Nutrition of Infants with Very Low Birth Weight using Human and Bovine Based Milk Fortifier: Benefits and Costs
Received: 21-Oct-2021 / Accepted Date: 04-Nov-2021 / Published Date: 11-Nov-2021
Abstract
Objectives: Small infants require adequate enteral nutrition to achieve continuous growth. Therefore, breast milk should be supplemented with fortifier. In addition to the cost-efficient fortifiers produced from bovine milk, an expensive fortifier derived from human milk has been available. We compared, whether preterm infants benefit from human fortifier supplementation and whether the higher purchase costs are economically viable for hospitals.
Methods: Preterm infants of <32+0 gestational week and <1000 g birth weight, were enrolled. The newborns were nourished with human milk. Supplementation with human fortifier or bovine fortifier was initiated once oral milk intake reached 100 mL/kg BW/d. Standardized documentation of body weight, respiratory situation, Intraventricular Hemorrhage (IVH), Periventricular Leukomalacia (PVL), Necrotizing Enterocolitis (NEC) and Retinopathy of Prematurity (ROP) and duration of the in-hospital stay was undertaken at day of life 7, 14, 21, 35 and 42. For each individual the revenue was calculated.
Results: Between 01/2019 and 12/2020, 23 children were enrolled. 10 preterms received human and 13 bovine fortifier. 2 infants developed BPD and one required ligature for a Patent Ductus Arteriosus Botalli (PDA) in the children who were supplemented with human milk-based fortifier. Three children in the group fed the bovine fortifier developed BPD, PVL was documented in one, ROP in 2, higher-grade NEC in one and ductus ligature was required by two children. Stool-calprotectin values measured on study days 35 and 42 were significant lower in infants given human fortifier. Nevertheless, the in-hospital stay was shorter in the human fortifier group (median of 75.5 days) than in the bovine fortifier (median of 80 days) group.
Total revenue gain was +39854.20 € (+5958.20 € per patient) in patients fed the human fortifier versus +20573.42 € (-346.00 € per patient) in individuals who received bovine fortifier. The costs for human fortifier supplementation were in total € 41005.00. Bovine fortifier was less expensive (total cost: € 250.00).
Conclusion: Fortifiers produced from human milk entail higher therapeutic costs but are offset by shorter in-hospital stays and fewer morbidities among preterm infants. Preterm infants tolerate human milk-based fortifiers significantly better than bovine-based fortifiers.
Keywords: Human milk-based diet; Bovine-based fortifier; Human milk-based fortifier; Preterm; Human milk
Abbreviations
AP: Anus Praeternaturalis; BW: Birth Weight; BPD: Bronchopulmonary Dysplasia; C-Section: Caesarean Section; DRG: Diagnosis Related Group; F: Female; GA: Gestational Age; GW: Gestational Week; G: Gram; IQTIG: Institute for Quality Assurance and Transparency in Health Care; IRB: Institutional Review Board; IVH: Intraventricular Hemorrhage; M: Male; μg: Microgram; NEC: Necrotizing Enterocolitis; PDA: Patent Ductus Arteriosus Botalli; PVL: Periventricular Leukomalacia; ROP: Retinopathy of Prematurity
Introduction
Breast milk is regarded as the best and most cost-effective form of nutrition for preterm and term neonates worldwide [1]. At approximately 70 kilocalories (kcal) or 294 kilojoules (kJ) per 100 mL, the energy and nutritional content is not sufficient for preterm infants to achieve growth parallel to the percentile lines for body weight, length and head circumference. To compensate for the inadequate calorie intake from breast milk alone, the industry has developed special nutritional supplements (fortifiers). These fortifiers are produced almost exclusively from cow’s milk (bovine fortifiers). Reports of a correlation between bovine fortifiers and food intolerances appear repeatedly in the literature, including even an increased incidence of Necrotizing Enterocolitis (NEC) in preterm infants [2-4]. Since 2006, a fortifier based on human milk (human fortifier) as an alternative to bovine fortifiers has been commercially available [5]. In building up the enteral diet of a preterm infant, breast milk and human fortifiers constitute a physiological unit which in the literature is ascribed positive clinical effects in the child’s development [6]. This is contrasted by high production and purchasing costs [7].
Calprotectin is an S100 protein found in neutrophil cytoplasm which is released in the presence of various inflammatory diseases. Several publications have described fecal calprotectin as a potential diagnostic test for early detection of inflammatory processes in the gastrointestinal tract of preterm infants and thus as a possible early indication of food intolerance [8-10]. There is only limited data on the clinical outcome of high-risk preterm infants with a birth weight <1000 g fed with a human fortifier during their first weeks of life where economic aspects are also considered. We summarized a case series of preterm infants of <32+0 Gestational Weeks (GW) and Body Weight (BW) <1000 g who received breast milk supplemented either with a human or a bovine fortifier. Using serial examination of the fecal calprotectin concentration as a clinical marker for food intolerance with an inflammatory component among preterm infants, we examined the cost-benefit correlation and the clinical course in preterm infants who were nourished with human milk supplemented with human fortifier in contrast to preterm infants supplemented with bovine fortifier.
Materials and Methods
Institutional review board approval
This case series was approved by the Institutional Review Board (IRB) of the University of Regensburg (No.: 18-1048-101). All the children received care on the neonatal intensive care unit at KJF Clinic Saint Elisabeth, Neuburg an der Donau, Germany. No changes were made to the standards for nutrition, nursing, and care.
Patient characteristics and nutrition
Preterm infants of <32+0 GW and <1000 g BW receiving care on the level I perinatal center of the KJF were enrolled. The parents were provided with explanations concerning bovine and human milk-based fortifiers, respectively, and the fecal tests for calprotectin. All preterm infants were given breast milk and/or human donor milk as of their first day of life and initially an additional parenteral nutritional solution. The parenteral nutritional solution was reduced and discontinued once the oral fluid intake exceeded 125 mL/kg BW. The daily macromolecule requirement (carbohydrates (up to 12 g/kg BW/d [11]), protein (up to 3.5 g/kg BW/d [12]), fats (up to 4.0 g/kg BW/d [13])) was calculated in accordance with the guidelines of the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN). Supplementation with human fortifier (Prolacta from Bioscience®, Duarte, California 91010, USA) or bovine fortifier (Aptamil FMS®, Nutricia Milupa GmbH, Frankfurt, Germany, consisting of protein hydrolysate and casein hydrolysate from cow’s milk) was initiated once oral milk intake reached 100 mL/kg BW/d. Data collection was terminated on attaining a BW of 1250 g.
Standardized clinical documentation of the body weight, respiratory situation (oxygen requirement at day 28), occurrence of complications (Intraventricular Hemorrhage (IVH), Periventricular Leukomalacia (PVL), Necrotizing Enterocolitis (NEC), Retinopathy of Prematurity (ROP), Bronchopulmonary Dysplasia (BPD) and pneumothorax was undertaken at day of life 7, 14, 21, 35 and 42.
Measurement of calprotectin
A stool sample was taken from the first stool a day passed on day of life 7, 14, 21, 35 and 42 and was tested for calprotectin. Stool sample collection was standardized with the aid of CALEX® Cap extraction devices. The sample pin included with the extraction tube permits analysis of a defined volume of stool only. Any excess stool is removed by the insertion aid and thus is not available for analysis. The stool sample is dissolved in the extraction buffer, 60 μL of which is applied to the test cassette (Quantum Blue® fCAL extended). The Quantum Blue® fCAL Point-of-Care Testing (PoCT) device (Bühlmann Laboratories AG, 4124 Schönenbuch, Switzerland) was used to measure the fecal calprotectin levels. The analysis method entails a sandwich immunoassay. The signal obtained with the test method is quantified using the reader and can be read from the display of the device after no more than 15 minutes. The calprotectin concentration is displayed in micrograms (μg) per gram (g) of stool.
Cost-calculation
The case costs per study subject were calculated using the MetaKIS® software application (Cerner Health Services Deutschland GmbH, 65510 Idstein, Germany) of Diagnosis Related Group (DRG) data and a bidirectional interface with CGM MEDICO® (CompuGroup Medical Deutschland AG, 56070 Koblenz, Germany). In the DRG-system, each patient case is classified based not only on severity but also on a shorter, average or longer hospital stay. In the context of a mixed calculation, revenue gains upon discharge prior to the average length of stay are assumed. If discharge occurs after this cutoff date, the incurred costs will tip the balance.
Statistical analysis
To calculate the statistical significances, the Mann-Whitney-U test for two independent samples was used. A difference of p<0.05 was defined as significant.
To calculate the homogeneity of two attributes, a Fisher-Yates test was used based on a 2 × 2 contingency table. Calculations with the Fisher-Yates test are indicated in the publication by χ2. A significance level of p<0.05 also applies in this test procedure.
Results
Patients
Between January 1, 2019 and December 31, 2020 23 children with a BW of <1000 g and <32+0 GW were enrolled. 10 preterm infants were fed breast milk (mother’s milk or donor milk) with human fortifier during, whereas 13 preterm infants received breast milk (mother’s milk or donor milk) plus the bovine fortifier. The details of the preterm infants studied are presented in the study subject flow chart (Figure 1) and in Table 1 (basic characteristics). There was no significant difference in the basic characteristics between the two study groups.
| Population | Human fortifier (n=10) | Bovine fortifier (n=13) | Significance | ||
|---|---|---|---|---|---|
| Absolute (%) | Absolute (%) | Absolute (%) | Absolute (%) | ||
| Sex | 7 f (70) | 3 m (30) | 7 f (53.9) | 6 m (46.1) | χ2=0.66 |
| Completed GWs | 27.5 | ± 2.2 | 25 | ± 2.2 | 0.843* |
| Median BW (g) | 705 | ± 169 | 820 | ± 149 | 0.495* |
| APGAR 5 | 7 | ± 1.6 | 7 | ± 1.8 | 0.352* |
| APGAR 10 | 8.5 | ± 1 | 9 | ± 1.5 | 0.262* |
| Chorioamnionitis | 6 no (60) | 4 yes (40) | 8 no (61.5) | 5 yes (38.5) | c2=1 |
| C-section | 0 (0) | 10 (100) | 0 (0) | 13 yes (100) | c2=1 |
| PPROM | 7 no (70) | 3 yes (30) | 10 no (76.9) | 3 yes (23.1) | c2=1 |
| Surfactant day 1 | 1 no (10) | 9 yes (90) | 2 no (15.4) | 11 yes (84.6) | c2=1 |
| MV within 72 h | 4 no (40) | 6 yes (60) | 4 no (30.8) | 9 yes (69.2) | c2=0.685 |
Note: * Mann-Whitney-U, χ2 : Fisher Chi-squared.
Abbreviations: BW: Birth Weight; GW: Gestational Week; MV: Mechanical Ventilation; PROM: Premature Rupture of Membranes.
Table 1: Subject characteristics in preterm infants given a human milk-based fortifier or bovine fortifier.
Fecal calprotectin
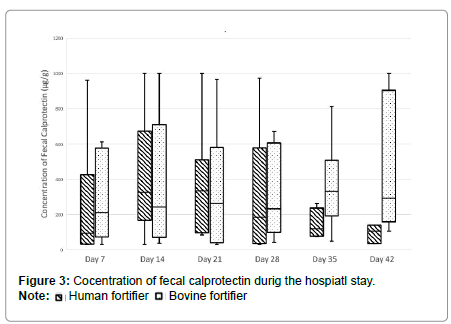
It is evident from the period studied, that the calprotectin levels during the first 35 to 42 days of life in the children given the human fortifier approximate the normal levels for children (3-4 years, <75 μg/g 20), unlike the children given the bovine fortifier (Figure 2). The median in these cases far exceeds the normal level for children (day 35: 331.5 μg/g; day 42: 292 μg/g). The calprotectin values found on study days 35 and 42 differ significantly from each other (day 35 p=0.02, day 42 p=0.03) and significantly less calprotectin was found in the stool of the preterm infants given the human fortifier (Figure 3).
Complications
Out of the 10 preterm infants who were fed the human milk-based fortifier, 2 were found to have BPD according to the diagnostic criteria of the Institute for Quality Assurance and Transparency in Health Care (IQTIG) [14]. The number of hours of ventilation (mechanical ventilation, continuous positive airway pressure, high-flow ventilation) in this group totaled 12333 (median 1064.5 hours/patient (± 572.3)). One child required ligature for a hemodynamically relevant Patent Ductus Arteriosus Botalli (PDA) at 28 days of age. There were no cases of ROP, PVL or NEC in the group of preterm infants given human milk-based fortifier.
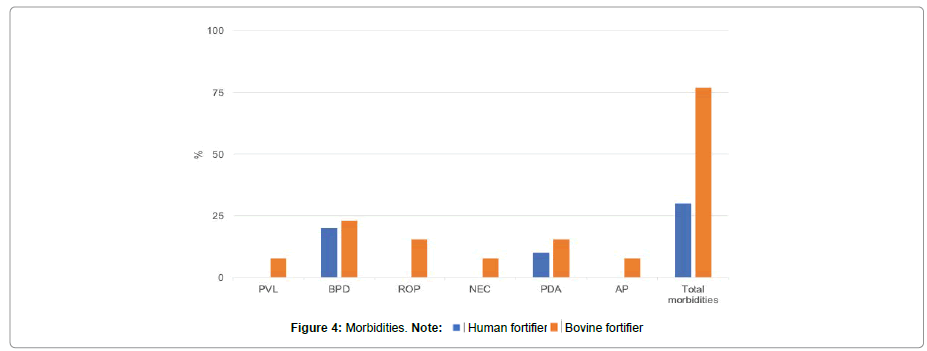
Three children in the group fed the bovine fortifier were noted to have BPD. The total number of ventilation hours (absolute) in this respect was 17187 (median 1125 hours/patient (± 605.8)). PVL was documented in one child, ROP in 2 children, and higher-grade NEC according to Bell in another child. Ductus ligature was required by one child at age 19 days and another child at 30 days. One child required Anus Praeternaturalis (AP) construction at day 20 of life due to impaired enteral food transit with no evidence of morphologic or anatomic anomalies. All the data, with significance levels, is presented in Table 2 and Figure 4.
| Human milk (n=10) | Cow’s milk (n=13) | Significance | |||
|---|---|---|---|---|---|
| Weight development | Median | Standard deviation | Median | Standard deviation | |
| Birth weight (g) | 705 | ± 169.1 | 820 | ± 149 | 0.495 |
| First dose of fortifier | 8 | ± 3.2 | 11 | ± 2.6 | 0.034 |
| Day of life (d) | |||||
| Weight when starting fortifier (g) | 717.5 | ± 139 | 725 | ± 160 | 0.576 |
| Human fortifier discontinued after days (d) | 24.5 | ± 12.8 | - | - | - |
| Weight after human fortifier (g) | 1116.5 | ± 179.9 | - | - | - |
| Weight when starting bovine fortifier (g) | 1168 | ± 181.4 | 725 | ± 160 | - |
| Delta weight gain/day (g/d) | 18.9 | - | 19.6 | - | - |
| Weight at discharge (g) | 2130.5 | ± 847.2 | 2390 | ± 510 | 0.563 |
| Quality indicators | - | ||||
| Oxygen requirement ≥ 22% at age 28 days | 4 | 40% | 8 | 61.50% | χ2=0.238 |
| PVL | 0 | 0 | 1 | 7.70% | - |
| BPD | 2 | 20% | 3 | 23.10% | - |
| ROP | 0 | 0 | 2 | 15.40% | - |
| NEC (operation) | 0 | 0 | 1 | 7.70% | - |
| PDA (operation) | 1 | 10 | 2 | 15.40% | - |
| Creation of AP | 0 | 0 | 1 | 7.70% | - |
| Total morbidities | 3 | 30% | 10 | 76.90% | χ2= 0.04 |
| Inpatient days/patient | 75.5 | ± 33.6 | 80 | ± 19 | 0.514 |
| Ventilation hrs/patient | 1064.5 | ± 572.3 | 1125 | ± 607.3 | 0.563 |
| Gain in days | 7.1 | ± 12 | -2.5 | ± 17.5 | 0.182 |
| Revenue gain (€) | 5958 | ± 12,052 | -346 | ± 20176 | 0.307 |
| Positive revenues | 7 | 70% | 5 | 38.50% | χ2=0.214 |
Note: €: Euro; χ2: Fisher Chi-squared.
Abbrevations: PVL: Periventricular Leukomalacia; BPD: Bronchopulmonary Dysplasia; ROP: Retinopathy of Prematurity; NEC: Necrotizing Enterocolitis; PDA: Patent Ductus Arteriosus Botalli;
Basic specification for service providers [14].
Case cost calculations and revenues
Preterm infants fed the human fortifier remained in hospital for a median of 75.5 (± 33.6) days and neonates fed the bovine fortifier for a median of 80 (± 19) days.
In the group given the human fortifier, the revenue gain was +39854.20 € and +5958.20 € per patient. Making the same calculation for the group on bovine fortifier, the revenue gain was +20573.42 € and -346.00 € per patient.
The additional daily costs for human fortifier supplementation were on an average € 147.50/day (€ 115-180/day). During the observation period, the children were offered human fortifier for a total of 278 days of treatment. The generated revenue (absolute) of +39854.20 € must therefore be offset against the additional costs (absolute) of 41005.00 €.
Bovine fortifier was even given for 278 days, and the total cost is € 250.00, i.e., approx. € 20.00 per patient, corresponding to € 0.94 per day.
Discussion
Fortifiers are essential when feeding preterm and term neonates if an adequate growth percentile is to be achieved. To date, fortifiers used to feed preterm and term neonates in Germany have been based purely on bovine sources (daily treatment costs of approx. € 0.94/patient). Since 2018, a human milk-based fortifier has been commercially available in Germany (daily treatment costs of approx. € 147.50/patient). This fortifier is designed to provide preterm infants with a nutritional concept based exclusively on human milk. This new concept should be beneficial to early childhood development.
We used two different nutritional concepts in preterm infants of <32 GW with a BW of <1000 g. The parents could choose between supplementation of human fortifier or bovine fortifier supplementation to human milk as standard oral nutrition. There were no other differences in the remaining nutritional and care concept. The data analyses were designed to deliver insights into food tolerance, clinical outcome, and economic calculation.
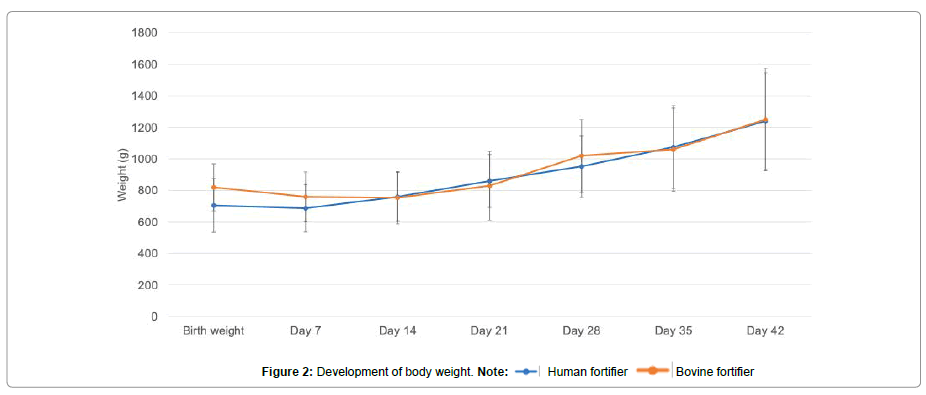
During the observation period, 10 preterm infants were fed with a human fortifier and 13 with a bovine fortifier. The weight curves after commencing the respective enteral supplementation in each group were roughly identical (Figure 2).
Fecal calprotectin is regarded as a sensitive marker for an incipient or existing inflammation in the gastrointestinal tract not only in the care of patients with acute or chronic inflammatory bowel diseases [8,10]. The concentration of calprotectin measured on study days 35 and 42 was significantly lower than in the bovine fortifier group. In addition, this can be considered as reduced inflammation and consequently as improved gastrointestinal tolerance. A link between nutrition and calprotectin levels has already been reported, but not in relation to human fortifier [15-18]. It is evident from the other values obtained in this respect that stable weight gain can be achieved with the administration of a human fortifier, as with bovine fortifier, but nutrition comprising a human fortifier possibly reduces the risk of inflammation.
Co-Morbidities such as PVL, BPD, ROP, NEC, PDA and AP are generally rare in the care of preterm infants. Judging by the total number of events observed in our preterm neonates, typical morbidities in the human fortifier group were significantly less frequent (30%) than in the bovine fortifier group (77%, p=0.04). Overall, preterm infants given a human milk-based fortifier were discharged from hospital after a median of 75.5 days versus 80 days in the bovine fortifier group. Taking together, neonates treated with human milk-based fortifier showed positive effects in cost-effectiveness justified in a shorter hospital stay and reduced complication rate in contrast to preterms fed with bovine fortifier. Nevertheless, the total revenue in the human milk-based fortifier group of € 39854.20 was wiped out by the high cost of the human milk-based fortifier (€ 41005.00). Given the small number of cases, the calculated gain with the two fortifier groups in treatment days and revenues covers not the additional costs of the human milk-based diet. At larger perinatal centers, where there are higher case numbers, however, there could be a distinct economic advantage from earlier discharges, a better balance of treatment costs, and earlier provision of neonatal beds with, in turn, earlier reoccupation. Even at small perinatal centers, the use of human fortifiers may create additional capacities which, in turn, could have a positive impact on cost-effectiveness.
Conclusion
No adverse effects or incompatibilities were observed with the human fortifier. Preterm infants of <1000 g birth weight who received a human fortifier in the first weeks of life instead of bovine supplementation were found to have a significantly lower fecal calprotectin concentration after day 35 of life, tended to require oxygen less frequently by the age of 28 days, and had significantly fewer preterm neonate morbidities over the entire clinical course compared with preterm infants given bovine fortifier. From an economic perspective, the higher overall revenues generated with the preterm infants receiving the human fortifier were consumed by the higher acquisition costs for the human fortifier. Maybe, high case numbers in combination with reduced comorbidities and shorter in-hospital stay can increase the cost-effectiveness of the human fortifier.
Limitation
Hence, the children in the human milk-based fortifier group weighed less (birth weight 705 g versus 820 g) but were of higher Gestational Age (GA) (27.5 GA versus 25 GA). Neither of these factors is statistically significant. Since birth weight rather than gestational week is decisive in economic considerations, nutritional concepts that are based on birth weight and not on maturity may well prove to be a disincentive with nutritional concepts. To obtain sound data in this respect, a blinded, randomized study should be oriented to gestational age rather than birth weight to make more reliably assess complications in preterm neonates such as BPD, ROP and NEC, which may be associated with nutrition and also have a significant impact on economic considerations.
Conflicts of Interest
The authors have no potential conflict of interest or financial support as it relates to the subject of this study.
Author contribution
Merem Osmanova and Matthias J Müller are equal authors to this manuscript.
References
- Karall DNG, Zittera ID, Bier A, von der Ohe G, Guóth-Gumberger M, et al.(2020) Stillen und stillberatung. Monatsschr Kinderheilkd 168:547-560.
- Huston RK, Markell AM, McCulley EA, Pathak M, Rogers SP, et al.  (2014) Decreasing necrotizing enterocolitis and gastrointestinal bleeding in the neonatal intensive care unit: The role of donor human milk and exclusive human milk diets in infants ≤ 1500 g birth weight. Infant Child Adolesc Nutr 6:86-93.
- Herrmann K, Carroll K (2014) An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed Med 9:184-190.
- Hair AB, Peluso AM, Hawthorne KM, Perez J, Smith DP, et al. (2016) Beyond necrotizing enterocolitis prevention: Improving outcomes with an exclusive human milk-based diet. Breastfeed Med 11:70-74.
- Schanler RJ (1998) Fortified human milk: Nature's way to feed premature infants. J Hum Lact 14:5-11.
- Premkumar MH, Pammi M, Suresh G (2019) Human milk-derived fortifier versus bovine milk-derived fortifier for prevention of mortality and morbidity in preterm neonates. Cochrane Database Syst Rev 11.
- Jochum F, Bührer C, Kauth T, Körner A, Koletzko B, et al. (2019) Commercial breast milk enhancers made from human milk: Insufficiently documented benefits and high costs. Monatsschrift Kinderheilkunde 167:145-148.
- Qu Y, Xu W, Han J, Zhou W, Wu H (2020) Diagnostic value of fecal calprotectin in necrotizing enterocolitis: A meta-analysis. Early Hum Dev 151:105170.
- Aydemir O, Aydemir C, Sarikabadayi YU, Emre Canpolat F, Erdeve O, et al. (2012) Fecal calprotectin levels are increased in infants with necrotizing enterocolitis. J Matern Fetal Neonatal Med 25:2237-2241.
- Yoon JM, Park JY, Ko KO, Lim JW, Cheon EJ, et al. (2014) Fecal calprotectin concentration in neonatal necrotizing enterocolitis. Korean J Pediatr 57:351-356.
- Mesotten D, Joosten K, van Kempen A, Verbruggen S (2018) ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Carbohydrates. Clin Nutr 37:2337-2343.
- Van Goudoever JB, Carnielli V, Darmaun D, Sainz de Pipaon M (2018) ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Amino acids. Clin Nutr 37: 2315-2323.
- Lapillonne A, Fidler Mis N, Goulet O, van den Akker CHP, Wu J, Koletzko B, et al. (2018) ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Lipids. Clin Nutr 37:2324-2336.
- Institute for Quality Assurance and Transparency in Health Care (2021) QA basic specification for service providers 2021 V06.
- Savino F, Castagno E, Calabrese R, Viola S, Oggero R, et al. (2010) High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology 97:299-304.
- Costa S, Patti ML, Perri A, Cocca C, Pinna G, et al. (2020) Effect of different milk diet on the level of fecal calprotectin in very preterm infants. Front Pediatr 8:552.
- Doshi H, Pandya S, Codipilly CN, Schanler RJ (2020) Does human milk fortifier affect intestinal inflammation in preterm infants? Breastfeed Med 15:776-77
- Groer M, Ashmeade T, Louis-Jacques A, Beckstead J, Ji M (2016) Relationships of feeding and mother's own milk with fecal calprotectin levels in preterm infants. Breastfeed Med 11:207-212.
Citation: Osmanova M, Müller MJ, Habisch B, Hippe A, Seeliger S (2021) Nutrition of Infants with Very Low Birth Weight using Human and Bovine Based Milk Fortifier: Benefits and Costs. Neonat Pediatr Med S10:003.
Copyright: © 2021 Osmanova M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Usage
- Total views: 3264
- [From(publication date): 0-2021 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2660
- PDF downloads: 604

 Human fortifier
Human fortifier Bovine fortifier
Bovine fortifier