Research Article Open Access
Occurrence of Orientia Tsutsugamushi Genotypes in Areas of Union Territory of Puducherry and Tamil Nadu State, India
Patricia Anitha K1, Hoti SL1*, Reba Kanungo2, Jambulingam P1, Yasin Nazeer3, Shashikala Nair2 and Sudhagar Mookappan41Vector Control Research Centre, Indira Nagar, Puducherry, India
2Department of Microbiology, Pondicherry Institute of Medical Sciences, Ganapathichettikulam, Kalapet, Puducherry, India
3Regional Medical Research Centre, Nehru Nagar, Belagavi, Karnataka, India
4Department of Medicine, Pondicherry Institute of Medical Sciences, Ganapathichettikulam, Kalapet, Puducherry, India
- *Corresponding Author:
- Hoti SL
Vector Control Research Centre
Indira Nagar, Puducherry, India
Tel: 918105536970
E-mail: slhoti@yahoo.com
Received Date: January 30, 2017; Accepted Date: February 16, 2017; Published Date: February 26, 2017
Citation: Anitha PK, Hoti SL, Kanungo R, Jambulingam P, Nazeer Y, et al.(2017) Occurrence of Orientia Tsutsugamushi Genotypes in Areas of Union Territory of Puducherry and Tamil Nadu State, India. J Emerg Infect Dis 2:124.doi: 10.4172/2472-4998.1000124
Copyright: © 2017 Anitha PK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Disease and Pathology
Abstract
Scrub typhus, caused by the proteobacterium, Orientia tsutsugamushi, is re-emerging in several regions of Asia, particularly in India. Severity of infection and clinical presentations are known to vary with the infecting prototype/genotype. Hence genotypic characterization and distribution of various genotypes is of clinical and epidemiological importance. One hundred and forty five suspected scrub typhus cases, reporting to a tertiary care centre in Puducherry, India were investigated for O. tsutsugamushi specific IgM antibodies by ELISA out of which, 135 (93%) were positive. Most cases occurred post-monsoon, peaking in September, and there was a preponderance in young adults. Forty samples tested positive by Nested PCR based on 56kDa gene of O. tsutsugamushi and amplicons from PCR positive samples were sequenced. Phylogenetic tree constructed using 35 sequences (from nt 20 to nt 130) obtained in the study, along with those retrieved from NCBI site, formed two major branches; one comprising 15 sequences from this study, which aligned with Karp genotype, and the other containing 6 sequences which aligned with a sequence from Vellore, India. The second major branch had 12 sequences, which formed an independent group from the Gilliam prototype. Analysis of association of clinical manifestations with genotypes obtained in the present study showed that the sequences that formed an independent group, were associated with a wide spectrum of clinical manifestations involving multiple organs and more severe manifestations like ARDS and hypotension. The sequences, which branched with Karp prototype were associated with hepatitis, meningitis and Multi-organ disorder syndrome (MODS). The present study thus indicated that different genotypes of O. tsutsugamushi occur in southern regions of India, which were associated with various clinical manifestations. Studies on the occurrence of genotypes will help in development of diagnostic methods and vaccines to curtail the infection.
Keywords
Scrub typhus; Orientia tsutsugamushi; Genotypes; Clinical manifestations; South India.
Introduction
Scrub typhus, also called ‘tsutsugamushi disease’, is an acute febrile illness caused by Orientia tsutsugamushi and transmitted by trombiculid mites; predominantly Leptotrombidium deliense, which accounts for about 20% of pyrexias in endemic regions [1,2]. Although scrub typhus was restricted to the so-called called ‘Tsutsugamushi triangle’, which included most of the South East Asian countries, it is recently reported from areas outside this region; most notable among these being Dubai, Africa and South America [3-5]. In India the disease is reported from wide geographic areas such as Jammu and Kashmir, Rajasthan, Himachal Pradesh North Eastern states and Southern parts of the country [6-8].
The most common clinical presentations of scrub typhus are high fever, headache and myalgia. However, it can also present with varied nonspecific symptoms often leading to a clinical dilemma during the course of infection. Scrub typhus can also result in severe complications that may prove fatal. Although O. tsutsugamushi has been the only known species of the genus, recently a new species, Orientia chuto has been reported from Dubai [3,5]. Based on the variation in the variable domain of the immunodominant surface 56 kDa antigen, prototypes and serotypes of O. tsutsugamushi have been identified. Among these, Karp, Gilliam and Kato are considered as protoypes of Orientia, while there are genotypes such as Kuroki, Shimokoshi, Irie, Hirano, Kawasaki, Boryong etc. [9-11]. Information on the clinical presentations of these prototypes/ serotypes/genotypes is scanty. A few reports indicate that the clinical presentation and severity of scrub typhus varies with the infecting strain of O. tsutsugamushi [12]. A study from Korea highlighted the differences in clinical manifestations between Boryong and Karp genotypes of O.tsutsugamushi. Patients infected with the Boryong genotype were found to present with significantly more generalized weakness, conjunctival injection and skin rash than in the group infected with Karp genotype [10]. Studies conducted in mice model also demonstrated differences in the virulence among strains of O tsutsugamushi, and the strains were classified into a high virulence group (Karp, Kato and KN-3 genotypes), an intermediate virulence group (Gilliam genotype) and a low virulence group (Kuroki, Kawasaki and KN-2) [13,14]. In view of the differences in clinical manifestations with the infecting strain, it is important to investigate the distribution of genotypes across endemic areas and their association with clinical manifestations [10,12,15]. Although scrub typhus is reported from several geographic areas of India, there is a paucity of information on the occurrence of common genotypes of O. tsutsugamushi and the associated clinical presentations. Increasing incidence of scrub typhus with varying clinical manifestations has been reported recently in Puducherry, a coastal city in southern peninsular India, and its adjoining areas of Tamil Nadu state [8,16]. We investigated the occurrence of different genotypes of O. tsutsugamushi among clinically suspected cases of scrub typhus from this wide geographic area (about 2000 sq. km), who reported to a tertiary care hospital in Puducherry.
Materials and Methods
Puducherry is located between 11°56’ North and 79°50’ East, in south-eastern coastal peninsular India. All suspected cases of scrub typhus reporting to a tertiary care centre (Pondicherry Institute of Medical Sciences) in Puducherry, between March 2011 to March 2013 were recruited to the study. The inclusion criteria were fever for a period of 5 days or more for which the cause was not known or fever with a clinical suspicion of Scrub typhus according to WHO guidelines, in which the cases had an eschar, headache, lymphadenopathy or conjunctival injection. The exclusion criteria were cases with fever due to other common infections in this region like malaria, typhoid and dengue. A sample size of 145 was arrived at, assuming a prevalence rate of 43% with 8% margin of error and 95% confidence interval [17]. The approval of Institutional Ethics Committee was obtained prior to the start of the study (No. IEC/RC/12/54). Blood samples were collected from 145 patients, after obtaining written informed consent. Serum was separated and aliquots of sera and blood were stored at -70°C for further use. Other possible causes of febrile illnesses like Malaria, Typhoid, Dengue, Leptospirosis and Filariasis, were ruled out based on appropriate laboratory investigations as follows; Malaria was ruled out based on microscopic examination of stained peripheral blood smear and Parascreen Rapid test for Malaria (Zephyr Biomedicals, India), Leptospirosis and Dengue were ruled out based on Leptospira IgM and IgG card test (SD Bioline, India) and Dengue day 1 test (J. Mitra, India), respectively. Typhoid was ruled by both conventional blood culture and Widal test, which was done using stained Salmonella antigens (Span, India), and lymphatic filariasis by stained peripheral blood smear and BinaxNow Filariasis card test (Alere, USA).
Detection of IgM antibodies
A commercially available kit (Inbios International Inc. Seattle, USA) was used for detecting anti O. tsutsugamushi IgM antibodies. The kit utilizes recombinant 56kDa antigen of O. tsutsugamushi which was coated on wells of microtiter plate and the ELISA was performed as per the instructions of the manufacturer. An O.D value of the sample more than the cut-off (as per kit insert) was considered as positive for IgM antibodies.
Genotyping of samples
DNA was extracted from blood clots or whole blood samples using the Blood Genomic Extraction kit (Sigma-Aldrich, USA) and stored at -20oC until further use. The DNA was used in nested PCR targeting 56 kDa antigen of O. tsutsugamushi, employing the method described by Furuya et al. [18] and modified by Saisongkorh et al. [19].
Amplicons of 483 bps obtained were purified using PCR clean-up gel extraction kit (Macherey-Nagel, Germany) and the product was subjected to cycle sequencing reaction with either the forward or reverse primer. This product was further sequenced; using ABI automated Genetic analyzer 3130 XL (ABI-Applied Biosystem, CA, USA). The amplicons were sequenced in forward direction, and if the sequence was not readable, sequencing was performed in the reverse direction also.
Analysis of sequences
The fragment of 56 kDa gene located between variable domains II and III was sequenced from all the PCR positive samples. The nucleotide sequences obtained were edited using BioEdit (Version 7.0.0) and analyzed using Basic local alignment search tool (BLAST) in the NCBI website. Phylogenetic analysis was carried out along with reference sequences obtained from GenBank, in MEGA 6 software. After alignment using BioEdit Sequence alignment editor, 110 nucleotides (nt20 to nt130), which aligned in a similar manner were used for construction of phylogenetic tree. The tree was constructed by Maximum Likelihood method using Tamura-Nei model, with bootstrapping (1000 iterations) and gap positions were included in the analysis. The nucleotide sequences obtained have been submitted to Genbank (Accession numbers KT 970944-KT 970979 and KU 000002- KU 000004).
Clinical analysis
The demographic parameters and clinical presentations of 145 patients were documented by physicians. The clinical presentations were grouped into undifferentiated fever, hepatitis, Acute Respiratory Distress Syndrome (ARDS), meningitis, Multiorgan Disorder Syndrome (MODS) and Disseminated Intravascular Coagulation (DIC) [20,21].
Results
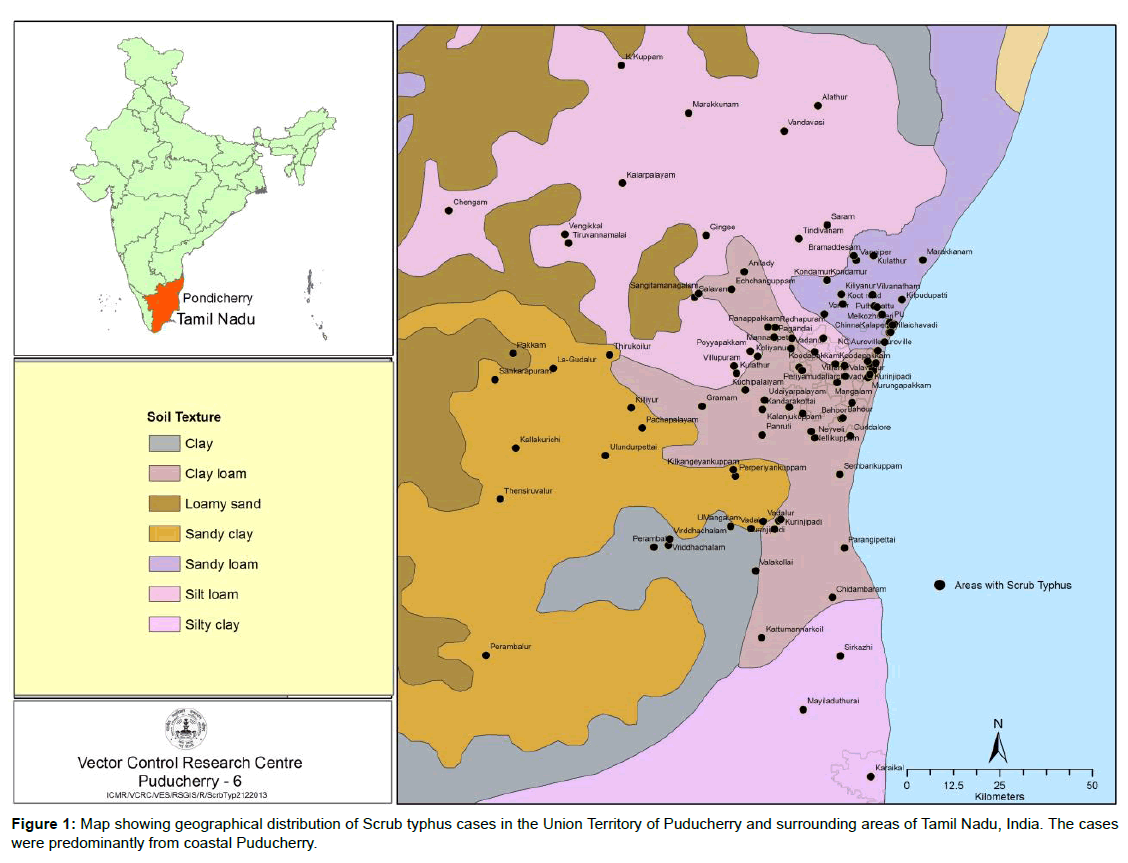
Scrub typhus cases reported in the study came from 22 different rural and urban locations in the Union territory of Puducherry and surrounding areas of Tamil Nadu state, India. About 25% of the cases were from coastal hamlets of Puducherry such as Kalapet, Pillaichavady and Keezhputhupet, while the remaining cases were from neighboring inland areas of Tamil Nadu state (Figure 1). O. tsutsugamushi specific IgM antibodies were detected in 135 (93%) cases out of 145 suspected scrub typhus cases. The remaining 10 IgM negative cases were diagnosed as scrub typhus based on the clinical presentations suggestive of the disease and the presence of the characteristic eschar. Nested PCR targeting 56 kDa gene was done on all the 145 samples of which 40 samples were positive. The amplicons were sequenced and sequences obtained were confirmed to be that of O. tsutsugamushi upon BLAST search.
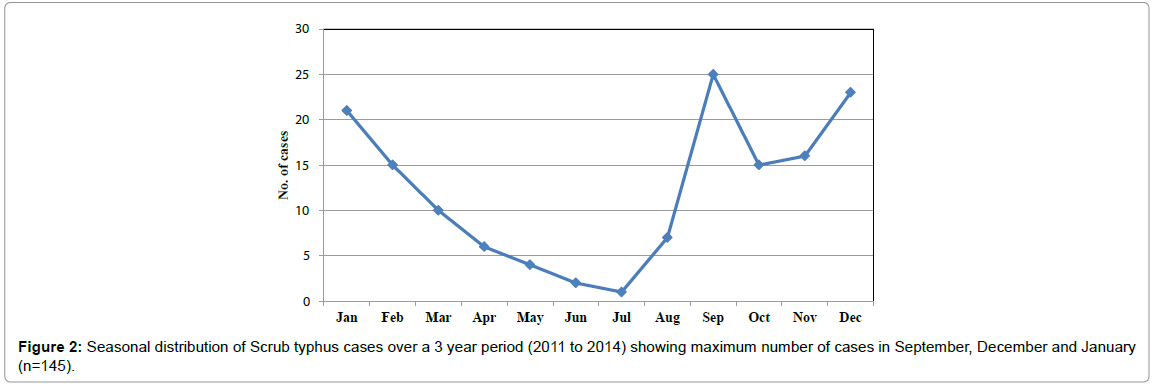
Seasonal variation in the incidence of scrub typhus cases was observed, with a rise of cases in relatively cooler months, peaking in September numbering 25 (17.2%) and another peak in December and January with 23 and 21 cases respectively (15.8 and 14.4%). The period between June and July months recorded lesser number of cases (Figure 2). The analysis of age profile of 145 suspected cases of scrub typhus showed an adult preponderance with 65.5% of the cases being adults. Among adults with scrub typhus, 49 were male and 46 were female. The youngest cases were three 1 year infants, while the oldest case was an 86 years old male, who succumbed to the infection due to complications.
Clinical manifestations and laboratory parameters of the cases
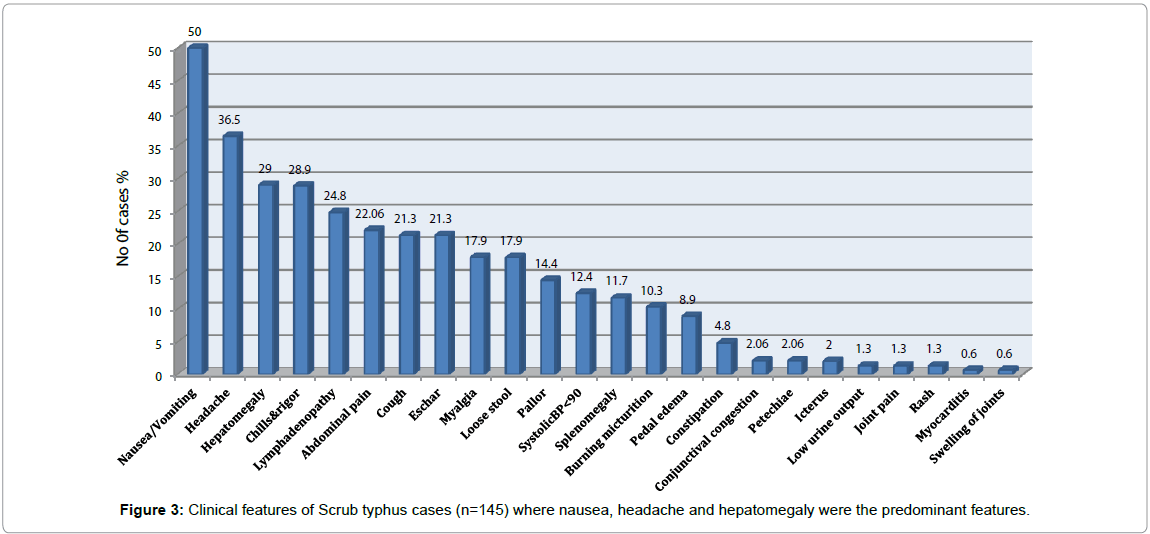
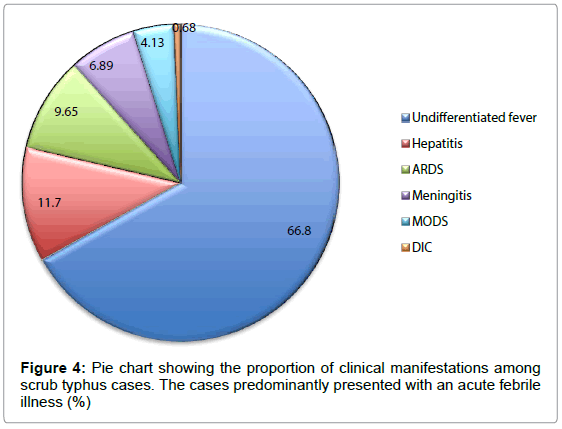
The clinical manifestations and laboratory parameters of cases are presented in Figure 3 and Table 1. About 84% of the cases had highgrade intermittent fever of less than 2 weeks duration. Majority of the patients presented with nausea and headache which was seen in 50% and 36.5% of the cases, respectively. The other common features seen were hepatomegaly and abdominal pain. Hepatomegaly, eschar and lymphadenopathy were seen in 29%, 21.3% and 24.8% of the cases, respectively. Manifestations such as chills, rigor and abdominal pain were also observed in some cases. The laboratory parameters found to be elevated included SGPT (48.9%), and bilirubin (51.7%). Anemia was observed in 40.6% of the cases. The clinical classification of cases is given in fig. 4. Although 67% of the cases had an acute febrile illness with no other complications, hepatitis, Acute Respiratory distress syndrome (ARDS), meningitis and multi-organ disorder syndrome (MODS) were also seen in some cases (Figure 4). One case presented with disseminated intravascular coagulation (DIC).
| Laboratory marker (Normal Value) | Positivity (%) |
|---|---|
| Elevated Bilirubin (up to 0.2mg/dl) | 51.7 |
| Elevated SGPT (15-37u/L) | 48.9 |
| Elevated ESR (2-20 mm/hr) | 44 |
| Anemia (11-16.5g/dl) | 40.6 |
| Thrombocytopenia(1.5-4.5lakhs/cu.mm) | 30.3 |
| Elevated Alkaline PO4ase (40-129u/L) | 26.2 |
| Elevated Gamma GlutamylTranspeptidase | 24.1 |
| Leukocytocis (>11,000/cu.mm) | 15.8 |
| Serum Creatinine (0.7-1.4mg/dl) | 11 |
| Elevated Urea (15-40mg/dl) | 11 |
| Leukopaenia (<3,500/cu.mm) | 6.2 |
Table 1: Laboratory parameters associated with Scrub typhus cases (n=145), the major marker being elevation of liver enzymes.
The clinical manifestations found in majority of the cases such as nausea/vomiting, headache, chills, rigor, and lymphadenopathy are non-specific symptoms that could be found in other case of febrile illnesses. Characteristic eschar, which occurs at the site of mite bite and reported with varying frequencies, was seen only in a minor proportion of the cases. Thirty one cases (21.3%) had an eschar, predominantly in the thigh, infra axillary or inguinal region. Out of these cases with an eschar, 21 were IgM positive.
Gastrointestinal symptoms like abdominal pain, nausea, vomiting and loose stools, which are reported to be common among scrub typhus cases, were also found to occur in cases in the present study. Lymphadenopathy is another common feature seen in this study. Elevated ESR (44%), anemia (40.6%) and thrombocytopenia (30.3%) were the other significant laboratory findings. Burning micturition was found to occur in 10.3% of the cases and all these cases had negative urine culture, which is not reported so far in scrub typhus cases. The symptoms more common in children when compared to adults were nausea, presence of an eschar, cough and lymphadenopathy.
Elevated levels of bilirubin, SGPT and thrombocytopenia were seen more among adults while anemia was more common in children.
Liver damage as indicated by simultaneous high levels of serum alkaline phosphatase, SGPT, bilirubin and gamma-glutamyltransferase and ARDS was the most common complication seen in patients included in the study. A single case had co-infection with Chikungunya, 2 with Malaria and 11 cases with Dengue. Most of the patients responded to treatment with doxycycline (100 mg bid for 7 days), however three cases, who reported to the hospital in later stages of the disease and were not on any antibiotics expired due to myocarditis, severe liver dysfunction and shock.
Phylogenetic analysis and clinical features of genotypes
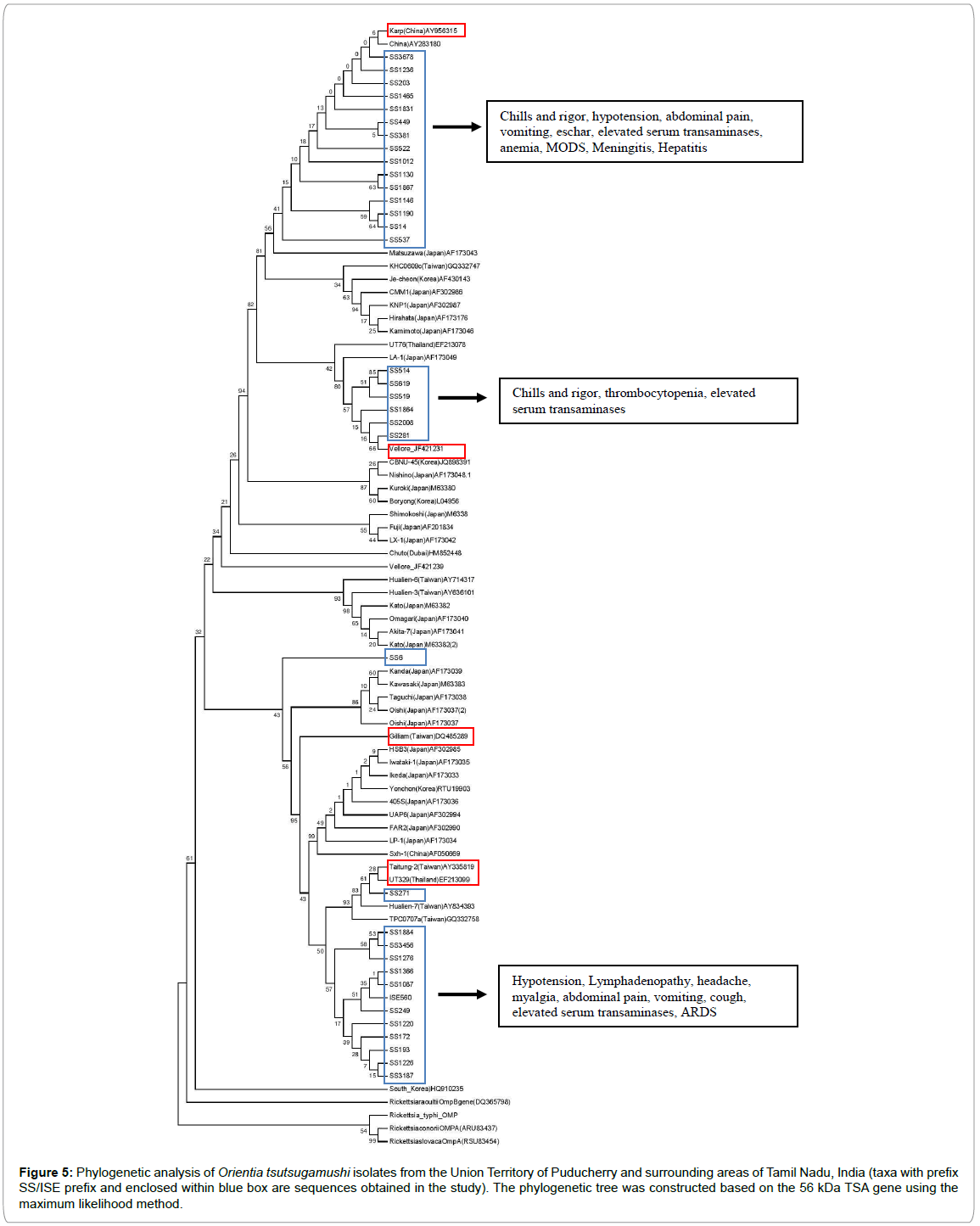
Thirty five nucleotide sequences of 56 kDa gene of O. tsutsugamushi generated in the present study were aligned along with those of O. tsutsugamushi and Rickettsia species retrieved from the NCBI and used for constructing phylogenetic tree (Figure 5). The tree broadly formed two major branches. One of these major branches formed two major sub-branches, one comprising the Karp prototype and 15 sequences of the present study and another containing 6 sequences of the present study and a sequence reported from Vellore, India (Vellore_JF421231). This major branch also had Kuroki, Boryong, Chuto and Kato genotypes as independent minor branches. The second major branch divided into two sub-branches; one sub-branch with Gilliam prototype (DQ485289), and the second sub-branch with 12 sequences of the present study as one minor branch. In addition to this, one sequence (SS6) formed a distinct minor branch aligning with an isolate from Japan, and another isolate (SS271) fell under another minor branch along with sequences from Taiwan and Thailand. Thus the O. tsutsugamushi encountered in the study belonged to several different prototypes/genotypes, with 15 isolates belonging to Karp prototype and interestingly 12 isolates forming an independent group from other genotypes.
Figure 5: Phylogenetic analysis of Orientia tsutsugamushi isolates from the Union Territory of Puducherry and surrounding areas of Tamil Nadu, India (taxa with prefix SS/ISE prefix and enclosed within blue box are sequences obtained in the study). The phylogenetic tree was constructed based on the 56 kDa TSA gene using the maximum likelihood method.
An analysis of clinical features among various genotypes of the present study showed that each genotype is associated with a different set of clinical manifestations. The isolates that formed an independent group along with Taiwan isolates had wider spectrum of manifestations involving multiple organs and more severe manifestations like ARDS and hypotension, whereas 6 isolates forming a sub-branch with Vellore isolate were characterized by comparatively less severe and narrow spectrum of clinical manifestations (Figure 5). Strains that closely aligned with Karp prototype were associated with manifestations like hepatitis, meningitis and MODS.
Discussion
Scrub Typhus is being increasingly reported from South India, with a definitive seasonal occurrence [22]. The present study also documented a seasonal trend, beginning from the end of monsoon (September), to the cooler months of December and January. Cases declined during the hotter season. Land cover with secondary or transitional vegetation is an important risk factor in determining the occurrence of the disease [23]. Post-monsoon, the rich growth of vegetation favors the breeding of mites, unlike the dry season, leading to increased man-vector contact and hence scrub typhus cases. Pondicherry Institute of Medical Sciences is situated in the outskirts of Puducherry town and patients who present to the hospital are mostly from Union Territory of Puducherry and bordering areas of Tamil Nadu state. Hence, the cases were found to be predominantly concentrated in the coastal areas of Puducherry such as Kalapet, Pillaichavady and Keezhputhupet and scrub typhus has been reported from coastal regions earlier [24]. More number of cases was also found to occur in adjoining areas of Tamil Nadu such as Panruti, Villupuram, Tiruvannamalai and Thirukoilur which are characterized by the presence of scrub forests and cashew groves [25].
Adult preponderance seen among cases in this study, could be related to outdoor exposure and work habits, although occupation related exposure was seen only in 4 cases, who were farmers. Also, there is encroachment of farm areas for residential purposes. The eschar, which is said to be a pathognomonic sign of scrub typhus and reported with varying frequencies in earlier studies [7,8,14] was seen only in a minor proportion of cases. The areas in the body where these eschars were seen suggest that the mite clings to tight clothing in these areas as observed in other studies [26]. The unique observation of the present study is burning micturition seen in a small proportion of cases, which needs further clinical evaluation. Some clinical symptoms were more common in children than in adults indicating that the clinical course could be different in different age groups. Co-infections that were seen in the study like Dengue, Malaria and Chikungunya can be expected, as the vectors of these diseases also breed in high densities during the post-monsoon season, increasing the possibilities of coinfections. Mortality, which was observed in three cases, could be due to the organism gaining virulence due to extremes of age, or due to underlying disorders like cirrhosis of liver, which was seen in one case who died.
Scrub typhus is emerging as a major public health problem in India with reports of its occurrence from several wide geographic areas. But, the information on the occurrence of genotypes/prototypes is sketchy. In the present study, genotyping based on 56kDa gene, showed that majority of the study isolates clustered into two groups viz., the Karp prototype and an independent group; probably a new variant. A few belonged to minor independent groups; one sequence (SS6) distinctly branched with isolates from Japan, and another sequence (SS271) with a Thailand isolate. Varghese et al. reported the occurrence of two prototypes viz., Kato and Karp from South India, similar to the former group in the present study [27]. Another study, reported by the same group, genotyped samples from 3 scrub typhus endemic geographic regions viz., in southern, northern and northeastern India and found Kato, Karp, Gilliam, Ikeda and also Neimeng-65 genotype like strains in the South and Northeast regions [28]. These findings indicate that all the prototypes and several genotypes are prevalent in different regions of India. However, in the present study Karp prototype and a new variant genotype were found in addition to other genotypes that have been reported from other endemic areas in South Eastern Asia.
It may be noted that clinical manifestations vary with the genotypes of O. tsutsugamushi. Earlier studies have shown that patients infected with Boryong strain had significantly more generalized weakness, eschar, skin rash, conjunctival congestion, high albumin levels, elevated ESR and fibrinogen levels compared to patients infected with the Karp strain. Response to treatment was also significantly slower in the Karp group as compared to Boryong group [10]. In an experiment done in BALB/c mice using O. tsutsugamushi isolates, remarkably different fatality rates were observed between Boryong strains and it was concluded, that fatality rates due to these strains, correlated with differences in both serotypes and virulence genes. In another study in BALB/c mice, Karp strain was found to multiply intraperitoneally within macrophages and kill the animal, whereas Kuroki strain invaded macrophages but were eliminated from the cells, allowing mouse survival [29,30]. The clinical presentations of the genotypic groups identified in the present study varied considerably and the group which formed an independent cluster along with Taiwan and Thailand isolates presented a broad range of clinical symptoms which ranged from mild features like headache, cough, abdominal pain and vomiting to more severe manifestations like hypotension and hepatitis. Two new genotypes have been reported from Himalayan region of India in an earlier study [31,32]. Yang et al. reported 4 prototype/genotype strains (Karp, Kato, Kawasaki and Gilliam) and eleven different Taiwanese genotypes (Taiwan-A, B, C, D, E, G, H, J, N, O and P) based on RFLP analysis of the 56-kDa type-specific antigen (TSA) gene [33]. Taiwan-H was the major genotype in eastern Taiwan and it was designated as the Taiwan Gilliam-variant (TG-v) cluster. Further, there are now evidences to show that recombinant strains of this bacterium are emerging [10,28,29].
One of the limitations of the study was that, only patients presenting to only one tertiary care hospital were included. Similar cases presenting to neighbouring hospitals could not be enrolled in the study due to logistic reasons. Nevertheless the findings of the present study suggest that there is a great genetic diversity and hence strain diversity among O.tsutsugamushi within and between different scrub typhus endemic regions. A wider and complete networking of hospitals to identify cases and the genotypes infecting amongst them, would give a more comprehensive view of the predominant genotypes. Genotypic variations will greatly impact the diagnosis of scrub typhus as the 56 kDa gene of O. tsutsugamushi and the protein coded by it are targets for PCR and immunological assays. These variations could be the reason for variation in the sensitivity of the 56 kDa protein based ELISA reported by different workers [34-37]. Similarly, development of vaccine for scrub typhus is beset with problems of polymorphism among O. tsutsugamushi strains. Hence, it is worthwhile to study the genetic diversity of O. tsutsugamushi in different regions and countries where the disease is endemic.
Conclusions
Information on the clinical presentations due to infection with different genotypes of O. tsutsugamushi is scanty. Scrub typhus is endemic in several geographic regions of India and the present study suggests that there is a great genetic diversity among O. tsutsugamushi strains within and between different scrub typhus endemic regions of Puducherry and surrounding Tamil Nadu areas. The occurrence of genotypes with varying clinical features such as that found in the present study and which overlap with other diseases is an alarming situation, which poses a major challenge to clinicians in the treatment/ management of scrub typhus cases. Genetic variability of O. tsutsugamushi will also impact the development and use of diagnostics and vaccine research. An understanding of the distribution of O. tsutsugamushi genotypes and their association with clinical variations will help in accurate diagnosis and treatment of the disease, respectively. Studies on global genetic/strain variation of O. tsutsugamushi need to be taken up to facilitate development of appropriate diagnostics and vaccines for scrub typhus.
Acknowledgements
This study was supported by the Indian Council of Medical Research in the form of intra mural research funding. The authors thank Mr. Hari Kishan Raju, Technical Assistant, VCRC for his help in drafting the map depicting distribution of Scrub typhus.
References
- Seong SY, Choi MS, Kim IS (2001) Orientiatsutsugamushi infection: overview and immune responses. Microbes Infect 3:11-21.
- Brown GW, Robinson DM, Huxsoll DL, Ng TS, Lim KJ (1976)Scrub typhus: a common cause of illness in indigenous populations. Trans R Soc Trop Med Hyg 70:444-448.
- Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, et al. (2010) Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J ClinMicrobiol48:4404-4409.
- Maina AN, Farris CM, Odhiambo A, Jiang J, Laktabai J, et al. (2016) Q Fever, Scrub Typhus, and Rickettsial Diseases in Children, Kenya, 2011-2012. Emerg Infect Dis 22:883-886.
- Balcells ME, Rabagliati R, Garcia P, Poggi H, Oddo D, et al. (2011) Endemic scrub typhus-like illness, Chile. Emerg Infect Dis 17: 1659-1663.
- Chogle AR (2010) Diagnosis and treatment of scrub typhus--the Indian scenario. J Assoc Physicians India58:11-12.
- Mathai E, Lloyd G, Cherian T, Abraham OC, Cherian AM (2001) Serological evidence for the continued presence of human rickettsioses in southern India. Ann Trop Med Parasitol95:395-398.
- Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, et al. (2010) Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India 58:24-28.
- Ree HI, Kim TE, Lee IY, Jeon SH, Hwang UW, et al. (2001)Determination and geographical distribution of Orientiatsutsugamushi serotypes in Korea by nested polymerase chain reaction. Am J Trop Med Hyg65:528-534.
- Kim DM, Yun NR, Neupane GP, Shin SH, Ryu SY, et al. (2011) Differences in clinical features according to Boryoung and Karp genotypes of Orientiatsutsugamushi. PLoS One 6:e22731.
- Lee YM, Kim DM, Lee SH, Jang MS, Neupane GP (2011) Phylogenetic analysis of the 56 kDa protein genes of Orientiatsutsugamushi in Southwest Area of Korea. Am J Trop Med Hyg84:250-254.
- Browning JS, Raphael M, Klein EF, Coblenz A (1945) Scrub typhus. Amer J Trop Med 25:481-492.
- Groves MG, Osterman JV(1978) Host defenses in experimental scrub typhus: genetics of natural resistance to infection.Infect Immun19: 583-588.
- Nagano I, Kasuya S, Noda N, Yamashita T(1996)Virulence in mice of Orientiatsutsugamushi isolated from patients in a new endemic area in Japan. MicrobiolImmunol40:743-747.
- Silpapojakul K, Varachit B, Silpapojakul K (2004) Paediatric scrub typhus in Thailand: a study of 73 confirmed cases. Trans R Soc Trop Med Hyg98:354-359.
- Prabhagaravarthanan R, Harish BN, Parija SC(2008) Typhus fever in Pondicherry. J Comm Dis 40:159-160.
- Prakash JA, Kavitha ML, Mathai E(2011) Nested polymerase chain reaction on blood clots for gene encoding 56 kDa antigen and serology for the diagnosis of scrub typhus. Indian J Med Microbiol 29:47-50.
- Furuya Y, Yoshida Y, Katayama T, Yamamoto S, Kawamura (1993) Serotype-specific Amplification of Rickettsia tsutsugamushi DNA by nested polymerase chain reaction. JClinMicrobiol 31: 1637-1640.
- Saisongkorh W, Chenchittikul M, Silpapojakul K (2004) Evaluation of nested PCR for the diagnosis of scrub typhus among patients with acute pyrexia of unknown origin. Trans R Soc Trop Med Hyg 98:360-366.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular EvolutionaryGenetics Analysis version 6.0. MolBiolEvol 30: 2725-2729.
- Fauci, Anthony S, Braunwald, Eugene, Kasper (2009) Harrison's Principles of Internal Medicine, 17th Edn, (eBook), McGraw-Hills.
- Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, et al. (2003) Outbreak of Scrub typhus in southern India during the cooler months. Ann N Y AcadSci990: 359-364.
- Wardrop NA, Kuo CC, Wang HC, Clements AC, Lee PF, et al. (2013) Bayesian Spatial modeling and the significance of agricultural land use to scrub typhus infection in Taiwan. Geospat Health 8: 229-239.
- Domrow R, Cook I (1967) Recent studies of the epidemiology of scrub typhus in North Queensland. Act Med Biol (Niigata) 15: 43-48.
- Areendran G, Rao P, Raj K, Sahu L (2010) Vegetation types of the endangered Eastern Ghats mountain ecosystem–A Remote Sensing perspective,10th International Symposium on High Mountain Remote Sensing Cartography 63-72.
- Gentry JW, Yueh CS, Wah PO (1963) Preliminary observations on Leptotrombidium (Leptotrombidium) akamushi and Leptotrombidium (Leptotrombidium) deliensis in their natural habitat in Malaya: (Acarins: Trombiculidae). Am J Hyg78: 181- 190.
- Varghese GM, Janardhanan J, Trowbridge P, Peter JV, Prakash JA, et al. (2013) Scrub typhus in South India: clinical and Laboratory manifestations, genetic variability, and outcome. Int J Infect Dis 17: 981-987.
- Varghese GM, Janardhanan J, Mahajan SK, Tariang D, Trowbridge P, et al. (2015) Molecular epidemiology and genetic diversity of Orientiatsutsugamushi from patients with scrub typhus in 3 regions of India. Emerg Infect Dis 21:64-69.
- Chu H, Park SH, Kim EJ, Hwang KJ, Shim SK (2010) Phylogenetic clustering of 4 prevalent virulence genes in Orientiatsutsugamushi isolates from human patients. J Microbiol 48:124-128.
- Fukuhara M, Fukazawa M, Tamura A, Nakamura T, Urakami H (2005) Survival of two Orientiatsutsugamushi bacterial strains that infect mouse macrophages with varying degrees of virulence. MicrobPathog 39: 177-187.
- Mahajan SK, Rolain JM, Kashyap R, Bakshi D, Sharma V, et al. (2006)Scrub typhus in Himalayas. Emerg Infect Dis 12:1590-1592.
- Bakshi D, Singhal P, Mahajan SK, Subramaniam P, Tuteja U, et al. (2007) Development of a real- time PCR assay for the diagnosis of scrub typhus cases in India and evidence of the prevalence of new genotype of O. tsutsugamushi. Acta Trop 104:63-71.
- Yang HH, Huang IT, Lin CH, Chen TY, Chen LK(2012) New genotypes of Orientiatsutsugamushi isolated from humans in Eastern Taiwan. PLoS One 7: e46997.
- Blacksell SD, Tanganuchitcharnchai A, Nawtaisong P, Kantipong P, Laongnualpanich A, et al.(2015) Diagnostic Accuracy of the InBios Scrub Typhus Detect Enzyme-Linked Immunoassay for the Detection of IgM Antibodies in Northern Thailand. Clin Vaccine Immunol 23:148-154.
- Prakash JAJ, Abraham OC, Mathai E (2006) Evaluation of tests for serological diagnosis of scrub typhus. Trop Doctor 36: 212-213.
- Isaac R, Varghese GM, Mathai E, Manjula J, Joseph I (2004) Clinical Infectious Diseases 39: 1395-1396.
- Gupta N, Chaudhry R, Thakur CK (2016) Determination of cutoff of ELISA and immunofluorescence assay for scrub typhus. J Global Infect Dis 8: 97-99.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 5314
- [From(publication date):
March-2017 - Apr 16, 2025] - Breakdown by view type
- HTML page views : 4343
- PDF downloads : 971