications of OGM have significantly declined due to technical advances in surgical management of intracranial tumors. Mortality rates inpublished series ranged from 0 to 17% [8]. In our series, two patients (8%) passed away.
Most frequent complications associated with OGM surgery are CSF leak, meningitis, hydrocephalus, worsening vision, motor deficit and anosmia [31]. The occurrence of post-operative complications doesn’t seem to be correlated with tumor size [31].
Complications are thought to be associated with surgical approach. In the series of Nakamura, the most frequent post-operative complications were subdural hygroma (17.6%), seizures (11.8%), hydrocephalus (5.8%) and hemorrhage (2.9%) when patients were operated with frontolateral approach. The bifrontal approach was associated with more frequent hemorrhage (10.9%), hydrocephalus (8.7%) and less frequent hygromas (2.2%) and seizures (4.3%) [11]. In contrast to these findings, Zygourakis and Co didn’t find difference between extended bifrontal approach and a less invasive unilateral approach [31].
CSF leak is a worrying complication regarded risk of infections. Komotar and Co found, a statistically significant higher rate of CSF leak when patients were operated via EES [32]. Regarding different series, risk of CSF leak was 26,2% following an EES and 6,2% after a transcranial approach [33].
Anosmia is a frequent post-operative complication which is due to manipulation of olfactory structures during surgery. Loss of smell impacts life quality. Thus, preservation of anatomical and functional olfactory structures is challenging. Anosmia might be the consequence of intracranial pressure increase or neuronal loss due to infection and reoperation [13].
For some authors, olfactory function is better preserved with lateral approach than bifrontal approach [34,35]. In fact, frontolateral route offers a good visualization of neural and vascular structures. Besides, contralateral olfactory tract is preserved unless tumor size is regular [13]. For Hendrix, occurrence of post-operative anosmia was correlated with contralateral and bilateral olfactory deterioration. Tumor volume and quality of resection weren’t risk factor of smell loss [13]. In our series, olfactory disorders were found in 80% of patients.
Visual outcomes depend on surgical approach. According to Komotarand Co, vision remained stable in 41.5% of patients and improved in 54.2% when patients were operated via a transcranial approach [32]. In a systematic review by Shetty and Co, visual outcomes were better with EES compared to transcranial approach: Vision improved in 80.7% of patients operated by EES V.S 12, 8% of patients operated via a transcranial approach [36]. In our series, vision was stable in 12 patients and was improved in 3 patients.
Conclusion
OGM are rare tumors with a particular clinical presentation. Advances of microsurgical techniques improved morbidity and mortality rates. The choice of surgical approach depends on surgeon’s experience, tumor size and extent. A complete tumor removal is the aim of surgery which is often challenging due to anatomical and functional constraints.
References
- Ciurea AV, Iencean SM, Rizea RE, Brehar FM. (2012) Olfactory groove meningiomas: a retrospective study on 59 surgical cases. Neurosurg Rev 35: 195-202.
- Tuna H, Bozkurt M, Ayten M, Erdogan A, Deda H. (2005) Olfactory groove meningiomas. J Clin Neurosci 12: 664-668.
- Cushing H, Eisenhardt L. (1939) The olfactory meningiomas with primary anosmia. Meningiomas Springfield 1938: 250-82.
- Fountas KN, Hadjigeorgiou GF, Kapsalaki EZ, Paschalis T, Rizea R, et al. (2018) Surgical and functional outcome of olfactory groove meningiomas: Lessons from the past experience and strategy development. Clin Neurol Neurosurg 171: 46-52.
- Koutourousiou M, Fernandez-Miranda JC, Wang EW, Snyderman CH, Gardner PA. (2014) Endoscopic endonasal surgery for olfactory groove meningiomas: outcomes and limitations in 50 patients. Neurosurg Focus 37: E8.
- Aguiar PH, Tahara A, Almeida AN, Simm R, Silva AN, et al. (2009) Olfactory groove meningiomas: approaches and complications. J Clin Neurosci 16: 1168-1173.
- Turazzi S, Cristofori L, Gambin R, Bricolo A. (1999) The pterional approach for the microsurgical removal of olfactory groove meningiomas. Neurosurgery 45: 821-826.
- Hentschel SJ, DeMonte F. (2003) Olfactory groove meningiomas. Neurosurg Focus 14: 1-5.
- Barzaghi LR, Spina A, Gagliardi F, Boari N, Mortini P. (2017) Transfrontal-sinus-subcranial approach to olfactory groove meningiomas: surgical results and clinical and functional outcome in a consecutive series of 21 patients. World Neurosurg 101: 315-324.
- Black P, Morokoff A, Zauberman J, Claus E, Carroll R. (2007) Meningiomas: science and surgery. Clin Neurosurg 54: 91-99.
- Nakamura M, Struck M, Roser F, Vorkapic P, Samii M. (2007) Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery 60: 844-852.
- Welge-Luessen A, Temmel A, Quint C, Moll B, Wolf S, et al. (2001) Olfactory function in patients with olfactory groove meningioma. J Neurol Neurosurg Psychiatry 70: 218-221.
- Hendrix P, Fischer G, Linnebach AC, Krug JB, Linsler S, et al. (2019) Perioperative olfactory dysfunction in patients with meningiomas of the anteromedial skull base. Clin Anat 32: 524-533.
- Tsikoudas A, Martin-Hirsch DP. (1999) Olfactory groove meningiomas. Clin Otolaryngol Allied Sci 24: 507-509.
- Spektor S, Valarezo J, Fliss DM, Gil Z, Cohen J, et al. (2005) Olfactory groove meningiomas from neurosurgical and ear, nose, and throat perspectives: approaches, techniques, and outcomes Neurosurgery 57: 268-280.
- Durante F. (1885) Estirpazione di un tumore endocranico. Arch Soc Ital Chir 2: 252-255.
- Rachinger W, Grau S, Tonn J-C. (2010) Different microsurgical approaches to meningiomas of the anterior cranial base. Acta Neurochir (Wien ) 152: 931-939.
- Obeid F, Al-Mefty O. (2003) Recurrence of olfactory groove meningiomas. Neurosurgery 534-543.
- Hassler W, Zentner J. (1989) Pterional approach for surgical treatment of olfactory groove meningiomas. Neurosurgery 25: 942-947.
- Maiuri F, Salzano FA, Motta S, Colella G, Sardo L. (1998) Olfactory groove meningioma with paranasal sinus and nasal cavity extension: removal by combined subfrontal and nasal approach. J Craniomaxillofac Surg 26: 314-317.
- Khan OH, Krischek B, Holliman D, Klironomos G, Kucharczyk W, et al. (2014) Pure endoscopic expanded endonasal approach for olfactory groove and tuberculum sellae meningiomas. J Clin Neurosci 21: 927-933.
- Mathiesen T, Lindquist C, Kihlström L, Karlsson B. (1996) Recurrence of cranial base meningiomas. Neurosurgery 39: 2-9.
- Simpson D. (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20: 22-39.
- Adegbite AB, Khan MI, Paine KW, Tan LK. (1983) The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg 58: 51-66.
- Miller DC. (1994) Predicting recurrence of intracranial meningiomas. A multivariate clinicopathologic model--interim report of the New York University Medical Center Meningioma Project. Neurosurg Clin N Am 5: 193-200.
- Ojemann RG. (1993) Management of cranial and spinal meningiomas (honored guest presentation). Clin Neurosurg 40: 321-383.
- Derome PJ, Guiot G. (1978) Bone problems in meningiomas invading the base of the skull. Clin Neurosurg 25: 435-451.
- Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. (1985) Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 62: 18-24.
- Zygourakis CC, Sughrue ME, Benet A, Parsa AT, Berger MS, et al. (2014) Management of planum/olfactory meningiomas: predicting symptoms and postoperative complications. World Neurosurg 82: 1216-1223.
- Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. (2012) Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World Neurosurg 77: 713-724.
- Purohit A, Jha R, Khalafallah AM, Price C, Rowan NR, et al. (2019) Endoscopic endonasal versus transcranial approach to resection of olfactory groove meningiomas: a systematic review. Neurosurg Rev.
- Jang WY, Jung S, Jung TY, Moon KS, Kim IY. (2013) Preservation of olfaction in surgery of olfactory groove meningiomas. Clin Neurol Neurosurg 115: 1288-1292.
- Nanda A, Maiti TK, Bir SC, Konar SK, Guthikonda B. (2016) Olfactory Groove Meningiomas: Comparison of Extent of Frontal Lobe Changes After Lateral and Bifrontal Approaches. World Neurosurg 94: 211-221.
- Shetty SR, Ruiz-Trevino AS, Omay SB, Almeida JP, Liang B, et al. (2017) Limitations of the endonasal endoscopic approach in treating olfactory groove meningiomas. A systematic review. Acta Neurochir (Wien) 159: 1875-1885.
Citation: Mejbri M, Karmani N, Ayadi K, Slimene A, Abderrahmen K, et al. (2020) Olfactory Groove Meningioma: Clinical Presentation and Surgical Outcomes of Subfrontal Approach, Experience of The National Institute of Neurology of Tunisia Otolaryngol (Sunnyvale) 10: 408. DOI: 10.4172/2161-119X.1000408
Copyright: © 2020 Mejbri M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3314
- [From(publication date): 0-2020 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 2548
- PDF downloads: 766