Olanzapine Suppresses Ferroptosis via its Anti-oxidant Activity
Received: 01-May-2024 / Manuscript No. cmb-24-138170 / Editor assigned: 04-May-2024 / PreQC No. cmb-24-138170 / Reviewed: 18-May-2024 / QC No. cmb-24-138170 / Revised: 25-May-2024 / Manuscript No. cmb-24-138170 / Published Date: 30-May-2024
Abstract
Ferroptosis is an iron dependent cell death, triggered by Fe2+ mediated oxidation of polyunsaturated fatty acids (PUFAs). Dysregulation of ferroptosis is associated with several human diseases, such as neurodegenerative diseases, multi-organ ischemia-reperfusion injuries and cancers. Hence modulating ferroptosis would provide beneficial effect in these related diseases and identification drugs especially clinically proved drugs that have anti-ferroptotic effect would provide new treatment options for ferroptosis related diseases. The effect of Olanzapine on ferroptotic inducers RAS-selective-lethal-3(RSL3) and imidazole ketone erastin (IKE) was measured by Cell Counting Kit 8(CCK8) and Hoest33342 staining. Level of lipid peroxidation and Fe2+ after Olanzapine treatment with or without RSL3 or IKE were determined by microscopy and flow cytometry assays. Influences of Olanzapine on the major anti-ferroptotic pathways were assayed by western blots. The anti-oxidant activity of Olanzapine in vitro was measured by DPPH assay. According cell viability assays, Olanzapine strongly inhibits RSL3 and IKE induced ferroptosis, but not other types of cell death induced by many chemotherapy drugs. Olanzapine showed anti-oxidant activity by DPPH assay. In this study, we have identified clinically used antipsychotic drug Olanzapine as a potent and specific inhibitor of ferroptosis. It suppresses ferroptosis in multiple cell lines, suggesting it is a general ferroptosis inhibitor.
keywords
Olanzapine; Ferroptosis; Anti-oxidant; Cell death
Introduction
Ferroptosis is a kind of cell death that has only recently been characterized. It is distinguished by severe lipid peroxidation caused by Fe2+, which eventually results in membrane damage and cell death. In order to counteract the detrimental effect of overwhelmed lipid peroxidation, cells have developed several anti-oxidant systems to deal with lipid peroxidation. The major anti-ferroptosis pathway is glutathione peroxidase 4 (GPX4)/solute carrier family 7 member 11 (SLC7A11)/glutathione (GSH) system. Of this pathway, GPX4 plays a major role in removing lipid peroxidation, using GSH as a cofactor. SLC7A11 mediates the transport of cystine, which is necessary for GSH production, hence supporting GPX4’s function to counteract ferroptosis. Besides GPX4/SLC7A11/GSH axis, several other important anti-ferroptosis pathways have been identified [1].
These include but not limited to: the ferroptosis suppressor protein 1 (FSP1)/coenzyme Q10(CoQ10) pathway, GTPcyclohydro-lase1(GCH1)/6-pyruvoyltetrahydropterinsynthase (PTPS)/sepiapterineductase (SR)/ tetrahydrobiopterin (BH4) pathway, dihydroorotate dehydrogenase (DHODH)/CoQ pathway, tearoyl-CoA desaturase (SCD)/monounsaturated fatty acid (MUFA) pathway. These pathways act together or in parallel with GPX4 axis to counteract ferroptosis. In addition, as a master transcription factor of anti-oxidant regulation, nuclear factor erythroid-2-related factor 2(NRF2), suppresses ferroptosis by inducing several antioxidant genes, including glutathione regulation as well as iron regulation. Cells equipped with these anti-oxidant pathways render strong anti-ferroptotic activity [2].
Recent advances in ferroptosis research have linked ferroptosis to several human diseases, where ferroptosis is critical in the research of pathogenesis. Before the concept of ferroptosis been coined in 2012, iron dysregulation and lipid peroxidation have already been known to associate with the pathology of Alzheimer’s disease and Parkinson’s disease. Now more and more researchers have discovered that ferroptosis plays a critical impact in these neurodegenerative disorders. Moreover, ferroptosis is proved to play a vital role in cardiac, renal, brain, hepatic, intestinal tissue ischemia-reperfusion injuries. Hence identifying clinical drugs that have strong anti-ferroptosis function will provide new therapeutic options for ferroptosis related diseases [3].
In this study, we have shown that olanzapine, an antipsychotic drug used clinically, is a strong ferroptosis inhibitor independent of the above-mentioned known ferroptosis regulatory pathways. Olanzapine strongly inhibited RSL3 and IKE induced ferroptosis in multiple cell lines. Furthermore, olanzapine also inhibited synergist ferroptosis inducing effect of RSL3 or IKE combined with FSP1 inhibitor iFSP1, DHODH inhibitor BQR, CoQ2 inhibitor 4-CBA (depletion CoQ10), SCD inhibitor CAY10566, NRF2 inhibitor ML385, and GCH1 inhibitor DAHP. Finally, we have shown that olanzapine had anti-oxidant activity by DPPH assay. All these data suggest olanzapine functions as an anti-oxidant to prevent ferroptosis [4].
Materials and Methods
Cell culture Materials
Procell provided the cells BT549, HL60, U937, NB4, Toledo, and HT1080 (China). They were cultured in Dulbecco's modified eagle medium (DMDM, Gibco) or Roswell Park Memorial Institute (RPMI) 1640 (Gibco), respectively, with 10% fetal bovine serum (FBS, Hyclone) and 1% penicillin-streptomycin liquid (Hyclone) added as supplements. The environments were 37 °C, 5% CO2, and humidified [5].
Cell viability test
Cell activity and proliferative potential were assessed using the Cell Counting Kit 8 (CCK 8; BIOGROUND, China). Briefly, 100μL of culture media was used to seed cells in 96-well plates. 10μL of CCK8 solution was added and incubated for 2-3 hours at 37°C with 5% CO2 after treatment for 24 or 48 hours. At 450 nm, absorbance was measured [6].
Hoechst nuclear staining
4000 cells per well were planted onto 96-well plates after the cells had been counted using a blood cell count plate. Following treatment with olanzapine and RSL3/IKE, the cells were washed three times in PBS, stained with Hoechst 33342 (C1022, Beyotime) at a concentration of 2μg/ mL for 20 minutes, and examined under a fluorescence microscope [7].
Apoptosis assay
To detect apoptosis in Toledo cells, an annexin V/propidium iodide (PI) kit (Vazyme, China) was employed. Collect 105 Toledo cells, divide into four groups, treat the cells for 2 hours with DMSO, OLA, RSL3 and RSL3+OLA, centrifuge at 1,800 rpm for 5 minutes at 4°C, and discard the supernatant; wash the cells twice with pre-cooled PBS, centrifuge at 1,800 rpm for 5 minutes at 4°C, and discard the supernatant. Add 100μL of 1 × Binding Buffer and gently blow to create a single cell suspension. Blowing gently, add 5μL of Annexin V-FITC and 5μL of PI Staining Solution. Incubate at room temperature (20-25°C) for 10 minutes before adding 400μL 1 Binding Buffer and gently mixing. A BD FACSCANTO II system was used to examine the stained samples (BD Biosciences, USA) within 1 h [8].
Lipid ROS assay
C11 BODIPY 581/591 molecular probe (Invitrogen, USA) and MitoPerOx (Cayman, USA) were used to detect cellular lipid ROS build-up. Cells were collected in four groups, 1*105 cells in each group, treated with DMSO, OLA, RSL3 and RSL3+OLA for 2h, incubated with BODIPY-C11 and MitoPerOx at an effect concentration of 1μM for 30min at 37℃ under light-proof conditions. The cells were collected and washed it three times with PBS; finally, the cells were suspended in PBS and detected by flow cytometry, Microscope to observe the change of green fluorescence after treatment [9].
Measurement of Fe2+
105 cells were injected in 24-well plates and incubated overnight in an incubator. Cells were treated in groups with DMSO, OLA, RSL3 and RSL3+OLA and incubated for 2 hours at 37°C in a 5% CO2 incubator before being washed three times with serum-free media. FerroOrange (Dojindo, Japan) and Mito-FerroGreen (Dojindo, Japan) working solutions were added and incubated for 30 minutes at 37°C in a 5% CO2 incubator. Flow cytometry and fluorescence microscopy were used to examine the final results [10].
JC-1 staining
To measure mitochondrial membrane potential, use jc-1 (Dojindo, Japan) according to the manufacturer's instructions and prior studies. The working solution concentration of jc-1 was 2ug/mL, Flow cytometry and fluorescence microscopy were used to examine the samples [11].
DPPH assay
First, extract solutions (20μL) were combined with an 80μL prepared DPPH solution. The mixture was then vigorously mixed and incubated at room temperature in the dark for 15 minutes. Finally, the reaction system was put to a 96-well plate, and the mixture's absorbance at 517 nm was measured. Set up negative control DMSO group, positive control Lip1 group, ethanol readings alone are background readings and subtracted from calculations [12].
Western blot analysis
A 6-well plate was used to spread 105 cells per well, and then DMSO, RSL3, IKE and OLA were added for the corresponding treatments. Cells were collected and washed with PBS after treatment. The protein levels were determined using the BCA test kit (Beyotime, Shanghai). The proteins were put on cellulose acetate films after being separated by gel electrophoresis on 10% SDS-PAGE. The films were incubated overnight at 4°C with the following primary antibodies: anti-GPX4 (1:1000, A1933, ABclonal), anti-AIFM2 (1:1000, A12128, ABclonal), anti-DHODH (1:1000, A13295, ABclonal), anti-SLC7A11 (1:1000, A2413, ABclonal), and anti-GAPDH (1:1000, A2413 (1:5000, 60004-1-Ig, Proteintech). The blots were identified by Image Lab software (Bio-Rad, USA) the next day after incubation with peroxidase (HRP) goat anti-Rabbit IgG secondary antibody (Jackson, #111-035-003) [13].
Statistical investigation
Data was analyzed using the GraphPad Prism 9.0 program. The data is shown as means, with error bars showing standard deviation (SD). The independent samples t-test was used to analyze the differences between the two groups. Asterisks indicate significant differences (*P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001) [14].
Results
Olanzapine specifically inhibits ferroptosis.
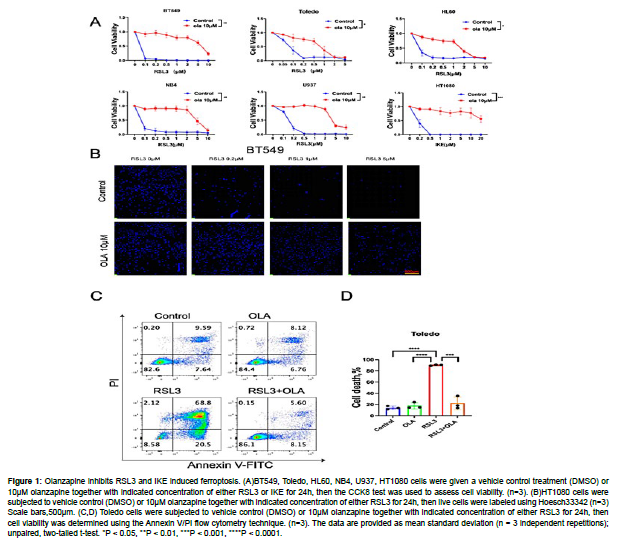
To investigate the role of olanzapine in ferroptosis regulation, we tested the influence of olanzapine on RSL3 or IKE induced ferroptosis in BT549, Toledo, HL60, NB4, U937 and HT1080 cell lines. For BT549, Toledo, HL60, NB4, U937 cell lines, we used RSL3 as they are responsive to it, whereas for HT1080 cell line we used IKE due to its sensitivity to IKE. In all these cell lines we have observed that olanzapine strongly inhibited RSL3 and IKE induced ferroptosis by CCK8 assay. The protective effect of olanzapine on RLS3 induced cell death is further supported by Hoechst33342 staining of live cells in BT549 cells and Annexin V/PI flow cytometry analysis in Toledo cells. All these data suggest olanzapine is a general ferroptosis inhibitor (Fig.1A-1D.) [15].
Figure 1: Olanzapine inhibits RSL3 and IKE induced ferroptosis. (A)BT549, Toledo, HL60, NB4, U937, HT1080 cells were given a vehicle control treatment (DMSO) or 10μM olanzapine together with indicated concentration of either RSL3 or IKE for 24h, then the CCK8 test was used to assess cell viability. (n=3). (B)HT1080 cells were subjected to vehicle control (DMSO) or 10μM olanzapine together with indicated concentration of either RSL3 for 24h, then live cells were labeled using Hoesch33342 (n=3) Scale bars,500μm. (C,D) Toledo cells were subjected to vehicle control (DMSO) or 10μM olanzapine together with indicated concentration of either RSL3 for 24h, then cell viability was determined using the Annexin V/PI flow cytometry technique. (n=3). The data are provided as mean standard deviation (n = 3 independent repetitions); unpaired, two-tailed t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
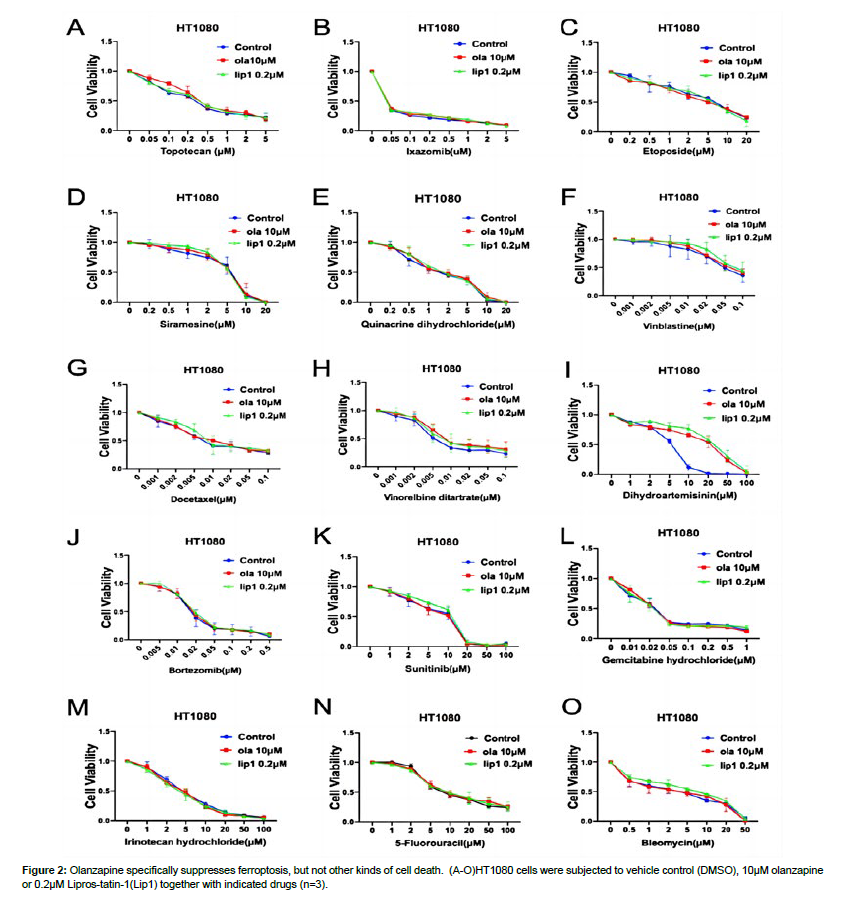
To further test whether olanzapine specifically inhibits ferroptosis, instead of other type of cell death, we tested the impact of olanzapine on several other cell toxic drugs, mostly chemotherapy drugs. Together we included a comparative control of Liproxstatin-1 (lip1), which is a widely used specific ferroptosis inhibitor. Among these drugs, dihydroartemisinin, a known ferroptosis inducing drug, induced cell death is rescued by lip1 and olanzapine at the same extent [16]. This data suggests olanzapine fully inhibited ferroptosis induced by dihydroartemisinin. Besides dihydroartemisinin, olanzapine and lip1 failed to rescue other drugs induced cell death. These drugs are topotecan, ixazomib, etoposide, siramesine, quinacrine, vinblastine, docetaxel, vinorelbine, bortezomib, sunitinib, gemcitabine, irinotecan, 5-fluorouracil, and bleomycin. Some of these drugs are known to inducing apoptosis, including topotecan, ixazomib, etoposide, siramesine, quinacrine, vinblastine, docetaxel, bortezomib, sunitinib, gemcitabine, 5-fluorouracil, and bleomycin among them, etoposide and gemcitabine also cause autophagy. Together, these data suggest olanzapine specifically inhibit ferroptosis (Fig. 2.) [17].
Olanzapine prevents lipid peroxidation and Fe2+ accumulation
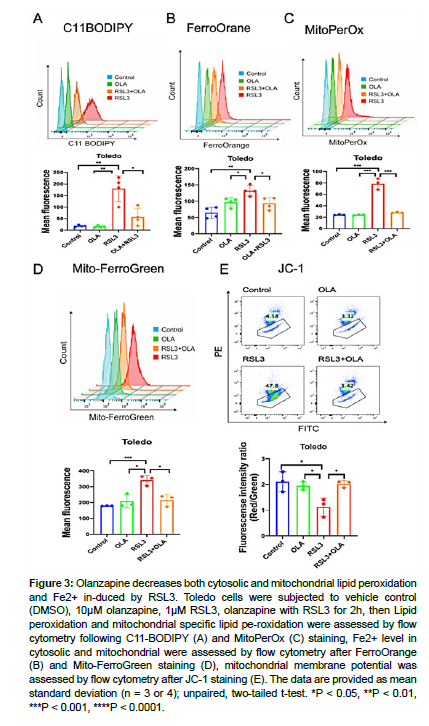
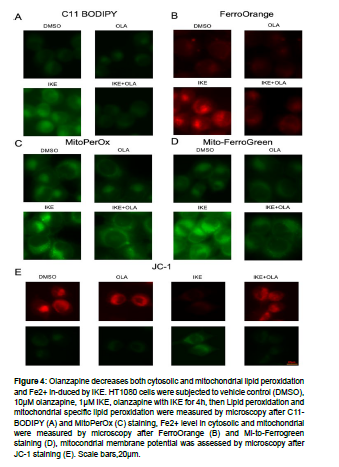
Ferroptosis is interpreted by Fe2+ increase and elevated lipid peroxidation. To test how olanzapine influence on ferroptosis, we further analyzed total lipid peroxidation by C11 BODIPY, mitochondrial lipid peroxidation by MitoPerOx, total Fe2+ level by FerroOrange, mitochondrial Fe2+ level by Mito-ferroGreen, and mitochondrial activity by JC-1 in Toledo and HT1080 cells. Toledo cells were treated DMSO control, olanzapine, RSL3 and olanzapine together with RSL3, and then above-mentioned parameters were measured by flow cytometry [18]. RSL3 treatment resulted an increased in total lipid peroxidation and mitochondrial lipid peroxidation, which were all decreased by olanzapine co-treatment. Similar results were observed with Fe2+ level, as RSL3 induced accumulation of total and mitochondrial Fe2+ were decreased by olanzapine co-treatment. Furthermore, olanzapine rescued mitochondrial defect caused by RSL3 treatment as measured by JC-1 assay. Similarly, IKE induced total and mitochondrial lipid peroxidation, Fe2+ accumulation, as well as mitochondrial defect, were rescued by olanzapine co-treatment in HT1080 cells, evidenced by microscopy analysis. These data suggest olanzapine modulates suppress lipid peroxidation and Fe2+ accumulation both in cytosolic and mitochondrial (Fig. 3, 4.) [19, 20].
Figure 3: Olanzapine decreases both cytosolic and mitochondrial lipid peroxidation and Fe2+ in-duced by RSL3. Toledo cells were subjected to vehicle control (DMSO), 10μM olanzapine, 1μM RSL3, olanzapine with RSL3 for 2h, then Lipid peroxidation and mitochondrial specific lipid pe-roxidation were assessed by flow cytometry following C11-BODIPY (A) and MitoPerOx (C) staining, Fe2+ level in cytosolic and mitochondrial were assessed by flow cytometry after FerroOrange (B) and Mito-FerroGreen staining (D), mitochondrial membrane potential was assessed by flow cytometry after JC-1 staining (E). The data are provided as mean standard deviation (n = 3 or 4); unpaired, two-tailed t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure 4: Olanzapine decreases both cytosolic and mitochondrial lipid peroxidation and Fe2+ in-duced by IKE. HT1080 cells were subjected to vehicle control (DMSO), 10μM olanzapine, 1μM IKE, olanzapine with IKE for 4h, then Lipid peroxidation and mitochondrial specific lipid peroxidation were measured by microscopy after C11- BODIPY (A) and MitoPerOx (C) staining, Fe2+ level in cytosolic and mitochondrial were measured by microscopy after FerroOrange (B) and Mi-to-Ferrogreen staining (D), mitocondrial membrane potential was assessed by microscopy after JC-1 staining (E). Scale bars,20μm.
Olanzapine inhibits ferroptosis independent of other major anti-ferroptotic pathways
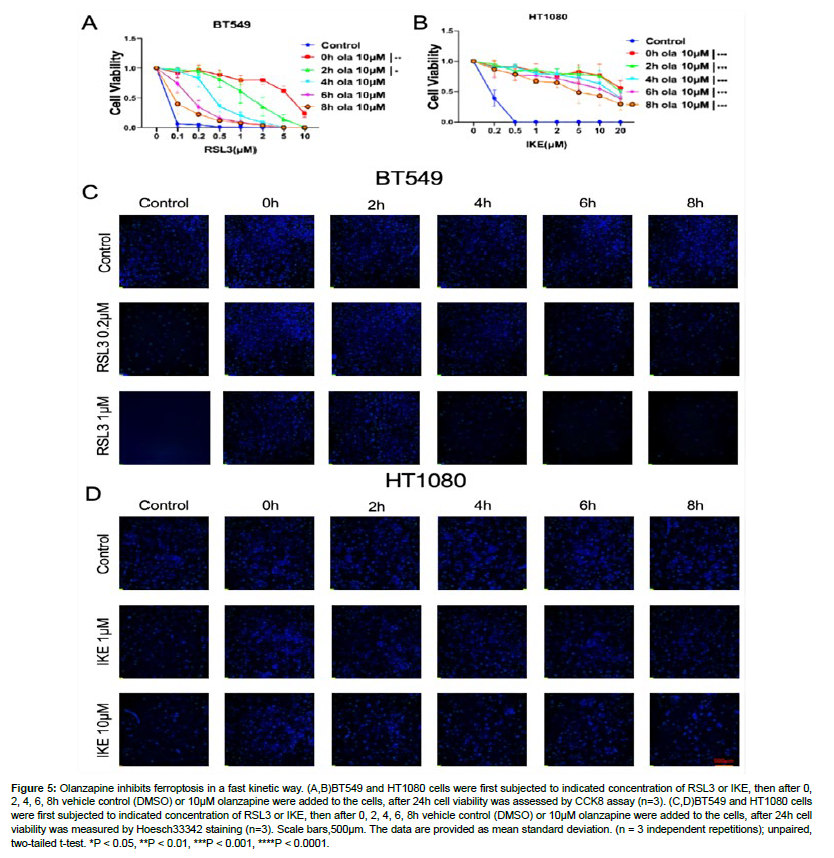
Given its clear role in ferroptosis regulation, we were interested in how olanzapine regulates ferroptosis. We first checked the kinetics of olanzapine’s anti-ferroptotic effect. To do this, RSL3 or IKE were first added to the cells, then olanzapine was added to the cells after 0h, 2h, 4h, 6h, 8h of RSL3 or IKE treatment. According to CCK8 assay, olanzapine can still rescue RSL3 or IKE induced ferroptosis even after ferroptosis induction was initiated. Live cell staining by Hoechst33342 in BT549 and HT1080 cells further confirmed the observation. In BT549 cells treated with RSL3, olanzapine added after RSL3 can still significantly rescue ferroptosis caused by RSL3 in a time-dependent way, while in HT1080 cells olanzapine rescued IKE induced ferroptosis in a similar manner. All these data indicate that olanzapine acts in a very fast motion and can rescue ferroptosis even it is initiated (Fig. 5A-5D.) [21].
Figure 5: Olanzapine inhibits ferroptosis in a fast kinetic way. (A,B)BT549 and HT1080 cells were first subjected to indicated concentration of RSL3 or IKE, then after 0, 2, 4, 6, 8h vehicle control (DMSO) or 10μM olanzapine were added to the cells, after 24h cell viability was assessed by CCK8 assay (n=3). (C,D)BT549 and HT1080 cells were first subjected to indicated concentration of RSL3 or IKE, then after 0, 2, 4, 6, 8h vehicle control (DMSO) or 10μM olanzapine were added to the cells, after 24h cell viability was measured by Hoesch33342 staining (n=3). Scale bars,500μm. The data are provided as mean standard deviation. (n = 3 independent repetitions); unpaired, two-tailed t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
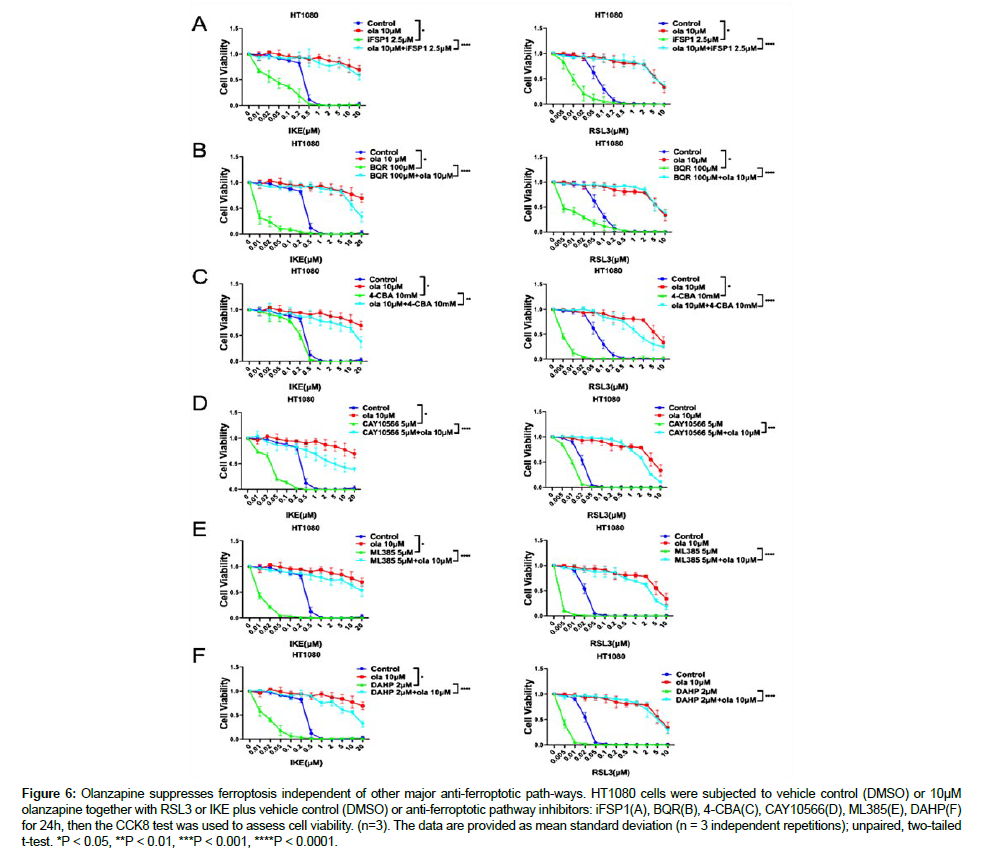
Next, we would like to know whether olanzapine modulate ferroptosis via other major ferroptosis regulatory pathway. To test this, we used inhibitors that targeting the other major pathways. This include FSP1/CoQ10 pathway inhibitor iFSP1, CoQ2 inhibitor 4-CBA which depletes CoQ10, DHODH inhibitor BQR, NRF2 inhibitor ML385, SCD inhibitor CAY10566, GCH1 inhibitor DAHP [22]. When combined with RSL3 or IKE, these inhibitors significantly increased the susceptibility of HT1080 cells to ferroptosis caused by RSL3 or IKE. Interestingly, olanzapine almost fully rescued the synergistic ferroptotsis inducing effect of these inhibitors combined with RSL3 or IKE. These data together with its fast acting kinetic suggest olanzapine inhibits ferroptosis independent of these major ferroptotic pathways (Fig. 6.) [23].
Figure 6: Olanzapine suppresses ferroptosis independent of other major anti-ferroptotic path-ways. HT1080 cells were subjected to vehicle control (DMSO) or 10μM olanzapine together with RSL3 or IKE plus vehicle control (DMSO) or anti-ferroptotic pathway inhibitors: iFSP1(A), BQR(B), 4-CBA(C), CAY10566(D), ML385(E), DAHP(F) for 24h, then the CCK8 test was used to assess cell viability. (n=3). The data are provided as mean standard deviation (n = 3 independent repetitions); unpaired, two-tailed t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Olanzapine has anti-oxidant activity
Given the fast acting kinetic and the independence of the major anti-ferroptotic pathways, we speculated it may have anti-oxidant activity. Hence, we applied the DPPH assay to test whether olanzapine by itself possess any anti-oxidant activity. As expected, olanzapine showed a clear anti-oxidant activity in vitro by DPPH assay (Fig. 7.) [24].
Figure 7: Olanzapine shows DPPH activity. DPPH assay was done with indicated drugs in vitro, the value was measured at 517nM (n=3). The data are provided as mean standard deviation (n = 3 or 6 independent repetitions); unpaired, two-tailed t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
Ferroptosis, since its discovery, has been linked to the cause of many diseases. In animal models, manipulating ferroptosis has been proposed as a potential therapeutic strategy for a number of human illnesses. Ferroptosis inhibitors, such as iron chelators and alpha-lipoic acid, have been shown to have protective effect in Alzheimer’s disease. Likewise, alpha-lipoic acid also has shown protective effects on ischemia reperfusion injuries, such as liver transplantation and simultaneous kidney-pancreas transplantation. Another ferroptosis inhibitor, ferrostatin-1, was reported to ameliorate ischemia-reperfusion induced heart failure. Also, ferroptosis inhibition attenuates renal injury by ischemia-reperfusion. Hence clinically used drugs that show potential strong anti-ferroptotic would have great translational value for ferroptosis related disease and conditions [25-28].
In this study, we have identified a clinical used antipsychotic drug olanzapine as a potent ferroptosis inhibitor. Moreover, olanzapine is also a specific ferroptosis inhibitor, as it does not inhibit other forms of cell death induced by chemo drugs. Mechanistically, olanzapine very likely inhibits ferroptosis by its anti-oxidant activity. There are several reasons for this speculation. First and most importantly, olanzapine showed potent DPPH activity in vitro. This indicates olanzapine have strong anti-oxidant activity by itself [29], which may be sufficient enough for its anti-ferroptotic effect in cells. Secondly, olanzapine act in a very fast motion, even if it is added several hours after RSL3 or IKE. This suggests it is unlike to activate other anti-ferroptotic pathways. Thirdly, olanzapine almost fully rescued the synergistic ferroptotic induction effect of RSL3 or IKE combined with other anti-ferroptotic pathway inhibitors, including iFSP1 for FSP1, BQR for DHODH, 4-CBA for CoQ10 depletion, ML385 for NRF2, CAY10566 for SCD, and DAHP for GCH1. These data further suggest olanzapine inhibits ferroptosis independent of these parallel anti-ferroptosis pathways. These findings suggest that olanzapine is a powerful and selective ferroptosis inhibitor, with its anti-oxidant activity to inhibit ferroptosis [30].
Given that olanzapine is proved and used already in clinics for the treatment of agitation associated with certain mental conditions, It may be useful in the treatment of ferroptosis-related diseases, such as Alzheimer’s disease, Ischemia-Reperfusion injury etc. However, this possibility should be validated in future experiments, using animal models [31].
Conclusions
In this work, we demonstrated that olanzapine, a commonly used antipsychotic medicine, is a powerful ferroptosis inhibitor irrespective of the previously reported ferroptosis regulatory mechanisms. The DPPH assay revealed that olanzapine exhibited anti-oxidant activity. These data imply that olanzapine, with its anti-oxidant effect, is a strong and selective ferroptosis inhibitor.
Author Contributions
Conceptualization, S.D., M.C., Q,Y. and J.P.; methodology, S.D., M.C., Q,Y. and J.P.; software, S.D. and M.C.; validation, S.D, M.C. and Y.S.; formal analysis, S.D, M.C. and Y.S.; investigation, S.D, M.C. and Y.Z.; resources, S.D, M.C. and Y.Z.; data curation, S.D. and M.C.; writing original draft preparation, S.D, M.C. and H.L.; writing review and editing, Q,Y. and J.P.; visualization, Q,Y. and J.P.; supervision, Q,Y. and J.P.; project administration, Q,Y. and J.P.; funding acquisition, Q,Y. and J.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81972564).
Data Availability Statement
All data are presented within the manuscript (figures).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575: 688-92.
- Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, et al. (2020) GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci 6: 41-53.
- Mao C, Liu X, Zhang Y, Lei G, Yan Y, et al. (2021) DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593: 586-90.
- Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, et al. (2019) Exogenous Monounsaturated Fatty Acids Promote a Fer-roptosis-Resistant Cell State. Cell Chem Biol 26: 420-432.
- Kang YP, Mockabee-Macias A, Jiang C, Falzone A, Prieto-Farigua N, et al. (2021) Non-canonical Glutamate-Cysteine Ligase Activity Protects against Ferroptosis. Cell Metab 33: 174-189.
- Boll MC, Alcaraz-Zubeldia M, Montes S, Rios C (2008) Free Copper, Ferroxidase and SOD1 Activities, Lipid Peroxidation and NO x Content in the CSF. A Different Marker Profile in Four Neurodegenerative Diseases. Neurochem Res 33: 1717-23.
- Masaldan S, Belaidi AA, Ayton S, Bush AI (2019) Cellular Senescence and Iron Dyshomeostasis in Alzheimer’s Disease. Pharmaceuticals 12: 93.
- Stockwell BR, Jiang X, Gu W (2020) Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol 30: 478-90.
- Zhang L, Jia R, Li H, Yu H, Ren K, et al.(2019) Insight into the Double-Edged Role of Ferroptosis in Disease. Biomolecules 11: 1790.
- Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, et al. (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16: 1180-91.
- Zhang H, Zhuo Y, Li D, Zhang L, Gao Q, et al. (2022) Dihydroartemisinin inhibits the growth of pancreatic cells by inducing ferroptosis and activating antitumor immunity. Eur J Pharmacol 926: 17-28.
- Wang L, Liu Y, Zhao TL, Li ZZ, He JY, et al. (2019) Topotecan induces apoptosis via ASCT2 mediated oxidative stress in gastric cancer. Phytomedicine 57: 117-28.
- Wang T, Zhang P, Chen L, Qi H, Chen H, et al. (2022) Ixazomib Induces Apoptosis and Suppresses Proliferation in Esophageal Squamous Cell Carcinoma through Activation of the c-Myc/NOXA Pathway. J Pharmacol Exp Ther 380: 15-25.
- Spirkoski J, Melo FR, Grujic M, Calounova G, Lundequist A, et al. (2012) Mast cell apoptosis induced by siramesine, a sigma-2 receptor agonist. Biochem Pharmacol 84: 1671-80.
- Wu X, Wang Y, Wang H, Wang Q, Wang L, et al. (2012) Quinacrine Inhibits Cell Growth and Induces Apoptosis in Human Gastric Cancer Cell Line SGC-7901. Curr Ther Res Clin Exp 73: 52-64.
- Calviño E, Tejedor MC, Sancho P, Herráez A, Diez JC (2015) JNK and NFκB dependence of apoptosis induced by vinblastine in human acute promyelocytic leukaemia cells: Kinases in Vinblastine Apoptosis of NB4 Cells. Cell Biochem Funct 33: 211-9.
- Attia RT, Tolba MF, Trivedi R, Tadros MG, Arafa HMM, et al. (2016) The chemomodulatory effects of glufosfamide on docetaxel cytotoxicity in prostate cancer cells. PeerJ 4: 21-68.
- Pérez-Galán P, Roué G, Villamor N, Montserrat E, Campo E, et al. (2006) The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood 107: 257-64.
- Chen Y, Lu Z, Qi C, Yu C, Li Y, et al. (2022) N6-methyladenosine-modified TRAF1 promotes sunitinib resistance by regu-lating apoptosis and angiogenesis in a METTL14-dependent manner in renal cell carcinoma. Mol Cancer 21: 111.
- Schmittgen T, Gissel K, Zakrajsek B, Lawrence B, Liu Q, et al. (2005) Diverse gene expression pattern during 5-fluorouridine-induced apoptosis. Int J Oncol 27: 297-306.
- Wu F, Ma Y, Wang J, Ou H, Dang H, et al. (2020) Bleomycin A5 suppresses Drp1‑mediated mitochondrial fission and induces apoptosis in human nasal polyp‑derived fibroblasts. Int J Mol Med 47: 346-60.
- Lee KI, Su CC, Yang CY, Hung DZ, Lin CT, et al. (2016) Etoposide induces pancreatic β-cells cytotoxicity via the JNK/ERK/GSK-3 signaling-mediated mitochondria-dependent apoptosis pathway. Toxicol In Vitro 36: 142-52.
- Gagnadoux F, Le Pape A, Urban T, Montharu J, Vecellio L, et al. (2005) Safety of Pulmonary Administration of Gemcitabine in Rats. J Aerosol Med 18: 198-206.
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, et al. (2017) Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171: 273-85.
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, et al. (2012) Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 149: 1060-72.
- Mou Y, Wang J, Wu J, He D, Zhang C, et al. (2019) Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 12: 34.
- Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, et al. (2019) Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chem Biol 26: 623-633.
- Dang X, Huan X, Du X, Chen X, Bi M, et al. (2022) Correlation of Ferroptosis and Other Types of Cell Death in Neurodegenerative Diseases. Neurosci Bull 38: 938-52.
- Belaidi AA, Bush AI (2016) Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem 139: 179-97.
- Ding Y, Zhang Y, Zhang W, Shang J, Xie Z, et al. (2021) Effects of Lipoic Acid on Ischemia-Reperfusion Injury. Oxid Med Cell Longev 5: 1-15.
- Fang X, Wang H, Han D, Xie E, Yang X, et al. (2019) Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci 116: 2672-80.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Yao Q (2024) Olanzapine Suppresses Ferroptosis via its Anti-oxidantActivity. Cell Mol Biol, 70: 332.
Copyright: © 2024 Yao Q. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 960
- [From(publication date): 0-2024 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 682
- PDF downloads: 278