Research Article Open Access
Odor Identification Function Differs between Vascular Parkinson ism and Akinetic-Type Parkinson's Disease
| Mutsumi Iijima*, Mikio Osawa, Shinichiro Uchiyama and Kazuo Kitagawa | |
| Department of Neurology, Tokyo Women’s Medical University School of Medicine, Japan | |
| Corresponding Author : | Mutsumi Iijima Department of Neurology Tokyo Women’s Medical University School of Medicine 8-1 Kawada-cho Shinjuku, Tokyo 162–8666, Japan Tel: +813–3353–8111 E-mail: iijima.mutsumi@twmu.ac.jp |
| Received January 08, 2016; Accepted January 22, 2016; Published January 29, 2016 | |
| Citation:Iijima M, Osawa M, Uchiyama S, Kitagawa K (2016) Odor Identification Function Differs between Vascular Parkinsonism and Akinetic-Type Parkinson’s Disease. J Alzheimers Dis Parkinsonism 6:207. doi: 10.4172/2161-0460.1000207 | |
| Copyright: © 2016 Iijima M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Abstract
Objective: Main clinical features of vascular parkinsonism (VP) are rigidity, fixed face, and short stepping gait. VP remains difficult to diagnose based on clinical features from patients with Parkinson’s disease (PD) without rest tremor. Olfactory dysfunction is a non-motor symptom in idiopathic PD. We investigated whether olfactory function can distinguish VP from PD.
Method: Participants were comprised of 13 patients with VP, 40 non-demented patients with akinetic-type PD, and 40 age-matched controls. Olfactory function was examined using the Odor Stick Identification Test for Japanese (OSIT-J), which evaluates the detection of 12 odorants familiar to Japanese participants.
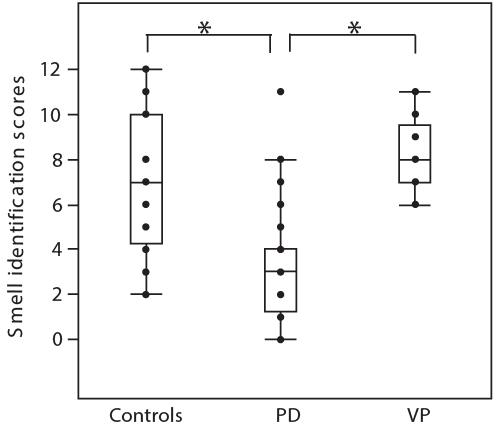
Results: Corrected odor identification scores in OSIT-J were 8.2 ± 1.5 (mean ± SD) points in patients with VP, 3.2 ± 2.5 in PD patients, and 7.1 ± 3.0 in normal subjects. These were significantly higher in VP than those in PD patients (p<0.001), but were not different from those in normal subjects.
Conclusion: The olfactory identification test is non-invasive, convenient, and useful to distinguish VP from PD as a screening test.
| Keywords |
| Vascular parkinsonism; Parkinson’s disease; Olfactory function; Odor stick identification test; MIBG cardiac scintigraphy |
| Introduction |
| The vascular parkinsonism (VP) was introduced arteriosclerotic parkinsonism occurring in cerebrovascular disease at first. It’s main clinical features were rigidity, fixed face, and short stepping gait [1,2]. Postmortem studies in VP showed commonly vascular pathology without Lewy bodies, and other neurodegenerative conditions associated with parkinsonism [2]. VP remains difficult to distinguish clinically from patients with elderly Parkinson’s disease (PD) without rest tremor. The helpful investigations in diagnosis for VP or PD, such as magnetic resonance imaging (MRI), presynaptic striatal dopamine transporter single photon emission computer tomography (SPECT), cardiac metaiodobenzylguanidine (MIBG) myocardial scintigraphy, or transcranial color-coded sonography were reported [1-7]. Previous MRI studies showed that two types of vascular lesion were multiple territory infarcts periventricular and subcortical white matter lesions, and basal ganglia ischemic lesions in patients with VP [1,3]. Patients with VP were significantly more have abnormal imaging from 90 to 100% compared with patients with PD, however, even in PD, MRI abnormalities were shown from 12 to 43% [1]. |
| In PD, olfactory dysfunction is a non-motor symptom with estimates of prevalence of up to 80% to 90%, and may appear years before the onset of motor symptoms [8-11]. Therefore, olfactory function tests were useful for detection of olfactory dysfunction in PD, and also for differentiating PD from patients with parkinsonism such as multiple system atrophy (MSA) and progressive supranuclear palsy (PSP) [11,12]. However, there was a few report of evaluation of olfactory functions in VP [13]. Therefore, we investigated whether olfactory functions distinguished VP from PD patients with akinetictype. |
| Method |
| Participants |
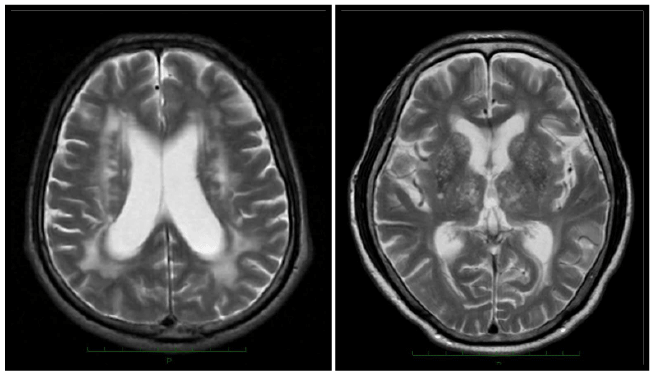
| Subjects comprised 13 patients with VP, (10 men and 3 women), ranging in age from 63 to 83 (mean ± SD; 75.6 ± 6.1) years. Diagnosis of VP was done by the following the three criteria [2,3], 1. Clinical features were bradykinesia (slowness of initiation of voluntary movement with progressive reduction in aped and amplitude of repetitive actions in either upper limb or lower limb, including the presence of reduced step length) and at least one of the following; muscle rigidity, rest tremor, or postural instability, 2. Brain MRI findings showed multiple lacunar infarcts in basal ganglia regions, or /and ischemic white matter changes (Figure 1), 3. Patients with PSP, MSA and drug-induced parkinsonism were excluded. Mini-Mental State Examination (MMSE) scores for the patients with VP ranged from 14 to 29 points (14 points in 1 patient, and other patients were above 25 points, mean ± SD: 26.1 ± 4.0). In order to ensure the diagnosis, patients were followed from one to two years. Forty non-demented patients with akinetic-type PD (25 men and 15 women, 74.7 ± 4.1 years), and 40 age-matched controls (22 men and 18 women, 75.0 ± 4.4 years). The diagnosis of PD was made based on the Criteria of the United Kingdom Brain Bank [14] and a negative family history of PD. Motor performance was assessed using the motor section (part III) of the Unified Parkinson’s Disease Rating Scale (UPDRS). A patient with akinetic-type PD was diagnosed by means of part III of the UPDRS on the basis of tremor and nontremor scores. The tremor score was derived from the sum of UPDRS items 20 (tremor at rest) and 21 (action or postural tremor of hands). The non-tremor score was obtained from the sum of UPDRS items 18 (speech), 19 (facial expression), 22 (rigidity), 27 (arising from chair), 28 (posture), 29 (gait), 30 (postural stability), and 31 (body bradykinesia and hypokinesia). PD was classified as akinetic-type if the non-tremor score was at least twice the tremor score. MMSE scores for the patients with PD ranged from 28 to 30 points. All patients were examined nasal sinus diseases by brain CT or MRI. Patients with nasal sinus diseases and with a history of olfactory diseases were excluded from the study. |
| Informed consent was obtained from all participants following a full explanation of the study. This study was done in accordance with the guidelines of the Committee of Medical Ethics of Tokyo Women’s Medical University. |
| Odor function |
| Odor stick identification test (OSIT-J): This test included 12 odorants: perfume, rose, condensed milk, Japanese orange, curry, roasted garlic, fermented beans/sweaty socks, cooking gas, menthol, India ink, wood, and Japanese cypress, by Takasago International Corporation Ltd. (Tokyo, Japan) [15,16]. Each odorant was enclosed in melamine resin microcapsules, which were mixed into an odorless solid cream and then shaped like a lipstick. The examiner painted each odor stick in a 2-cm circle on thin paraffin paper, folded the paper in half, rubbed it to grind the microcapsules, and then passed it to the participant. The participant opened the paraffin paper and sniffed it. He or she then chose one of six possible answers from four labeled pictures of entities associated with the odors, 1 of which was correct, and 2 others (unknown and not detected). Participants were directed to avoid eating and smoking 30 min prior to being examined. The order in which the odorants were presented was randomized. |
| Subjective symptoms: Self-rating ability to smell was evaluated with a 5-point scale ranging from normosmia to anosmia: (1) can smell normal-strength odors; (2) some decline in sense of smell; (3) can smell only strong odors; (4) much diminished sense of smell; and (5) no sense of smell. |
| 123I-MIBG myocardial scintigraphy |
| For MIBG myocardial scintigraphy, planar scintigraphic imaging in the anterior view was performed using a single head gamma camera (GCA7200, Toshiba, Japan), 15 minutes (early) and 4 hours (delayed) after intravenous injection of MIBG (111 MBq). To measure MIBG uptake, heart (left ventricle) and mediastinal regions of interest were drawn manually. The heart to mediastinum ratio (H/M) for both early and delayed images, and the myocardial washout rate (WR) were calculated. Normal values at our hospital are below 1.6 (mean – 2SD) for both early and delayed H/M ratio [17]. |
| Statistical analysis |
| Statistical comparisons among groups employed one ANOVA using age and smell identification scores. Where indicated by a significant F-value, post hoc comparisons were carried out using Tukey’s test. For comparison of H/M ratios and WR between VP group and PD group, first, it was assayed whether the variance of the two groups of data are equal, if it were different, they were compared using a welch test. Subjective olfactory symptom was compared between VP and PD groups using non-parametric analysis (Mann-Whitney U-test). |
| Results |
| Clinical manifestations of VP |
| Brain MRI showed in 3 patients with multiple lacunar patterns, 1 patient with ischemic white matter changes, and 9 patients in both patterns. Their comorbidities were hypertension in all 13 patients, dyslipidemia in 4, diabetes mellitus in 3. One patient had smoking history. Clinical features of VP were mainly gait disturbance, bradykinesia and rigidity on the lower limbs dominantly without laterality. There was not any patient with resting tremor (Table 1). Eight patients were took oral levodopa therapy. Bradykinesia were mildly improved in five patients, but any symptoms were not improved in three patients. UPDRS III score was 19.7 ± 13.9 in VP, 20.1 ± 8.5 in PD, respectively, and it was not significant difference between VP and PD. |
| Olfactory functions |
| Smell identification scores were 8.2 ± 1.5 (mean ± SD) in patients with VP, 3.2 ± 2.5 in PD patients, and 7.1 ± 3.0 in normal subjects, and were significantly different among three groups (F=29.7, P<0.001). Smell identification scores were in patients VP was significantly higher than that in patients with PD (P<0.001, post hoc Tukey’ test), but was not any different comparing with normal subjects (Figure 2). The scores of subjective symptoms were 2.5 ± points in PD patients and 1.2 ± 0.4 points in VP patients, and it was significantly higher in PD than in VP patients (<0.05). |
| Cardiac 123 I-MIBG scintigraphy |
| Eight patients with VP and 17 patients with PD were performed the cardiac 123I-MIBG scintigraphy. In patient with VP, early H/M ratio was 2.34 ± 0.75 (range: 1.33–3.88), delayed H/M ratio 2.13 ± 0.59 (range: 1.07–3.23) and WR was 37.3 ± 18.1% (range: 3.3–86 %) Early and delayed H/M ratios were reduced in 2 patients, one of who had been suffering from diabetes. In patients with PD, early H/M ratio was 1.57 ± 0.24 (range: 1.30–2.02), delayed H/M ratio 1.31 ± 0.25 (range: 1.03– 1.86) and WR was 65.5 ± 17.7 % (range: 34– 93 %). The H/M ratios were significantly decreased in PD than in VP (early H/M ratio; P<0.05, delayed H/M ratio; P<0.05, Welch test), and WR was significantly increased in PD than in VP (P<0.05, Welch test). |
| Discussion |
| VP can be clinically distinguished from PD characterized by lower body predominance postural instability with freezing gait and fall, pyramidal signs and dementia [1,2,4]. However, it is difficult for a patient without resting tremor to diagnose PD or VP just based on clinical features at a patient’s first visit. In this study, patients with VP showed also predominant lower body symptoms, but UPDRS III score was not significant difference between VP and age matched PD with akinetic-type. The previous report suggested that the testing olfactory function using the University of Pennsylvania smell identification test (UPSIT) may be helpful in differentiating VP from PD [13]. The UPSIT scores in VP were significantly better than in PD, but did not differ from the healthy controls [13]. In this study, the odor identification function using OSIT-J in patients with VP was better than in akinetictype of PD, and did not differ significantly from that in aged matched controls. Odor identification function differed among the clinical subtypes of PD, and was significantly lower in PD with akinetic-type that in tremor-dominant type [18]. A new point of this study was that of compared the odor identification function between patients with VP and PD patients with akinetic-type. |
| The olfactory processing in humans is transmitted from the olfactory receptors located in the nasal mucosa to the olfactory bulb and then to the primary olfactory cortices, consisting of the piriform cortex, the entorhinal cortex, and the periamygdaloid cortex [11,19]. The primary olfactory cortices project to the hippocampus and the mediodorsal nucleus of the thalamus [11,19]. Vascular or tumor lesions involving the oribitofrontal cortex, medial temporal lobe, or the thalamic nuclei may induce hyposmia or anosmia [19,20]. With regard to the pathophysiological basis on the normal olfactory function in our patients with VP, above olfactory related areas, or these network structures may have been maintained. |
| The other helpful investigations for differentiating between PD and VP that were neuroimaging, presynaptic striatal dopamine transporter SPECT, transcranial color- coded sonography, cardiac 123I-MIBG scintigraphy, quantitative EEG, and immunological markers in plasma and CSF reported [1]. Cardiac 123I MIBG scintigraphy is a noninvasive tool for assessing myocardial sympathetic nerve terminals, and its sensitivity and specificity by the delayed H/M ratio in early stage (Hoehn-Yahr stage 1 or 2) PD was 94.1% and 80.2%, respectively [7]. In this study, the H/M ratios in patients with PD were significantly decreased than that in patients with VP. However, cardiac 123I-MIBG uptakes are affected in patients with cardiac autonomic neuropathy of diabetes, and with taking the MAO-B inhibitors. Patients with VP could have arteriosclerotic risk factors such as hypertension, diabetes, and dyslipidemia. In this study, two patients with VP showed the reduction of H/M ratios, and one patient of those had been suffering from diabetes. In addition, cardiac 123I MIBG scintigraphy is pretty high cost and restricted to use by countries. Cardiac 123I-MIBG uptake was significantly correlated with scores of odor identification tests in patients with early stage PD [17,21,22]. If it is difficult to examine cardiac 123I-MIBG scintigraphy in patients with parkinsonism, evaluation of odor function is one of the useful tool for distinguishing VP from PD. [123I] FP-CIT SPECT (DaTSCAN) imaging of dopamine transporters (DaT) with specific tracers gives a guide to the attenuation of functional dopaminergic neurons [5,6]. Reductions of DaT radiotrace uptake correlate with the loss of presynaptic nigrostriatal neurons and are more sensitive than clinical examination to detect nigrostriatal defects in PD, MSA, PSP, corticobasal degeneration, and dementia with Lewy bodies. Pooled accuracy of DaTSCAN measures in differentiating between PD and vascular or drug-induced parkinsonism were relatively high, with sensitivity and specificity values above 85% and 80%, respectively [5]. DaTSCAN has just become available to use from 2014 in Japan, but it is time- consuming, a pretty high cost, and is performed in limited hospitals |
| In addition, detailed examinations for characteristic other non- motor symptoms of PD, such as autonomic dysfunctions, REM behavior disorder, and color vision impairment [23] could help distinguish VP. Limitations of olfactory identification tests, results may not be accurately evaluated in patients with nose disease, advanced dementia and ultra-elderly more than 90 years of age. However, the olfactory identification test is non-invasive, convenient, and useful to distinguish VP from PD as a screening test. |
| Conclusion |
| This study clarified that the odor identification function in patients with VP was better than in akinetic-type of PD, and suggests that the olfactory identification test is useful to distinguish VP from PD. |
| Acknowledgement |
| We thank Dr. Satoru Shimizu for statistical support. This research is partially supported by the Promoting Clinical Trials for Development of New Drugs and Medical Devices from Japan Agency for Medical Research and development, AMED. |
| Conflict of Interests |
| The authors declare that there is no conflict of interests regarding the publication of this paper. |
References
- Kalra S, Grosset DG, Benamer HT (2010) Differentiating vascular parkinsonism from idiopathic Parkinson's disease: a systematic review. MovDisord 25: 149-156.
- Zijlmans JC, Daniel SE, Hughes AJ, Révész T, Lees AJ (2004) Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. MovDisord 19: 630-640.
- Zijlmans JC, Thijssen HO, Vogels OJ, Kremer HP, Poels PJ, et al. (1995) MRI in patients with suspected vascular parkinsonism. Neurology 45: 2183-2188.
- Vale TC, Caramelli P, Cardoso F (2015) Clinicoradiological comparison between vascular parkinsonism and Parkinson's disease. J NeurolNeurosurg Psychiatry 86: 547-553.
- Brigo F, Matinella A, Erro R, Tinazzi M (2014) [¹²³I]FP-CIT SPECT (DaTSCAN) may be a useful tool to differentiate between Parkinson's disease and vascular or drug-induced parkinsonisms: a meta-analysis. Eur J Neurol 21: 1369-1369e90.
- Booth TC, Nathan M, Waldman AD, Quigley AM, Schapira AH, et al. (2015) The role of functional dopamine-transporter SPECT imaging in parkinsonian syndromes, part 2. AJNR Am J Neuroradiol 36: 236-244.
- Orimo S, Suzuki M, Inaba A, Mizusawa H (2012) 123I-MIBG myocardial scintigraphy for differentiating Parkinson's disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism RelatDisord 18: 494-500.
- Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, WoltersECh, et al. (2004) Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol 56: 173-181.
- Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, et al. (2008) Association of olfactory dysfunction with risk for future Parkinson's disease. Ann Neurol 63: 167-173.
- Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, et al. (2007) Olfactory loss may be a first sign of idiopathic Parkinson's disease. MovDisord 22: 839-842.
- Doty RL (2012) Olfaction in Parkinson's disease and related disorders. Neurobiol Dis 46: 527-552.
- Suzuki M, Hashimoto M, Yoshioka M, Murakami M, Kawasaki K, et al. (2011) The odor stick identification test for Japanese differentiates Parkinson's disease from multiple system atrophy and progressive supra nuclear palsy. BMC Neurol 11: 157.
- Katzenschlager R, Zijlmans J, Evans A, Watt H, Lees AJ (2004) Olfactory function distinguishes vascular parkinsonism from Parkinson's disease. J NeurolNeurosurg Psychiatry 75: 1749-1752.
- Hughes AJ, Daniel SE, Blankson S, Lees AJ (1993) Aclinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol 50: 140-148.
- Saito S, Ayabe-Kanamura S, Takashima Y, Gotow N, Naito N, et al. (2006) Development of a smell identification test using a novel stick-type odor presentation kit. Chem Senses 31: 379-391.
- Iijima M, Kobayakawa T, Saito S, Osawa M, Tsutsumi Y, et al. (2008) Smell identification in Japanese Parkinson's disease patients: using the odor stick identification test for Japanese subjects. Intern Med 47: 1887-1892.
- Iijima M, Osawa M, Momose M, Kobayakawa T, Saito S, et al. (2010) Cardiac sympathetic degeneration correlates with olfactory function in Parkinson's disease. MovDisord 25: 1143-1149.
- Iijima M, Kobayakawa T, Saito S, Osawa M, Tsutsumi Y, et al. (2011) Differences in odor identification among clinical subtypes of Parkinson's disease. Eur J Neurol 18: 425-429.
- Leboucq N, Menjot de Champfleur N, Menjot de Champfleur S, Bonafé A (2013) The olfactory system. DiagnInterv Imaging 94: 985-991.
- Rousseaux M, Muller P, Gahide I, Mottin Y, Romon M (1996) Disorders of smell, taste, and food intake in a patient with a dorsomedial thalamic infarct. Stroke 27: 2328-2330.
- Lee PH, Yeo SH, Kim HJ, Youm HY (2006) Correlation between cardiac 123I-MIBG and odor identification in patients with Parkinson's disease and multiple system atrophy. MovDisord 21: 1975-1977.
- Oka H, Toyoda C, Yogo M, Mochio S (2010) Olfactory dysfunction and cardiovascular dysautonomia in Parkinson's disease. J Neurol 257: 969-976.
- Piro A, Tagarelli A, Nicoletti G, Fletcher R, Quattrone A (2014) Color vision impairment in Parkinson's disease. J Parkinsons Dis 4: 317-319.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 10690
- [From(publication date):
March-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9890
- PDF downloads : 800
