Observed Effects of Acidity and Basicity on Clam Shell Composition
Received: 03-Apr-2023 / Manuscript No. jee-23-94286 / Editor assigned: 05-Apr-2023 / PreQC No. jee-23-94286 (PQ) / Reviewed: 19-Apr-2023 / QC No. jee-23-94286 / Revised: 21-Apr-2023 / Manuscript No. jee-23-94286 (R) / Published Date: 28-Apr-2023 DOI: 10.4172/2157-7625.1000391
Abstract
Air pollution has effectively increased the acidity of marine and freshwater ecosystems by yielding increased amounts of greenhouse gases like carbon dioxide or nitrogen oxide, which can react with water to form acids. Acidification of surrounding aqueous environment can effectively corrode the calcareous shells of bivalves by protonating dissociated carbonate ions (CO3 2-) to increase the solubility of the constituent calcium carbonate (CaCO3). In this study, Asian clam (Corbicula fluminea) aragonitic shells were subjected to aqueous solutions of different pH for eight days. Solubility of aragonite (CaCO3) from the shell of each environment was measured by titration, and hardness was measured before and after the shells were exposed to their respective environments. Results for this study conflicted with formulated hypotheses based on contemporary chemical models. It was hypothesized that only acidic conditions would change shell aragonite solubility by protonating carbonate ions to increase solubility, but the collected data suggest that the solubility only changed (decreased) in basic conditions.
Introduction
Due to a higher frequency of air pollution in recent times, aquatic ecosystems have been subjected to increased acidity due to the condensation of acid rain [5]. Atmospheric carbon dioxide (CO2) molecules may spontaneously react with water molecules to yield carbonic acid (H2CO3) [8].
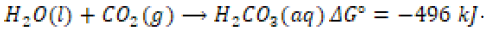
Other atmospheric gases like nitrogen monoxide (NO) and sulfur
oxide (SO) may also react with water molecules to produce nitric acid
(HNO3) and sulfuric acid (H2SO4) respectively [5]. A relatively high
abundance of carbonic acid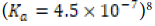 and the formation
of strong acids like nitric acid and sulfuric acid would suggest that
any aqueous mixture subjected to these acids would experience an
effective increase in hydronium concentration (and thus a decrease in
pH). It should be noted that prior to any sort of air pollution, there
was acidification of aquatic bodies due to the presence of such gases as
carbon dioxide, nitrogen oxide, and sulfur dioxide in the atmosphere;
with the rise of greenhouse gas emissions, there has thus been greater
amounts of acidification in the ocean and other aquatic bodies.
Significant changes in the acidity of aquatic ecosystems may negatively
impact the health of native species physiologically adapted to a narrow
pH range. Bivalves like the Asian clam (Corbicula fluminea) are among
the organisms to be negatively impacted by the acid rain deposition.
In addition to its impacts on bivalve physiological properties, acidic
conditions are expected to effectively corrode clam shells. Clam shells
are primarily composed of calcium carbonate (CaCO3),
an insoluble
salt in standard conditions [1, 3, 4].
and the formation
of strong acids like nitric acid and sulfuric acid would suggest that
any aqueous mixture subjected to these acids would experience an
effective increase in hydronium concentration (and thus a decrease in
pH). It should be noted that prior to any sort of air pollution, there
was acidification of aquatic bodies due to the presence of such gases as
carbon dioxide, nitrogen oxide, and sulfur dioxide in the atmosphere;
with the rise of greenhouse gas emissions, there has thus been greater
amounts of acidification in the ocean and other aquatic bodies.
Significant changes in the acidity of aquatic ecosystems may negatively
impact the health of native species physiologically adapted to a narrow
pH range. Bivalves like the Asian clam (Corbicula fluminea) are among
the organisms to be negatively impacted by the acid rain deposition.
In addition to its impacts on bivalve physiological properties, acidic
conditions are expected to effectively corrode clam shells. Clam shells
are primarily composed of calcium carbonate (CaCO3),
an insoluble
salt in standard conditions [1, 3, 4].
Bivalve shells are reported to be composed of 95% calcium carbonate (CaCO3) by weight; the same report states that calcareous (calcium carbonate-rich) shells have hardness that is about 3000 times that of their pure respective calcium carbonate polymorph [1]. It should be noted that the other 5% of bivalve shell mass primarily consists of proteins and carbohydrates [1]. Depending upon their environmental conditions, bivalve shells may be primarily composed of either calcite or aragonite, two anhydrous polymorphs of calcium carbonate most likely to be incorporated into mollusk shells [1]. At lower pressures and higher temperatures, calcite is the more stable polymorph characterized by its trigonal structure and block-like crystals [6]. The denser aragonite has an orthorhombic conformation and needle-like crystals that are spontaneously formed at higher pressures. 6 In aquatic environments inhabited by bivalves, the formation shells based on either calcite or aragonite depends upon the contents of the surrounding aqueous mixture [7]. Aqueous environments rich in magnesium cation (Mg2+) specifically promote the formation of aragonite-based shells from dissociated calcium carbonate.7 Mg2+ is reported to slow down calcite growth and incorporate itself into calcite structure while aragonitic shell formation is not affected by Mg2+ in aqueous mixture [7].
Even though calcium carbonate is considered “insoluble” in aqueous solution, pure calcium carbonate in crystal lattice form will experience an equilibrium reaction in aqueous solution in which some of the ions will leech off and reattach to the lattice structure:
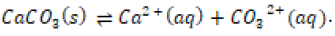
The solubility product constant (Ksp) of calcium carbonate in
aqueous solution at 25°C is 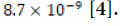 [4]. Thus calcium carbonate in
aqueous solution has a solubility of
[4]. Thus calcium carbonate in
aqueous solution has a solubility of 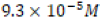 in such conditions.
Solubility of ionic solids in aqueous solutions can be directly affected
and manipulated by changes in pH or common ion concentration with
the addition of species that can induce such changes. In an aqueous
environment experiencing increases in acidity, hydronium ions (H3O+)
are expected to react with carbonate ions (CO3 -) from the clam shell to
form bicarbonate ion or carbonic acid by protonation:
in such conditions.
Solubility of ionic solids in aqueous solutions can be directly affected
and manipulated by changes in pH or common ion concentration with
the addition of species that can induce such changes. In an aqueous
environment experiencing increases in acidity, hydronium ions (H3O+)
are expected to react with carbonate ions (CO3 -) from the clam shell to
form bicarbonate ion or carbonic acid by protonation:
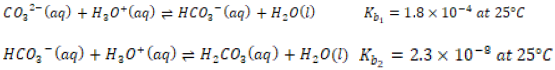
Note that the reversible chemical equations above are occurring in aqueous solvent [8].
In this study, effects of acidity or basicity aqueous solutions on C. fluminea shell composition were observed. Asian clam shells were subjected to aqueous environments of either pH 3.18, pH 7.00 or pOH 3.18 at about 20°C. Clam shell hardness, a measure of durability, was measured before and after eight days of exposure to aqueous environment. Calcium carbonate concentration (or “water hardness”) of the surrounding solution was measured after eight days to detect whether significant amounts of calcium carbonate had left the clam shell specimens. It was hypothesized that the clam shell hardness would decrease in the acidic environment, because bicarbonate ( HCO3-) ion and carbonate ion (CO32- ) are weak bases that can be protonated by hydronium (HCO3+ ), a very strong acid in aqueous solution. Basic environment is not expected to affect the clam shell hardness; because calcium hydroxide could not be formed from dissociated Ca2+ ions from the clam shell as calcium hydroxide is a strong base that spontaneously dissolves in aqueous solution.
Procedure and Materials
In this experiment, C. fluminea clam shells were subjected to different
aqueous solutions and observed for changes in composition. Clam
shells used in this experiment would be measured for their hardness,
a measure of how sturdy the shells are, before and after exposure to the
aqueous solutions. After eight days in aqueous solution, each clam shell
would be measured again for hardness, and a sample of their aqueous
solution would be titrated. In preparation for this experiment, materials
gathered include Corbicula fluminea clam shells, 250-mL beakers, a
durometer, a 50-mL buret, a buret clamp, a ring stand, funnel, 100-mL
Erlenmeyer flasks, stir plates, stir bar, 25-mL volumetric pipet, 5-mL
serological pipet, distilled water, 0.1-M calcium chloride aqueous
solution, 0.03-M magnesium chloride aqueous solution, Eriochrome
black T indicator, 0.01-M EDTA solution, and river water collected from
the American River. Three aqueous environments were prepared for
each of the clam shells. One was a control environment only including
250 milliliters of deionized water. An “acidic” environment (pH 3.18)
was prepared using a 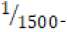 dilution of 1.0 M hydrochloric acid
aqueous solution to yield 250 milliliters of
dilution of 1.0 M hydrochloric acid
aqueous solution to yield 250 milliliters of 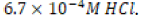 “basic”
environment (pOH 3.18 or pH 10.72) was prepared by similar means
using a
“basic”
environment (pOH 3.18 or pH 10.72) was prepared by similar means
using a 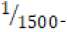 -dilution of 1.0 M sodium hydroxide aqueous solution to yield 250 milliliters of
-dilution of 1.0 M sodium hydroxide aqueous solution to yield 250 milliliters of 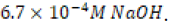
The acidic environment first created was initially 250 milliliters of 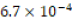 M hydrochloric acid (HCl), or one-six thousandth of a mole
of hydrochloric acid in one quarter of a liter of aqueous solution. A
-dilution of the original 1.0-M HCl stock solution was conducted
by adding 6.7 mL of stock solution to 93.3 mL of deionized water,
effectively producing 100. ML of
M hydrochloric acid (HCl), or one-six thousandth of a mole
of hydrochloric acid in one quarter of a liter of aqueous solution. A
-dilution of the original 1.0-M HCl stock solution was conducted
by adding 6.7 mL of stock solution to 93.3 mL of deionized water,
effectively producing 100. ML of 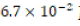 HCl. After the new dilute
solution was prepared, it was mixed with a stir rod. 25.0 milliliters of
this dilution was soon transferred to a 250-mL glass container, and an
additional 225 mL of deionized water was also added to the container.
The container was sealed and then shaken to homogenize the mixture.
This 250-mL solution then served as the acidic environment of pH 3.18.
A nearly identical series of steps was taken to prepare the basic solution
HCl. After the new dilute
solution was prepared, it was mixed with a stir rod. 25.0 milliliters of
this dilution was soon transferred to a 250-mL glass container, and an
additional 225 mL of deionized water was also added to the container.
The container was sealed and then shaken to homogenize the mixture.
This 250-mL solution then served as the acidic environment of pH 3.18.
A nearly identical series of steps was taken to prepare the basic solution 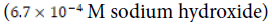 of pH 10.72. The control environment
was prepared simply by adding 250. mL of deionized water to an empty
250-mL container.
of pH 10.72. The control environment
was prepared simply by adding 250. mL of deionized water to an empty
250-mL container.
Clam shells were measured for hardness throughout the duration of this experiment using a durometer that reported using the D scale. Hardness was measured at the most superficial, medial, and somewhat dorsal region of clam shell periostracum by placing the point of the durometer perpendicular to a flat surface that the clam shell rested upon. Clam shell hardness measurement were taken before the clam shells were submerged in their aqueous solution and eight days after exposure to their surrounding solution to account for confounding variables. Clam shells were set in aqueous solution with the superficial, rough side faced upwards, like the superior shell of a live clam.
Samples of the environments containing clam shells were measured for their respective calcium carbonate (CaCO3) concentrations after eight days. A hard water titration was performed using Eriochrome Black T indicator. 0.03-M magnesium chloride (MgCl2) solution and pH 10 buffer solution were also used to activate the Eriochrome Black T indicator and maintain a stable pH respectively. 25 milliliters of the specific solution of interest were added to a 100-mL Erlenmeyer flask with a stir rod. 15 drops of 0.03 M-magnesium chloride and 3 drops of Eriochrome Black T indicator were added to the analyte. The analyte was placed upon a stir plate at 300 RPM throughout the duration of the titration. 0.01-M EDTA solution was used as the titrant and placed into a 50-mL buret position above the Erlenmeyer flask containing analyte solution. Eriochrome Black T indicator made analyte solution a “wine red” color2 at the beginning of the experiment. Addition of titrant was terminated when the analyte solution became a “sky blue” color.1 Stoichiometric calculations were conducted to determine the original calcium carbonate concentration of analyte before titration preparations, and the “Water hardness” was measured using a scale as provided by the CHS First Protocol (Table 1) [2]. Given that there was about eight days for each shell-solution system to achieve chemical equilibria for different reactions, calcium carbonate concentration was thus used as an approximation of the calcium carbonate solubility of our clam shells. The solubility product constant of shell calcium carbonate in each aqueous environment were obtained using the derived approximation for solubility.
| Calcium carbonate concentration (parts of per million) | Designation |
|---|---|
| Less than 43 ppm | Soft |
| 43‒150 ppm | Slightly hard |
| 150‒300 ppm | Moderately hard |
| 300‒450 ppm | Hard |
| Greater than 450 ppm | Very hard |
Table 1: Water hardness: Calcium carbonate concentration in aqueous solution (Adapted from CHS First Hard Water Titration Protocol2) .
Data/Results
In this study, only one C. fluminea clam shell was used per prepared aqueous environment due to environmental regulations of American River. Generally, no significant differences were observed the aqueous mixtures or clam shells when only comparing between the acidic and control environments. With regards to both qualitative and quantitative results, only the clam subjected to basic conditions was observed to have significant deviations from the control clam. Table 2 provides an overview of the contents of each prepared aqueous environment before addition of the respective clam shell. Figure 1 depicts an image of three harvested Asian clam shell specimens neither subjected to any prepared aqueous conditions nor used in this experiment. In (Figure 2), there was an observable color change to a slight red or burgundy hue in the periostracum layer (the superficial, rough layerCITE) of the clam shell subjected to the basic conditions for eight days at about 22°C; the clam shells subjected to the acidic or control conditions retained their original yellow hue after exposure to their respective environments for eight days. The same durometer was used to measure shell hardness for each shell before and after the duration of exposure to the appropriate environment. In contrast to expectations, an increase in measured hardness was observed among all three clam shells (Table 3) the clam shell in basic solution experienced the smallest increase in measured hardness.
| Clam shell environment | Concentration of Solute | pH of environment |
|---|---|---|
Control (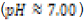 ) ) |
N/A | 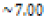 |
Acidic (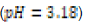 ) ) |
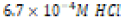 |
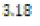 |
Basic (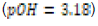 ) ) |
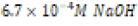 |
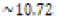 |
Table 2: Contents of each aqueous environment of differing acidity or basicity.
| Clam shell environment | Initial Hardness value (D scale) |
Hardness value after eight days of exposure (D scale) |
|---|---|---|
Control (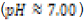 ) ) |
3.5 | 5.0 |
Acidic (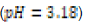 ) ) |
3.5 | 5.0 |
Basic (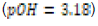 ) ) |
3.5 | 4.5 |
Table 3: Clam shell hardness before and after exposure to respective environment for eight days.
The solubility product constant of Asian clam shell aragonite in each environmental condition was estimated for the aragonite of each clam shell. Concentrations of calcium cation (Ca2+) were determined by use of titration results and stoichiometric conversions. Ca2+ ion concentrations and the derived solubility product constants (Ksp) are observable in (Table 4). The clam shells in control or acidic conditions had aragonite solubilities that were approximately equal, but the shell in basic conditions was observed to have an aragonite solubility about one half of that of the shell in control conditions. Note that “calcareous hardness” is simply a measure of how rich a tested aqueous solution is in calcium carbonate.
| Clam shell environment | Calcium concentrations based on titration results | Calcareous hardness of aqueous environment | Derived solubility product constant (Ksp) |
|---|---|---|---|
Control (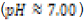 ) ) |
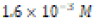 |
Soft (1.2 ppm) | 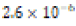 |
Acidic (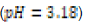 ) ) |
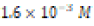 |
Soft (1.2 ppm) | 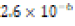 |
Basic (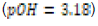 ) ) |
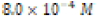 |
Soft (0.58 ppm) | 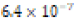 |
Table 4: Calcium ion concentrations in aqueous environments exposure to clam shell for eight days and derived solubility product constants (Ksp) of Asian clam shell aragonite (CaCO3) in such conditions.
Discussion
The objective of this study was to observe Asian clam shell hardness and aragonite solubility to use such measurements to quantify shell corrosion. While quantitative data of interest was obtained for this experiment, the validity of these quantitative data should be inspected with great caution. Due to concerns of ethical treatment and limited resources, only one trial was performed for each clam shell in aqueous solution. An ideal sample for each condition would contain a quantity far exceeding fifty and no more than ten percent of the entire population of interest. Our sample were limited to, one and thus quantitative analyses would yield P-values too large to be of statistical significance.
Hardness measurements were observed to increase across the shells of all three conditions. Unless the bio macromolecules comprising about 5% of clam shell mass were hydrated by surrounding solvent, solute of the respective aqueous solution, hydronium, or hydroxide, no increase in clam shell composition should have been expected. Contemporary chemical models suggest8 that the sodium cations from the basic environment would not interact with the aragonite (CaCO3) of the shell or dissociated constituent ions, because Na+ is a relatively stable chemical species in aqueous solution derived from strong base NaOH. Likewise, the chloride ions (Cl-) in the acidic environment is not expected to interact with the aragonite of the clam shell, because Cl- is a relatively stable ion in solution derived from strong acid HCl.
It was hypothesized that the solubility of clam shells would increase
in acidic solution and remains the same in basic conditions. Dissociated
constituent ions from calcium carbonate would be present in a low
concentration at standard ambient temperature and pressure (25°C
and 1 atm respectively) because calcium carbonate is reported to have
a Ksp of 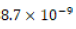 at 25°C in pure water solvent and thus a solubility
of
at 25°C in pure water solvent and thus a solubility
of 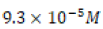 in those same conditions.8 Hard water titration data
suggested different interpretations than was hypothesized: The
estimated solubility of shell aragonite in the prepared basic conditions
was less than the estimated solubility of shell aragonite in pure water
solvent (control conditions). Meanwhile, the estimated solubility of
shell aragonite in acidic conditions was about the same as that of the
control condition. Reported literature values for the solubility product
constant of “calcium carbonate” (CaCO3) are most likely based on
results using calcite, the less dense and more stable polymorph of
calcium carbonate at standard ambient temperature and pressure. The
estimated solubility product constant for the control clam aragonite
in those same conditions.8 Hard water titration data
suggested different interpretations than was hypothesized: The
estimated solubility of shell aragonite in the prepared basic conditions
was less than the estimated solubility of shell aragonite in pure water
solvent (control conditions). Meanwhile, the estimated solubility of
shell aragonite in acidic conditions was about the same as that of the
control condition. Reported literature values for the solubility product
constant of “calcium carbonate” (CaCO3) are most likely based on
results using calcite, the less dense and more stable polymorph of
calcium carbonate at standard ambient temperature and pressure. The
estimated solubility product constant for the control clam aragonite 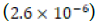 was not consistent with the literature Ksp value of
was not consistent with the literature Ksp value of 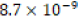 for “calcium carbonate’’ [8].
for “calcium carbonate’’ [8].
Further research is needed to better determine differences in the solubility of calcium carbonate in calcareous shells composed of aragonite or calcite. In pure aqueous solvent, a sample of pure aragonite is expected to spontaneously change to calcite. Because calcareous mollusk shells are reported to have substantially greater hardness than their respective pure anhydrous calcium carbonate polymorph, [1] it is likely that the proteins and carbohydrates in such shells give chemical and physical properties different from their pure polymorph.
The lone clam shell subjected to 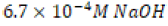 solution for
eight days experienced a change in color from a yellow hue to a red or
burgundy hue. This color change suggests that there was a chemical
reaction between the sodium hydroxide in solution. Additional research is needed to understand what bio macromolecules are incorporated in
mollusk shells and why sodium hydroxide may have induced such a
change in color.
solution for
eight days experienced a change in color from a yellow hue to a red or
burgundy hue. This color change suggests that there was a chemical
reaction between the sodium hydroxide in solution. Additional research is needed to understand what bio macromolecules are incorporated in
mollusk shells and why sodium hydroxide may have induced such a
change in color.
Conclusion
In this study, three 250-mL aqueous solutions of different pH values (3.18, ~7.00, and ~10.72 respectively) were created to mimic possible acidification or increases in basicity of aquatic ecosystems. Asian clam (Corbicula fluminea) shells were subjected to each environment for eight days to observe possible corrosion and effects on clam shell composition. Decreased shell hardness and increased calcium carbonate solubility were used as measures of corrosion. Clam shell calcium carbonate (CaCO3) solubility in pOH 3.18 solution was observed to decrease with respect to the control; shell CaCO3 solubility in pH 3.18 solution was not observed to be different from that of the control. Measured hardness values were observed to increase. A color change was observed in the periostracum layer of the shell subject to basic conditions, suggesting there was a chemical reaction between sodium hydroxide and shell components. Further research that explores the solubility of aragonite and calcite in clam shells would be useful to better quantify the effects of marine and freshwater ecosystem acidification on shell composition and hardness.
References
- Agbaje OBA, Shir IB, Zax DB, Schmidt A (2018) Bio macromolecules within Bivalve Shells: Is Chitin Abundant? Acta Biomaterialia 80: 176-186.
- California Northstate University: College of Health Sciences. CHS First: Hard Water Titration.
- Immel F, Broussard C, Catherinet B, Plasseraud L, Alcaraz G, et al.(2006) The Shell of the Invasive Bivalve Species Dreissena polymorpha. Biochemical, Elemental and Textural Investigations. PLoS One 11: 2-28.
- Kadar E, Lobo-da-Cunha A, Azevedo C (2009) Mantle-to-shell CaCO3 Transfer During Shell Repair at Different Hydrostatic Pressures in the Deep-sea Vent Mussel Bathymodiolus azoricus (Bivalvia: Mytilidae). Marine Bio 156: 959-967.
- Orr JC, Fabry VJ, Aumont O (2005) Anthropogenic Ocean Acidification Over the Twenty-First Century and Its Impact On Calcifying Organisms. Nature 473: 681-686.
- Sevcik R, Sasek P, Viani A (2018) Physical and Nanomechanical Properties of the Synthetic Anhydrous Crystalline CaCO3 Polymorphs Vaterite, Aragonite and Calcite. J Mater Sci 3: 4022-4033.
- Spann N, Harper E M, Aldridge DC (2010) The Unusual Mineral Vaterite in Shells of Freshwater Bivalve Corbicula fluminea from the UK.. Naturwissenschaften 97: 743-751.
- Zumdahl S, Zumdahl S (2012) Chapter 15: Solubility and Complex Ion Equilibria. Chemistry: An Atoms First Approach. 2nd Ed; Cengage Learning Boston MA 212: 972.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Talebzadeh A (2023) Observed Effects of Acidity and Basicity on Clam Shell Composition. J Ecosys Ecograph 13: 391. DOI: 10.4172/2157-7625.1000391
Copyright: © 2023 Talebzadeh A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2962
- [From(publication date): 0-2023 - Dec 13, 2025]
- Breakdown by view type
- HTML page views: 2561
- PDF downloads: 401


