Nutritional Management During and Beyond Diarrhea. Role of Rice-Based Foods
Received: 01-Sep-2022 / Manuscript No. nnp-22-73929 / Editor assigned: 03-Sep-2022 / PreQC No. nnp-22-73929(PQ) / Reviewed: 19-Sep-2022 / QC No. nnp-22-73929 / Revised: 24-Sep-2022 / Manuscript No. nnp-22-73929(R) / Accepted Date: 24-Sep-2022 / Published Date: 29-Sep-2022 DOI: 10.4172/2572-4983.1000263 QI No. / nnp-22-73929
Abstract
Diarrhea is the second leading cause of mortality. Diarrhea and malnutrition form a vicious cycle culminating in faltering growth, compromised gut immunity, delayed catch-up growth, and cognitive impairment. The physiological events associated with Diarrhea eventually warrant specific nutritional interventions beyond rehydration. The World Health Organization guidelines for selecting appropriate foods suggest using staples such as rice, which can be easily digested. This review outlines the role and benefits of a rice-based diet during and beyond diarrhea. In addition, we also highlight the importance of fortified rice-based cereals in reducing the risk of micronutrient deficiencies during the critical period of complementary feeding.
Keywords
Rice-based fortified food; Rice ORS; Digestibility, Micronutrients; Rice-based nutrition; Rice-based diet
Introduction
The Millenium Development Goal-4 (MDG-4) was to reduce the mortality in children less than five years by two-thirds between 1990 and 2015. [1] India’s under-five mortalities declined from 125 per 1,000 live births in 1990 to 43.5 per 1,000 live births in 2015 and 34.3 per 1,000 live births in 2019. [2-3] child mortality remains a severe concern, specifically in developing countries, as the MDG-4 was not achieved globally. Diarrheal diseases are the second most cause of mortality among children following acute respiratory tract infections, especially pneumonia. [4-5] In India, the incidence of diarrhea in children aged ≤5 years has increased from 9% in 2016 to 9.5% in 2020.6 Region-specific and cohort studies also substantiate the heavy burden of diarrhea in India. Further, diarrhea is a leading cause of malnutrition in children aged ≤ 5 years [4-7].
The vicious cycle of diarrhea and malnutrition: The impact during and after diarrhea
Malnutrition and diarrhea are a double-edged sword—malnutrition predisposes children to a higher risk of diarrhea, specifically recurrent or prolonged diarrhea that might aggravate malnutrition in children. [8] Malnutrition augments the severity, duration, and incidence of diarrhea. [9] There was a 37% and 73% increase in frequency and the duration of diarrhea, respectively, owing to malnutrition. Increased diarrhea frequency and span of diarrhea ultimately doubled the burden of diarrhea in malnourished children [10].
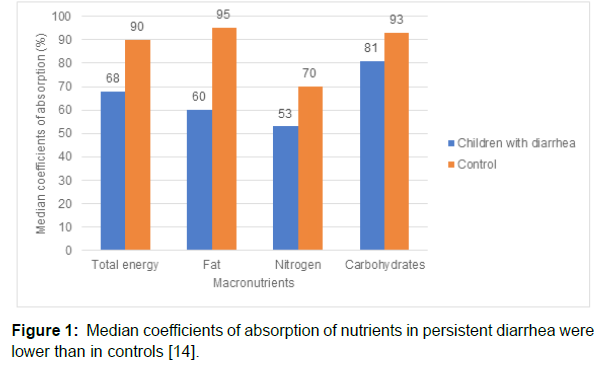
In the acute phase of diarrhea, the food intake and absorption decline by 30–40% and 10–30%, respectively, [11-12] thus setting the stage for malnutrition. In the face of sustained exposure to under nutrition, the prevailing maleficent cycle of infectious diarrhea and malnutrition leads to environmental enteropathy and hyper stimulation of the mucosal immune system. [11,13] Compared to normal children, children with persistent diarrhea showed a reduction in absorption of nutrients (Figure 1)[14].
Evidence suggests that diarrhea could induce a transient lactase deficiency due to intestinal inflammation or mucosal injury. Secondary lactose intolerance is a common complication of diarrhea in the milieu of malnutrition. As the mucosal injury heals, the lactase activity returns to normal and reverses the lactose intolerance [15-16].
A cumulative assessment of diarrhea and growth from seven longitudinal studies substantiates a 13% increase in stunting due to diarrhea. [17] Although it has been possible to establish the energy required for catch-up growth, no definitive value is pinned for the energy necessary for catch-up growth after diarrhea. On average, a twoyear- old child would need 2% of the recommended energy for growth. The catch-up growth after the diarrheal disease is estimated to be seven times the normal daily growth [12].
Consequently, a child would require 14% more energy to attain catch-up growth after the diarrheal illness. Therefore, 12% and 84% of daily protein energy are necessary for normal and catch-up growth, respectively [12].
Further evidence suggests that early childhood diarrhea and malnutrition (stunting) independently affect intellectual functioning during later childhood. Reduced food intake during acute diarrhea is associated with 55% and 85% reductions in calorie and protein intake in children. In the formidable first two years of life, the energy deprivation and diversion towards managing diarrhea could deprive the brain of the critical energy required for brain and neurological development [11].
Overall, it is evident that recurrent or persistent diarrhea has impending effects in later childhood. The intricate relationship between diarrhea and malnutrition results in nutritional deficiencies (micronutrient and macronutrient deficiencies), mucosal injury, compromised gut immunity, and growth and cognitive deficits. Hence, good nutrition before, during, and after diarrhea can interrupt the maleficent cycle of malnutrition and diarrhea [18].
Nutritional management in diarrhea
The Ministry of Health and Family Welfare, Government of India, recommends low osmolality oral rehydration salt solution (ORS), zinc, and continued feeding of energy-dense feeds in addition to breastfeeding as the nutritional strategy for managing diarrhea in infants and ORS in young children. [19] Energy-and nutrient-dense diets are recommended during convalescence to compensate for decreased food intake and nutrient/fluid loss during acute diarrhea. [20-21] the increased energy-protein requirements during acute diarrhea can be compensated by supplementing an energy-dense diet [21].
The foremost principle of nutritional intervention is not to withhold food following the episode of diarrhea. Oral or intravenous rehydration therapy is initiated to replenish water and electrolytes lost in the feces. Nevertheless, children should be offered an ageappropriate diet, regardless of the fluid used for oral rehydration or maintenance of hydration. [22] During diarrhea, breastfeeding should not be reduced or stopped. Infants must additionally receive oral rehydration to replace the fluid lost in the stool. The European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) advocates against withholding food longer than 4–6 hours after commencing rehydration. [23] Zinc supplementation as an adjunct to oral rehydration therapy (ORT) is recommended [22].
The daily energy requirement in infants and young children is usually more than the recommended energy owing to the weight loss due to diarrhea and the duration of the recovery period. [24] Hence, children should be offered food frequently but in small portions after correcting mild-to-moderate dehydration. An energy-dense and micronutrient-rich food comprising grains, eggs/meat, fruits, and vegetables should be offered. Energy intake should be increased following better tolerance.[22][25] The World Health Organization (WHO) guidelines for selecting appropriate foods suggest using staples such as rice which can be easily digested.[25] Infants above six months should be given staple food in a soft, mashed form. Tender coconut water, fresh fruit juices, and smashed plantain can also be included in the diet as a source of potassium.
Physiological considerations in the nutritional management of diarrhea
Abnormalities in food absorption during diarrhea are associated with certain pathophysiological changes that can be reversed through nutritional interventions (Table 1).The intestinal surface covered by the villi is usually renewed every three days. The renewal is prompted by diet and pancreatic secretions. The mucosal cell turnover and the production of intestinal hydrolytic enzymes are compromised during diarrhea if food is withheld or a child is subjected to starvation. Consequently, mucosal abnormalities prevail, and reintroduction of food after diarrheal episodes worsens malabsorption as well as diarrhea. [12] Early feeding accelerates mucosal repair and triggers early recovery of pancreatic function and production of brush-border hydrolytic enzymes. Thus, early feeding enables the resumption of normal digestion and improves the absorption of nutrients [25].
| Pathophysiology | Effect | Nutritional management |
|---|---|---|
| Villous atrophy | Reduction in the total absorptive surface —leading to poor absorption of nutrient | Initiate early re-feeding with micronutrient fortified food |
| Epithelial cell damage | Disaccharides deficiency, owing to impaired production of enzymes | No-milk containing foods can help overcome the initial lactase deficiency |
| Reduced intestinal concentrations of bile acids | Affects fat absorption | Provide energy-dense foods (fortified foods) |
| Intestinal permeability | Leaky gut, osmotic fluid loss | Food with low osmolality |
| Rapid transit of food through the gut | Insufficient time for digestion and absorption | Use of easy to digest, staple foods |
| Gut health beyond diarrhea | Recovery beyond diarrhea | Rice-based fortified feeds to support microbial regeneration |
Table 1: Physiological considerations in the nutritional management of diarrhea [12] [25].
Bile acids are critical for the absorption of fats. Bile acid excretion in the milieu of diarrhea is augmented, perhaps due to bacterial overgrowth in the small intestine and impaired ileal bile acid transport. Hence the low concentration of bile acids in the intestine results in fat malabsorption.12 Vegetable oil/ Ghee/ Butter (5–10 mL) may be mixed with staple food to augment the energy of the diet offered to compensate for the consequence of fat malabsorption. The rapid intestinal transit of food is associated with maldigestion and malabsorption, which can be compensated by frequently consuming easily digestible food [12].
The goal of feeding after the resolution of diarrhea is to correct malnutrition and minimize or correct any growth deficits. The nutritional deficit can be corrected by providing a regular diet with sufficient energy and other required nutrients. [25] The diet provided during diarrhea should not aggravate or irritate the gut. It should be easily digestible.
Digestibility of rice starch
In infants, the α-amylase activity in the intestine is not optimized, so starch digestion is slow. On the contrary, active maltase’s (sucrose and isomaltase) are fully developed at birth. Despite low levels of α-amylase, starch digestion appears to be sufficient in infants and also perhaps due to additional glucamylase activity of the maltase, which breaks down starch and dextrin’s directly into glucose [26].
The digestibility of starch is associated with the degree of branching and proportion of short chains in amylopectin, starch granule size, starch crystallinity, amylose content, and structural properties of starch granule-associated proteins. Rice contains Type-A starches with optimized digestibility. [27–29] among staples, the protein digestibility was highest with rice (Table 2) [30].
| Source | Mean | Digestibility relative to reference proteins |
|---|---|---|
| Rice, milled | 88 ± 4 | 93 |
| Wheat, whole | 86 ± 5 | 90 |
| Wheat endosperm (farina) | 96 ± 4 | 101 |
| Maize, whole | 85 ± 6 | 89 |
| Millet | 79 | 83 |
| Sorghum | 74 | 78 |
| Oatmeal | 86 ± 7 | 90 |
| Egg | 97 ± 3 | 100a |
| Milk | 95 ± 3 | |
| Meat, fish | 94 ± 3 |
Table 2: Calculated true digestibility by adults and children of various cereals as compared to animal protein [30].
However, it is also important to note that protein and amino acids hamper the digestion of rice starch because they form a physical barrier between starch and enzymes [31].
Role of Vitamin A, Zinc, and Selenium in diarrhea Clinical trials have supported the beneficial role of certain micronutrients such as vitamin A, zinc, and selenium in diarrhea management. One of zinc’s biological actions is maintaining the structure and function of the intestine. Experimental studies have confirmed mild villous atrophy in a zinc deficiency state. [32] Zinc deficiency led to a reduced intestinal weight with associated mucosal cell population and cell size reduction.33 Zinc deficiency also decreased brush border disaccharides’ activity besides inducing morphological changes in the intestine. [34] Further, faecal zinc loss diarrhea is also observed in acute or persistent diarrhea [18-35].
Zinc supplementation mitigates the duration and severity of acute and persistent diarrhea. On any given day, zinc supplementation lowered the probability of continuing diarrhea by 15% and 24% in children with acute or persistent diarrhea. There was a 42% reduction in treatment failure or death rate in children with persistent diarrhea [36].
Vitamin A plays a key role in the development and functioning of the visual system, immune system, reproduction, and maintenance of epithelial cell integrity. [37-38] Vitamin A’s latter effect helps maintain epithelial tissue’s physical and functional integrity against bacterial infections. [39] Vitamin A decreases the incidence and severity of diarrhea supposedly by preventing dehydration and persistence of diarrhea. In the intestine, vitamin A is linked to the production of cell glycoprotein and regulation of cell division, which enables the renewal of intestinal epithelial cells following acute enteric infections. Consequently, vitamin A also enables the absorption of water, electrolytes, and other nutrients.
Vitamin A supplementation during acute diarrhea, specifically in non-breastfed infants, is likely to alleviate the severity of diarrheal episodes and the risk of persistent diarrhea. Vitamin A significantly lowered the odds of risk of persistent diarrhea by 30% in children (1 to 5 years of age) with acute diarrhea (≤7 days) [40].
Selenium could play a role in gastrointestinal dysfunction, perhaps by mediating the adverse effect of oxidative stress in diarrhea. Selenium deficiency in the milieu of diarrhea augments oxidative stress and reduces the differentiation and proliferation of T cells.41 consequently, selenium might aid in the healing process of acute diarrhea. Further, diarrhea-induced lesions in the epithelial cells of the intestine can induce selenium deficiency [41-42].
Selenium supplementation in children (6 months to two years old) with acute watery diarrhea significantly reduced the diarrhea frequency compared with placebo. Further, the median time to recovery was lower among selenium-treated children [41].
Nutritional support beyond diarrhea
Young children should be given additional nutritious food during diarrheal episodes to prevent growth faltering and in the convalescence period for catch-up growth. Micronutrient malnutrition as a consequence of diarrhea is associated with impaired immune functioning. [43-44] Thus, infants and young children are predisposed to infections, delay in total recovery, and an increased likelihood of developing severe illness.
The gut microbiota has a critical role in developing immune and metabolic functions and growth. Altered gut microbiota is reported before, during, and after diarrheal episodes.45 Decreases in fecal bacterial diversity correlate with diarrheal frequency, duration, and severity. [45] According to the Global Enterics Multicenter Study (GEMS), moderate-to-severe diarrhea is associated with bacterial diversity and altered microbiota composition in children. [46] Hence, recurrent diarrheal disease is likely to impair the gut microbiota and affect the growth and immunity in children. Rice-based nutrition improves the growth of gut-beneficial microbiota. Therefore, for gut health beyond diarrhea, rice-based fortified foods could support microbial regeneration and restore normal functioning of the gut.
Rice-based feeds for diarrhea and beyond diarrhea
Easily digestible staple food, such as rice, is included in the WHO guidelines for selecting appropriate foods. Rice water is a conventional therapeutic food in diarrhea and is widely used as a substitute for glucose in ORS. Rice also shortens the duration of diarrheal episodes. The starch in rice delivers low glucose but higher energy density than glucose with a similar osmolality. The minor quantity of amino acids and proteins in rice helps repair diarrhea-associated mucosal damage. [47] Amylase-resistant starch is not well-digested in the small intestine but is fermented in the colon to short-chain fatty acids, which stimulate sodium and water absorption and prevent water loss [47-48].
Further, [49] rice has also been shown to inhibit intestinal secretions by inhibiting chloride channel secretion. Experimental studies have confirmed that the water secretory rates could be reduced by 45% in the jejunum and 38% in the ileum when 2.5% glucose is replaced with 2.5% rice glucose polymers. Hence, rice-based ORS is potentially more effective than glucose-based ORS in reducing the stool output and enabling greater absorption and retention of fluid and electrolytes [49].
Rice-based ORS contains long- and short-chain glucose polymers, and the latter’s osmolality is one-fourth of glucose. Thus, the benefits of rice-based ORS are related to its lower osmotic load and higher carbohydrate content.[50] conducted a meta-analysis of 13 randomized clinical trials comparing a rice oral rehydration salts solution (n=198) with a glucose-ORS in children (n=218) with infectious and noninfectious diarrhea.[50] Rice-based ORS was able to reduce the stool output by 32% (95% confidence interval [CI], 19–45%) and the rate of stool loss by 18% (95% CI, 6–30%).50 However, no such benefit was evident in the case of non-infectious diarrhea. Such a variation in the outcome could be attributed to variations in the quality of rice across geography. [47] Reported that the frequency of diarrheal episodes is related to the viscosity of the rice, which is linked to the amylose content. The amylose content of rice ranges from 3% to 25%, and the rice variety with the highest amylose content effectively reduced diarrhea. The rice variety that is usually rich in amylose content contains amylase-resistant starch.
Ragupathy randomly treated children (aged 6 months to 3 years; n=180) with acute watery diarrhea with glucose-ORS or amylaseresistant starch plus ORS.48 The study reported that by adding resistant starch to glucose-ORS, the duration of diarrhea could be significantly shortened compared with standard treatment. Compared to the standard treatment, the time to first formed stool was significantly shorter in children receiving additional amylase-resistant starch and ORS (median, 21.5 hours [95% CI, 17.26-25.74] vs.18.25 hours, [95% CI,13.09-23.41]; p=0.04). Also, the median time from enrolment into the study to the last unformed stool was shorter in children treated with amylase-resistant starch plus ORS vs. glucose-ORS (6.75 [95% CI, 4.27–9.23] vs. 12.7 [95% CI, 8.69–16.91]; p=0.03). Compared to the standard glucose-ORS group, the stool weight in the initial 24 h was lower in the group that received amylase-resistant starch plus ORS [48].
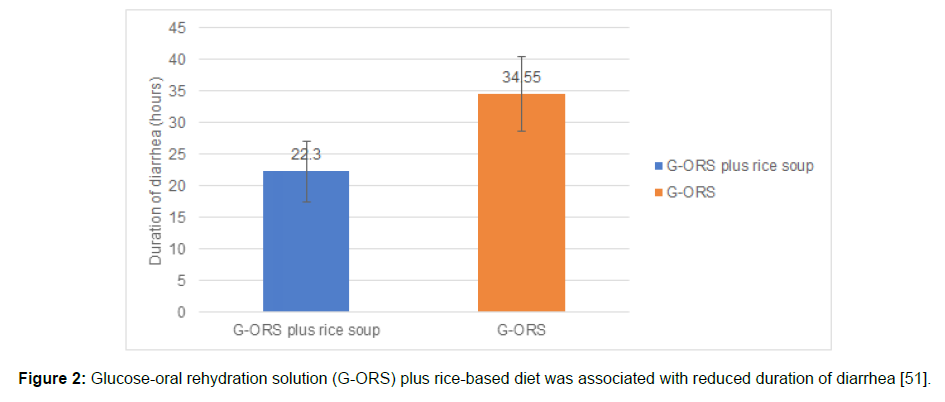
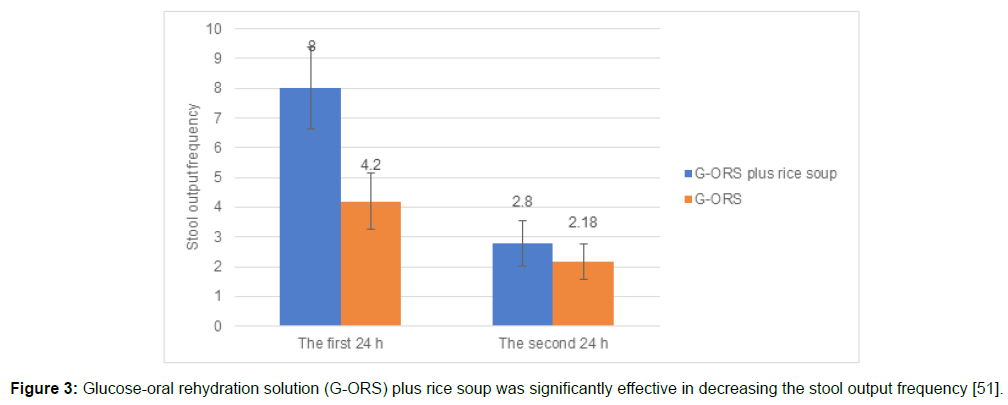
Roy recruited 26 infants (age: 4 to 18 months) suffering from persistent diarrhea and 25 age-matched healthy controls without diarrhea.14 All the infants were given a rice powder, egg white, glucose, and soya oil diet. The rice-based diet enabled 81% (n=21) of the children to recover from diarrhea seven days after initiating the diet. Nine of these patients (43%) had recovered as early as three days after the onset of diarrhea. Rapid recovery in 21 infants was attributed to the inexpensive and locally prepared rice-based diet [14]. Kianmehr [51] evaluated the combined effect of glucose-ORS and rice soup in children (aged 8–24 months) with acute diarrhea (n=40). Children were randomized to glucose-ORS plus rice soup or glucose- ORS (control group). Glucose-ORS and rice soup significantly reduced the duration of diarrhea in children than glucose-ORS only (Figure 2; p<0.001).51 Further, the fecal output during the first 24 h (p< 0.001) and the second 24 h was significantly lower (p=0.03) in the glucose- ORS plus rice soup group than that of the exclusive glucose-ORS group (Figure 3)[51].
The benefits of a rice-based diet beyond diarrhea could be related to its positive impact on intestinal microbiome growth. A study evaluating the effects of four cereal products compared to rice-based cereal has shown that the latter potentially supports the growth of probiotic bacteria. Rice-based cereal significantly increased Bacteroidaceae spp., Bifidobacteriaceae spp., and Lactobacillaceae spp. in the gut but reduced the abundance of the family Enterobacteriaceae spp. Ricebased cereal was associated with significantly lower (p < 0.001) pH levels than samples with oats, wheat, and sorghum-based cereals.
[52] Studies have shown that the growth of a major group of gramnegative bacteria can be inhibited at a mildly acidic pH of 5.5.[53] Hence, by lowering the pH, rice-based cereal could inhibit the growth of pathogenic Escherichia coli.
Importance of rice-fortification
The double burden of chronic or persistent diarrhea and malnutrition leads to growth and developmental failures, micronutrient deficiencies, and recurrent gut infections. Micronutrient deficiencies are associated with an increased risk of morbidity and perhaps mortality too. Food‐based interventions include dietary diversification, mass food fortification or point‐of‐use food fortification. [54-55] nearly 90% of the world’s rice is produced and consumed in Asia. More than 50% of the world’s population consumes rice as a staple food, but there is a concern that rice is deficient in minerals. Rice is the main source of carbohydrates, a moderate source of protein, and a good source of vitamins but with a limited mineral profile. [55-56] unfortunately, vitamins (thiamine and riboflavin) and minerals (such as iron, phosphorus, zinc, magnesium, and copper) are lost during rice de-hulling and polishing. Rice processing also affects the distribution of nutrients within the kernel. Rice polishing (frictional) leads to a 60-80% reduction in the concentration of minerals such as iron, magnesium, phosphorus, potassium, and manganese. Irrespective of the type of polishing, zinc, sulphur, calcium, copper, molybdenum, and cadmium concentration in rice decreased by less than 30%. [57] Prolonged parboiling also causes the loss of essential constituents in paddy rice. In the background of a micronutrient deficiency state, rice fortification with vitamins and minerals could improve the nutritive value of rice and meet the optimal micronutrient requirements esp. during the critical phase of growth & development (6 months- 2 years). [54–56] Fortification increases the micronutrient density of rice and hence improving the daily intake of essential vitamins and minerals. Micronutrient fortification is a cost-effective strategy for increasing the nutrient density of foods. A country-based experience showed that fortification of rice with iron, zinc, and vitamins B1 (thiamine), B3 (niacin), B6 (pyridoxine), B9 (folic acid), and B12 (cobalamin) significantly improved the recommended nutritional intake of the family [58].
Summary
Diarrhea in children is a significant problem in terms of mortality and morbidity. The intricate link between diarrhea and malnutrition affects the immune and nutritional status of children. Growth faltering is a significant consequence of diarrhea augmented in the milieu of malnutrition. Children with diarrhea are also predisposed to micronutrient deficiency due to inadequate nutrient intake and/ or malabsorption of nutrients. Oral rehydration solution is the cornerstone of managing dehydration in diarrhea. Nutritional management is warranted to address issues that prevail beyond dehydration. The diet provided during diarrhea should not aggravate or irritate the gut and should be gentle on developing gut. It should be easily digestible. Rice-based feeds during the diarrheal episodes and in the convalescence period is beneficial as rice contains starches that are easy to digest; provides a high energy density diet with minimal increase in osmolality; can furnish amino acids vital for mucosal repair; is nutrient dense which can replenish essential micronutrients lost during illness, and positively modulates gut microbiota. These benefits of a rice-based diet during and beyond diarrhea supports catch-up growth, improve children’s immune function, and eventually improve nutritional status. Clinical trials have supported the beneficial role of certain micronutrients such as vitamin A, zinc, and selenium in diarrhea management. Micronutrient fortification is a cost-effective strategy for increasing the nutrient density of these rice based feeds which support faster recovery during and beyond diarrhea.
Acknowledgement
The named author meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work, and have given final approval for the version to be published.
Conflict of Interest
The authors have no conflicts of interest relevant to this article.
References
- Millennium Development Goals (2021) UNDP in India.
- Demographics, Health&InfantMortality (2021) UNICEFDATA.
- India Data (2021) Mortality rate, under-5 (per 1,000 live births).
- WHO (2021) Diarrhoeal disease.
- Sultana M, Sarker AR, Sheikh N(2019) Prevalence, determinants and health care-seeking behavior of childhood acute respiratory tract infections in Bangladesh. PLOS ONE 14: 2104333-2104336.
- Ghosh K, Chakraborty AS, Mog M(2021) Prevalence of diarrhoea among under five children in India and its contextual determinants: A geo-spatial analysis. Clin Epidemiol Glob Health 12: 100813.
- Paul P (2020) Socio-demographic and environmental factors associated with diarrhoeal disease among children under five in India. BMC Public Health 20: 1886-2006.
- Patel U, Gedam DS (2014) Malnutrition – Diarrhea Cycle: How to break this? Int J Med Res Rev 2: 277-278.
- Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA, et al.(2008) Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev 66: 487-505.
- Guerrant RL, Schorling JB, McAuliffe JF, de Souza MA (1992) Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am J Trop Med Hyg 47: 28-35.
- MacIntyre J, McTaggart J, Guerrant RL, Goldfarb DM (2014) Early childhood diarrhoeal diseases and cognition: are we missing the rest of the iceberg? Paediatr Int Child Health 34: 295-307.
- National Research Council Subcommittee (1985) Nutrition and Diarrheal Diseases. Nutritional Consequences of Acute Diarrhea. National Academies Press 1: 1-4.
- Korpe PS, Petri WA (2012) Environmental Enteropathy: Critical implications of a poorly understood condition. Trends Mol Med 18: 328-336.
- Roy SK, Haider R, Akbar MS, Alam AN, Khatun M, et al. (1990) Persistent diarrhoea: clinical efficacy and nutrient absorption with a rice based diet. Arch Dis Child 65: 294-297.
- Daniel L. Swagerty J, Walling A, Klein RM (2002) Lactose Intolerance. Am Fam Physician 65: 1845-1849.
- Vesa TH, Marteau P, Korpela R (1999) Lactose intolerance. J Am Coll Nutr 19: 165-175.
- Richard SA, Black RE, Gilman RH (2013) Diarrhea in Early Childhood: Short-term Association With Weight and Long-term Association With Length. Am J Epidemiol 178: 1129-1138.
- (1993) Nutrition You can break the diarrhoea circle. Afr Women Health Safe Mother Mag 1: 28-29.
- Lakshminarayanan S, Jayalakshmy R (2015) Diarrheal diseases among children in India: Current scenario and future perspectives. J Nat Sci Biol Med 6: 24-28.
- National Research Council (US) Subcommittee on Nutrition and Diarrheal Diseases Control (2021) Nutritional Consequences of Acute Diarrhea. National Academies Press (US).
- Mazumder RN, Hoque SS, Ashraf H, Kabir I, Wahed MA (1997) Early Feeding of an Energy Dense Diet during Acute Shigellosis Enhances Growth in Malnourished Children. J Nutr 127: 51-54.
- Salam M, Lindberg G, Dite P (2012) Acute Diarrhea in Adults and Children: A Global Perspective. J Clin Gastroenterol 47: 12-20.
- Guarino A, Ashkenazi S, Gendrel D (2014) European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe. J Pediatr Gastroenterol Nutr 59: 132-152.
- National Research Council (US) Subcommittee on Nutrition and Diarrheal Diseases (2022) Energy Needs for Recovery from the Effects of Diarrhea. National Academies Press (US).
- World Health Organization (1992) Readings on Diarrhoea: Student Manual.
- De Vizia B, Ciccimarra F, De Cicco N, Auricchio S (1975) Digestibility of starches in infants and children. J Pediatr 86: 50-55.
- Gaenssle ALO, Satyawan CA, Xiang G, van der Maarel MJEC, Jurak E (2021) Long chains and crystallinity govern the enzymatic degradability of gelatinized starches from conventional and new sources. Carbohydrate Polym 260: 117801-117802.
- Syahariza ZA, Sar S, Hasjim J, Tizzotti MJ, Gilbert RG(1999) The importance of amylose and amylopectin fine structures for starch digestibility in cooked rice grains. Food Chem 136: 742-749.
- Ye X, Zhang Y, Qiu C, Corke H, Sui Z (2019) Extraction and characterization of starch granule-associated proteins from rice that affect in vitro starch digestibility. Food Chem 276:754-760.
- FAO (2022) Rice in human nutrition - Nutritional value of rice and rice diets Nutritional value of rice and rice diets.
- 31.Lu X, Chang R, Lu H, Ma R, Qiu L, et al. (2021) Effect of amino acids composing rice protein on rice starch digestibility. LWT 146: 111417-111418.
- Koo SI, Turk DE (1977) Effect of zinc deficiency on the ultrastructure of the pancreatic acinar cell and intestinal epithelium in the rat. J Nutr 107: 896-908.
- Roy SK, Tomkins AM (1989) The impact of experimental zinc deficiency on growth, morbidity and ultrastructure development of intestinal tissue. Bangladesh J Nutr Bangladesh 2: 1-7.
- Cui L, Takagi Y, Nezu R (1996) Prolonged zinc-deficient diet alters alkaline phosphatase and disaccharides activities and induces morphological changes in the intestine of rats. J Trace Elem Exp Med 8: 249-261.
- Castillo-Duran C, Vial P, Uauy R (1988) Trace mineral balance during acute diarrhea in infants. J Pediatr 113: 452-457.
- The Zinc Investigators’ Collaborative Group. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr 72: 1516-1522.
- Bates C (1995) Vitamin A. Lancet Lond Engl 345: 31-35.
- Bhan MK, Bhandari N (2022) The Role of Zinc and Vitamin A in Persistent Diarrhea Among Infants and Young Children. J Pediatr Gastroenterol Nutr 26: 446-453.
- De Luca L, Maestri N, Bonanni F, Nelson D (1972) Maintenance of epithelial cell differentiation: The mode of action of vitamin A. Cancer 30: 1326-1331.
- Bhandari N, Bahl R, Sazawal S, Bhan MK (1997) Breast-feeding status alters the effect of vitamin A treatment during acute diarrhea in children. J Nutr 127: 59-63.
- Sinaga M, Supriatmo S, Evalina R, Yudiyanto AR, Sinuhaji AB (2016) Selenium for acute watery diarrhea in children. Paediatr Indones 56: 139-143.
- Thomas AG, Miller V, Shenkin A, Fell GS, Taylor F(1994) Selenium and glutathione peroxidase status in paediatric health and gastrointestinal disease. J Pediatr Gastroenterol Nutr 19: 213-219.
- Fischer Walker CL, Black RE (2007) Micronutrients and Diarrheal Disease. Clin Infect Dis 4: 73-77.
- Giannattasio A, Guarino A, Lo Vecchio A (2016) Management of children with prolonged diarrhea. F1000Research 5: 1-5.
- Rouhani S, Griffin NW, Yori PP (2020) Diarrhea as a Potential Cause and Consequence of Reduced Gut Microbial Diversity among Undernourished Children in Peru. Clin Infect Dis off Publ Infect Dis Soc Am 71: 989-999.
- Pop M, Walker AW, Paulson J (2014) Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol 15: 76.
- Felipoff AL, Zuleta A, Sambucetti ME, Rio ME (2012) not any type of rice performs equally to improve lactose-induced diarrhea characteristics in rats: is amylose an antidiarrheal factor. Food Sci Technol 32: 323-328.
- Raghupathy P, Ramakrishna BS, Oommen SP (2006) Amylase-resistant starch as adjunct to oral rehydration therapy in children with diarrhea. J Pediatr Gastroenterol Nutr 42: 362-368.
- Goldberg ED, Saltzman JR1 (996) Rice inhibits intestinal secretions. Nutr Rev 54: 36-37.
- Gore SM, Fontaine O, Pierce NF (1992) Impact of rice based oral rehydration solution on stool output and duration of diarrhoea: meta-analysis of 13 clinical trials. BMJ 304: 287-291.
- Kianmehr M, Saber A, Moshari J, Ahmadi R, Basiri-moghadam M, et al. (2016) The Effect of G-ORS Along With Rice Soup in the Treatment of Acute Diarrhea in Children: A Single-Blind Randomized Controlled Trial. Nurs Midwifery Stud 5: 25852.
- Tetu SG, Chong RWW, Ashton J, Packer NH, Paulsen IT, et al. (2017) Cereal products derived from wheat, sorghum, rice and oats alter the infant gut microbiota in vitro. Sci Rep 7: 14312.
- Duncan SH, Louis P, Thomson JM, Flint HJ (2009) the role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol 11: 2112-2122.
- Olson R, Gavin-Smith B, Ferraboschi C, Kraemer K (2021) Food Fortification: The Advantages, Disadvantages and Lessons from Sight and Life Programs. Nutrients 13: 1118.
- Peña‐Rosas JP, Mithra P, Unnikrishnan B (2019) Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition. Cochrane Database Syst Rev: CD009902.
- Junaid-ur-Rahman S, Chughtai MFJ (2022) Khaliq A Rice: a potential vehicle for micronutrient fortification. Clin Phytoscience 8: 14.
- Hansen TH, Lombi E, Fitzgerald M (2012) Losses of essential mineral nutrients by polishing of rice differ among genotypes due to contrasting grain hardness and mineral distribution. J Cereal Sci 56: 307-315.
- WHO (2020) Fortification: Leveraging evidence for improving nutrition.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed atGoogle ScholarCrossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at Google scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at Google scholar, Crossref
Indexed at, Google scholar, Crossref
Citation: Acharyya BC, Srivastava PK (2022) Nutritional Management During and Beyond Diarrhea. Role of Rice-Based Foods. Neonat Pediatr Med 8: 263. DOI: 10.4172/2572-4983.1000263
Copyright: © 2022 Acharyya BC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3701
- [From(publication date): 0-2022 - Apr 25, 2025]
- Breakdown by view type
- HTML page views: 3276
- PDF downloads: 425