Research Article Open Access
NSC Hydrometallurgical Pressure Oxidation of Combined Copper and Molybdenum Concentrates
Corby G. Anderson1*, Todd S. Fayram2 and Larry G. Twidwell31Kroll Institute for Extractive Metallurgy, Colorado School of Mines, USA
2Continental Metallurgical Services, Butte, Montana, USA
3Montana Tech of the University of Montana, Butte, Montana, USA
- *Corresponding Author:
- Corby G. Anderson
Kroll Institute for Extractive Metallurgy
Colorado School of Mines, USA
Tel: 303-273-3580
Fax: 303-273-3795
E-mail: cgandersmines@gmail.com
Received Date: August 05, 2013; Accepted Date: October 03, 2013; Published Date: October 10, 2013
Citation: Anderson CG, Fayram TS, Twidwell LG (2013) NSC Hydrometallurgical Pressure Oxidation of Combined Copper and Molybdenum Concentrates. J Powder Metall Min 2:115. doi: 10.4172/2168-9806.1000115
Copyright: © 2013 Anderson CG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
The need for copper and molybdenum continues to grow as does the need for clean efficient metallurgical technologies capable of treating mixed metal concentrates. Currently, the use of hydrometallurgical pressure oxidation for copper concentrate treatment is growing. Furthermore, with limited molybdenum roasting capacity, stringent industrial molyb-denum concentrate roaster feed specifications, poor rhenium recoveries, and the inherent environmental issues asso-ciated with pyrometallurgical treatments, hydrometallurgical options are also now being pursued for molybdenum concentrates. Moreover, given the inherent grade, recovery and cost inefficiencies in the differential flotation process normally employed for molybdenum concentrates produced as a by-product of copper mining, there is a growing need to directly treat combined bulk copper and molybdenum concentrates. This minimizes molybdenum concentrate roasting limitations, specifications and requirements, while allowing simplification of increased efficiency of and cost reductions in upstream mineral processing circuits now producing separate copper and molybdenum concentrates by differential flotation. It would also allow more direct and efficient recovery of rhenium. Finally, hydro metallurgical technology will also reduce the need for costly final molybdenum concentrate impurity treatment circuits, thereby allowing for lower grade mixed metal molybdenum concentrates to be treated directly for a greater metal value realization. In summary, industrial nitrogen species catalyzed (i.e. NSC) hydro metallurgical pressure oxidation has many advantages over conventional pressure oxidation systems and offers a tangible process route to treat mixed bulk concentrates.This paper illustrates the fundamental concepts, confirmatory testing, application, proposed design and associated cost estimates for the development and industrial implementation of this proven mode of hydro metallurgical processing as applied combined to copper molybdenum rhenium concentrates.
Keywords
Hydrometallurgical; Oxidation; Copper; Molybdenum; Concentration
Introduction and Theoretocal Considerations
As a first step, the basics of nitrogen species catalyzed (i.e. NSC) hydrometallurgical pressure oxidation will be outlined. The commonly reported leach reaction of a sulfide mineral with nitric acid in conjunction with sulfuric acid is shown below.
3MeS (s) + 2HNO3 (aq) + 3H2SO4 (aq) → 3MeSO4 + 3S° (s) + 2NO (g) + 4H2O (1)
However, it has been postulated and confirmed that the actual reaction species is NO+ and not NO3- [1-4]. The addition of or presence of NO2- instead of NO3- accelerates the formation of NO+. As shown in Table 1, the NO+/NO couple is capable of an extremely high redox potential [5]. So, NO+ is readily formed from nitrous rather than nitric acid. For example, a convenient source of nitrous acid can be sodium nitrite [1,2]. When it is added to an acidic solution, nitrous acid isreadily formed.
| Oxidant | Redox Equation | E°h (pH = 0, H2 ref.) |
|---|---|---|
| Fe3+ | Fe3+ + e- à Fe2+ | 0.770 V |
| HNO3 | NO3- + 4H+ +3e- à NO + 2H2O | 0.957 V |
| HNO2 | NO2- + 2H+ + e- à NO + H2O | 1.202 V |
| O2 (g) | O2 + 4H+ + 4e- à 2H2O | 1.230 V |
| Cl2 (g) | Cl2 (g) + 2e- à 2Cl- | 1.358 V |
| NO+ | NO+ + e- à NO | 1.450 V |
Table 1: Relative Potentials of Hydrometallurgical Oxidizers.
NaNO2 (aq) + H+ → HNO2 (aq) + Na+ (2)
Nitrous acid further reacts to form NO+.
HNO2 (aq) + H+ → NO+ (aq) + H2O (3)
The NO+ then reacts with the mineral and oxidizes the sulfide to sulfur.
MeS (s) + 2NO+(aq)→ Me2+ (aq) + S° + 2NO (g) (4)
Of course, at higher temperatures and/or nitrogen species concentrations the sulfide can be fully oxidized to sulfate.
As can be seen, nitric oxide gas, NO, is produced from the oxidation of sulfides. As this gas has a limited solubility in aqueous solutions, it tends to transfer out of solution. In the pressure leach system, a closed vessel with an oxygen overpressure is used. The nitric oxide gas emanating from the leach slurry accumulates in the headspace of the reactor where it reacts with the supplied oxygen to form nitrogen dioxide gas. The NO is then regenerated to NO+. Overall this can be viewed as:
NO (g) + O2 (g) → 2NO2 (g) (5)
2NO2 (g) ←→ 2NO2 (aq) (6)
2NO2 (aq) + 2NO (aq) + 4H+ ←→ 4NO+ (aq) + 2H2O (7)
Since the nitrogen species is continuously regenerated, its role in the overall reaction as the actual oxidizer is not obvious. The net overall reaction has the sulfide mineral reacting with the acid solution and oxygen to solubilize the metal value into the sulfate solution and form some elemental sulfur.
2MeS (g) + 4H+ + O2 (g) → 2Me2+ (aq) + 2S° + 2H2O (8)
Of course, at higher temperatures and/or nitrous acid concentrations the sulfide would be fully oxidized to sulfate.
Overall, the nitrogen intermediates serve as an expedient means to transport oxygen to the surface of the solid particle and allow the resulting reaction to take place at a heightened redox potential. This inherent asset of the unique novel NSC system eliminates the need for the use of high temperatures and high pressures, which lead to higher costs in other pressure leach processes. For example, commonly available stainless steel can be used for the reactor vessel. And, complete oxidation of sulfide to sulfate can be achieved without the excessive conditions found in other pressure leach systems. Thus, the rapid kinetics of the system leads to smaller reactor volumes and higher unit throughputs. Finally, 99.9% of the nitrogen species utilized in the leach system report to the gas phase when the pressure vessel is flashed and they are readily destroyed and contained by commercially available scrubber systems. So, environmental impacts are minimized and the NSC leach plant solutions contain little or no nitrogen species. In summary, NSC was first used industrially from 1984 until 1995 on silver bearing copper concentrates. It has also been found to be applicable to and effective in the treatment of zinc, gold, lead, nickel, cobalt, copper PGM and molybdenum concentrates.
Copper and molybdenum flotation considerations
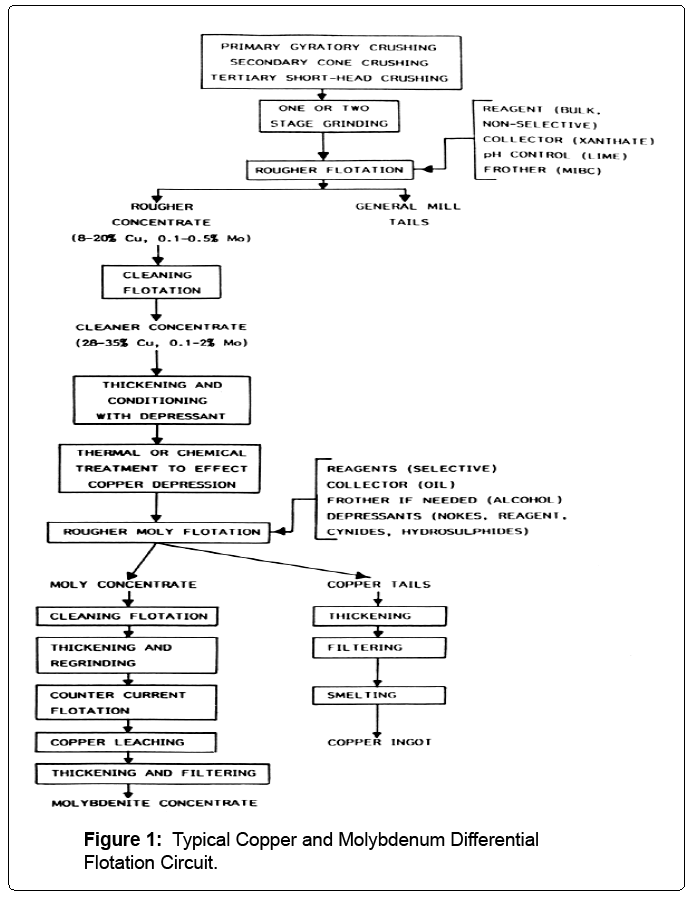
Traditional treatment of copper porphyry ores bearing molybdenum consists of differential floation to produce two concentrates. As illustrated in Figure 1 after comminution, a bulk copper and molybdenum concentrate is produced and then cleaned to increase grade but diminish recovery. Then, the copper is depressed from the bulk concentrate to produce a molybdenum concentrate and a copper concentrate. In order to meet grade constraints in the molybdenum concentrate, several stages of cleaner flotation followed by chemical leaching is required to minimize impurities. As such, as illustrated in Table 2 based on worldwide operating data, less than 50% of the molybdenum is finally recovered to the concentrate. This after full costs have been absorbed for mining and milling. Then typically, the molybdenum and copper concentrates are shipped for smelting. In the case of molybdenum, since global roasting capacity is limited, concentrate impurity penalties are stringent and smelting terms can be expensive. Further, little if any credit for by product rhenium is realized. As seen in Table 3 in general, there is a significant economic driver in floation plants for lowering both capital and operating costs by making a single concentrate. At a processing rate of 20,000 tonnes per day of ore, both the CAPEX and OPEX are reduced about 8%. The actual savings is about $12 million dollars in capital and and annual savings in operating cost of about $4 million dollars. Based solely on this cost saving consideration over ten years of processing, the next positive effect on NPV at a 5% discount rate before taxes is about $40 million dollars. At a processing rate of 40,000 tonnes per day, both the CAPEX drops about 6% while OPEX drops 8%. The actual savings is about $18 million dollars in capital expense and and and annual savings in operating cost of about $7 million dollars. Based solely on this cost saving consideration over ten years of processing, the next positive effect on NPV at a 5% discount rate before taxes is about $70 million dollars. Further, without the differential flotation or rigorous cleaning stages required, copper and, more importantly, molybdenum recoveries will increase. Given the hydrophobic nature of molybdenum, it is reasonable to assume that recoveries over 90 % would be readily achieved to a bulk concentrate. Lastly, as will be elucidated further on in the paper, on site hydrometallurgical treatment of the bulk concentrates can provide high quality copper cathode, high purity molybdenum trioxide and the potential for by product rhenium recovery credits that may not occur with traditional off site smelting.
| Copper Concentrate | Molybdenum Concentrate | |||||
|---|---|---|---|---|---|---|
| Company% | Cu % | Mo % | % Cu Recovery | % Mo | % Cu | % Mo Recovery |
| Anamax | 32 | 0.26 | 87 | 51 | 1.4 | 30 |
| Asarco | 28 | 0.29 | 90 | 52 | 0.66 | 43 |
| Asarco | 29 | 0.15 | 84 | 51 | 0.7 | 21 |
| Cyprus Bagdad | 32 | 1.45 | 74 | 54 | 0.75 | ---- |
| Cities Service | 27 | 0.05 | ---- | 53 | 1.25 | ---- |
| Duval | 25 | ---- | 90 | 53 | ---- | 75 |
| Gibralter | 29 | ---- | 81 | 53 | 0.6 | 30 |
| Inspiration | 42 | 0.7 | ---- | 53 | 0.5 | ---- |
| Kennecott | 21 | 0.5 | 77 | 50 | 1.15 | 29 |
| Kennecott | 21 | 0.41 | 85 | 49 | 1.4 | ---- |
| Kennecott | 27 | 0.25 | 89 | 55 | 0.5 | 66 |
| Lornex | 33 | ---- | 90 | 56 | 0.65 | 75 |
| Magma | 28 | 0.54 | 86 | 56 | 0.9 | 69 |
| Noranda | 30 | ---- | 86 | 55 | 0.3 | 78 |
| Cyprus Pima | 27 | 0.24 | 87 | 40 | 1.9 | 31 |
| Southern Peru | 28 | 0.4 | 86 | 53 | 1.2 | 30 |
| Utah Mines | 23.5 | 0.8 | 88 | 42 | 0.5 | 30 |
| Reported Average | 28.4 | 0.46 | 85.3 | 51.5 | 0.9 | 46.7 |
Table 2: Historical Concentration Data on Copper Molybdenum Differential Flotation Mills.
| Operating and Capital Costs Upper numbers are operating costs in dollars per tonne feed. Lower numbers are total capital costs. (2007 USA dollars) | |||||
|---|---|---|---|---|---|
| Ore Feed Rate (tonnes/day) | Carbon-in-Pulp Mills | Agitation Leach Mills | Flotation Mills Number of Concentrate Products | ||
| 1 | 2 | 3 | |||
| 100 | $54.22 $7,832,900 |
$ 57.73 $ 10,315,900 |
$ 62.13 $12,974,200 |
||
| 500 | $28.48 $15,227,000 |
$33.95 $16,547,400 |
$19.76 $13,265,400 |
$22.22 $17,335,800 |
$24.59 $20,918,100 |
| 1000 | $21.98 $23,669,000 |
$26.91 $45,866,400 |
$14.74 $18,210,300 |
$16.62 $23,448,600 |
$18.03 $27,486,700 |
| 2000 | &16.95 $37,362,400 |
$20.91 $45,866,400 |
$12.18 $25,675,200 |
$13,41 $30,870,900 |
$14.65 $36,924,800 |
| 5000 | $12.99 $69,796,500 |
$15,97 $92,619,700 |
$8.82 $43,112,800 |
$9.59 $49,341,300 |
$10.28 $56,072,900 |
| 10,000 | $7.60 $75,235,300 |
$8.30 $84,014,800 |
$8.79 $91,268,600 |
||
| 20,000 | $6.68 $140,546,100 |
$7.27 $152,225,000 |
$7.69 $164,022,900 |
||
| 40,000 | $6.19 $270,220,400 |
$6.71 $288,367,800 |
$7.08 $307,026,900 |
||
| 80,000 | $6.05 $502,409,200 |
$6.56 $537,948,600 |
$6.92 $570,274,000 |
||
Table 3: Flotation Mill Capital and Operating Cost Data.
Confirmatory nsc pressure oxidation combined copper and molybdenum concentrate testing
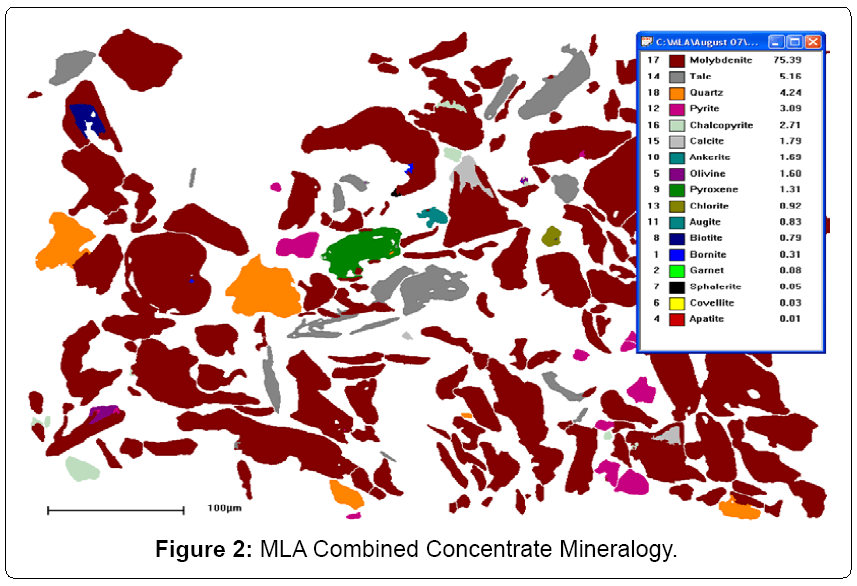
Confirmation testing of the application of industrial NSC pressure oxidation to a mixed chalcopyrite and molybdenite concentrate was undertaken. Previous studies and plant operating data had confirmed the applicability of NSC to chalcopyrite concentrates [6]. Furthermore, the applications of nitrogen species acid leaching systems for treatment of molybdenum concentrates are well documented and have been industrially piloted [7-9]. In addition, recent work suggests that the application of industrial hydrometallurgical pressure oxidation of molybdenum concentrates is currently being implemented [10,11]. Hence, a low grade, out of specification, molybdenite concentrate with appreciable chalcopyrite, pyrite and rhenium content was selected for the confirmatory testing Table 4. illustrates the elemental analysis of the chosen concentrate, while Figure 2. denotes the inherent quantitative mineralogical composition as determined by SEM Mineral Liberation Analysis.
| Mo, % | Cu, % | Re, % | Fe, % | TS, % | TC, % |
|---|---|---|---|---|---|
| 34.50 | 1.59 | 0.159 | 2.42 | 33.23 | 1.90 |
Concentrate Particle Size = 80 % passing 75 microns.
Table 4: NSC Tested Combined Molybdenum Copper Concentrate.
Design of NSC pressure oxidation mixed concentrate confirmatory testing experiments
Using Stat Ease Design Expert computer software, a statisticaly valid set of tests for NSC pressure leaching of the combined copper and molybdenum concentrate were designed as shown in Table 3. The key variable parameters studied included grind time (i.e. 0, 5 and 10 minutes) slurry solids concentration (i.e. 10, 30 and 50 g/L), initial sulfuric acidity (i.e. 25, 50 and 75 g/L), reaction time (i.e. 30, 60 and 90 minutes). For all these tests, total pressure was fixed at 90 psig, sodium nitrite addition at 2.0 g/L and autoclave stirring was done at 700 RPM. The testing was carried out and solids and liquids were analyzed by ICP for Re, Cu and Mo. Recoveries were then calculated and the data inserted into the Stat Ease program for optimization. The recovery data is also shown in Table 5.
| Std | Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| A: Grind | B: Solids | C: Initial | D: Temp | E: Reaction | Mo Rec | Re Rec | Cu Rec | |
| Time, min | g/L | Acidity, g/L | °C | Time, min | % | % | % | |
| 1 | 10 | 50 | 25 | 150 | 90 | 17.6 | 69.4 | 87.7 |
| 2 | 0 | 10 | 25 | 150 | 90 | 84.3 | 80.2 | 97.9 |
| 3 | 0 | 50 | 75 | 150 | 30 | 14.6 | 7.9 | 37.4 |
| 4 | 0 | 50 | 75 | 130 | 90 | 16.7 | 31.3 | 48.5 |
| 5 | 10 | 10 | 75 | 150 | 90 | 82.9 | 87.9 | 95.7 |
| 6 | 0 | 10 | 75 | 130 | 30 | 10.2 | 0.0 | 22.6 |
| 7 | 10 | 50 | 75 | 130 | 30 | 15.7 | 9.7 | 23.9 |
| 8 | 10 | 10 | 25 | 130 | 90 | 70.2 | 31.9 | 63.1 |
| 9 | 10 | 10 | 25 | 150 | 30 | 66.8 | 30.0 | 59.9 |
| 10 | 0 | 50 | 25 | 130 | 30 | 13.2 | 5.4 | 23.5 |
| 11 | 5 | 30 | 50 | 140 | 60 | 23.6 | 25.8 | 48.2 |
| 12 | 5 | 30 | 50 | 140 | 60 | 23.9 | 29.6 | 53.5 |
| 13 | 5 | 30 | 50 | 140 | 60 | 23.4 | 27.7 | 45.3 |
| Deposit | Indicated Resources (millions tonnes) | Grade | |
| Cu % | Mo % | ||
| North | 69.258 | 0.370 | 0.005 |
| South | 45.148 | 0.377 | No Data |
| Total | 114.406 | 0.373 | --- |
| Deposit | Inferred Resources (millions tonnes) | Grade | |
| Cu % | Mo % | ||
| North | 18.166 | 0.271 | 0.005 |
| South | 25.593 | 0.278 | No Data |
| Total | 41.759 | 0.275 | --- |
Table 5: Stat Ease Design of Experimentation Matrix and NSC POX Mo, Re & Cu Recovery Data.
Nsc Confirmatory Testing Results and Discussion
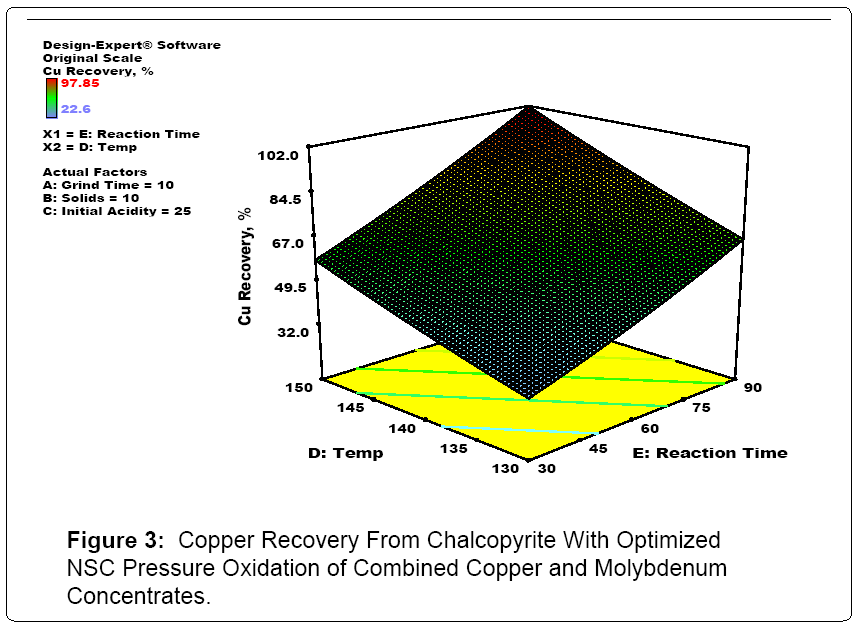
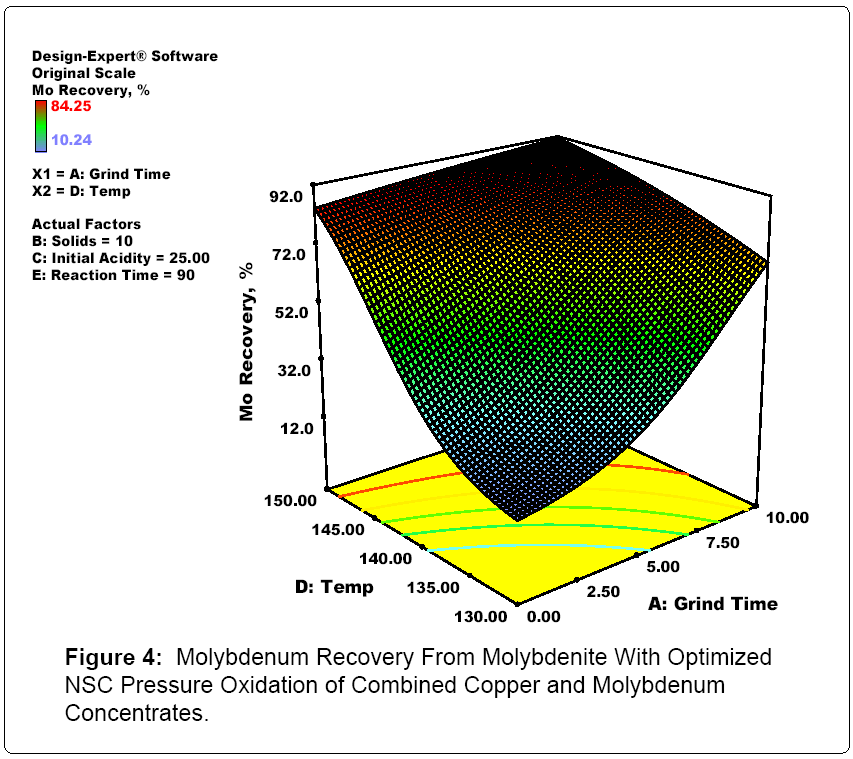
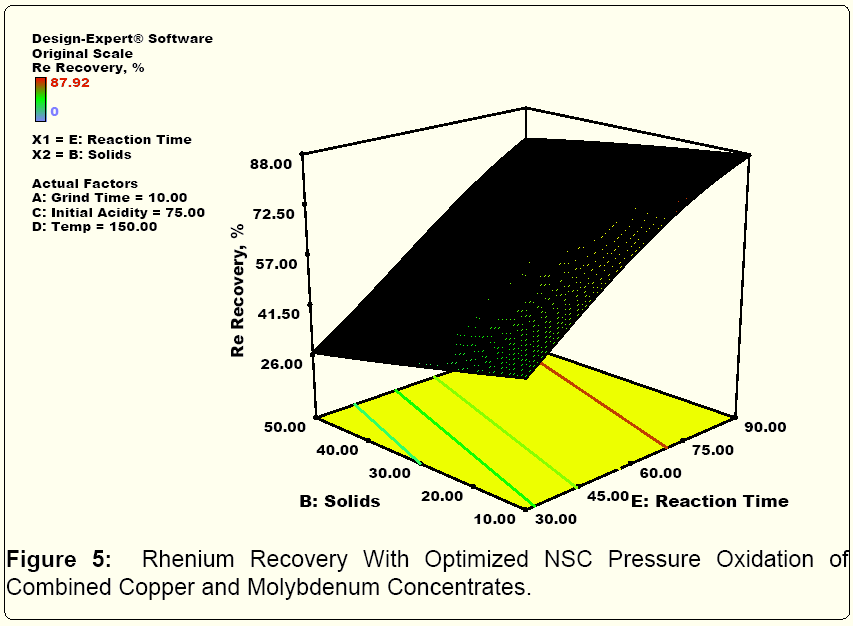
Figures 3, 4 and 5 summarize the Stat Ease optimization of modelling of the confirmatory NSC testing. In essence, the model fit for Mo, Cu and Re pressure leaching with NSC was excellent. For good molydenum recovery, grinding, higher temperatures and longer reaction times help at low molybdenum solids content. This is probably a function of molybdenum solubility limits. For good copper recovery, higher temperatuess and longer reaction times lead to better recoveries. Finally for rhenium, lower slurry solids densities and longer reaction times are the key elements for enhanced reovery. In addition, per the published literature and industrial operating practices, using both ion exchange and activated carbon, selective separation and concentration of both rhenium and molybdenum from copper, iron and other metal cations present in acidic mixed metal solutions produced in NSC testing was successfully carried out.
Industrial application and design considerations
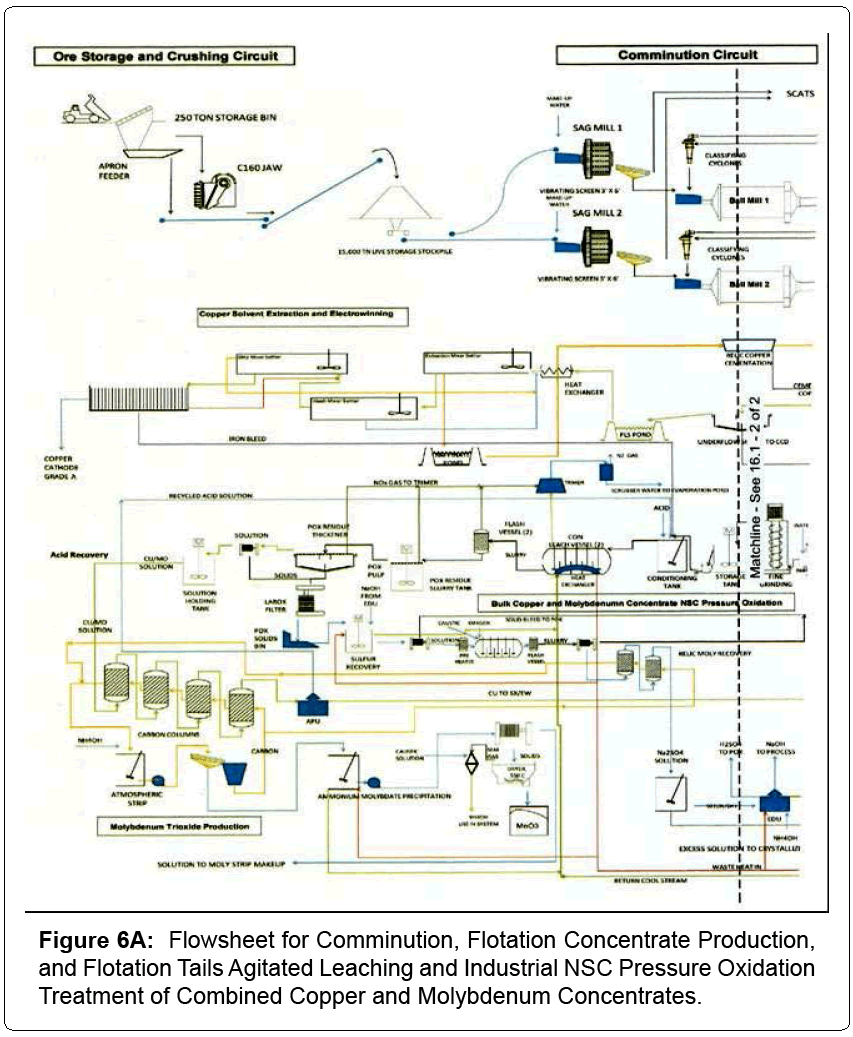
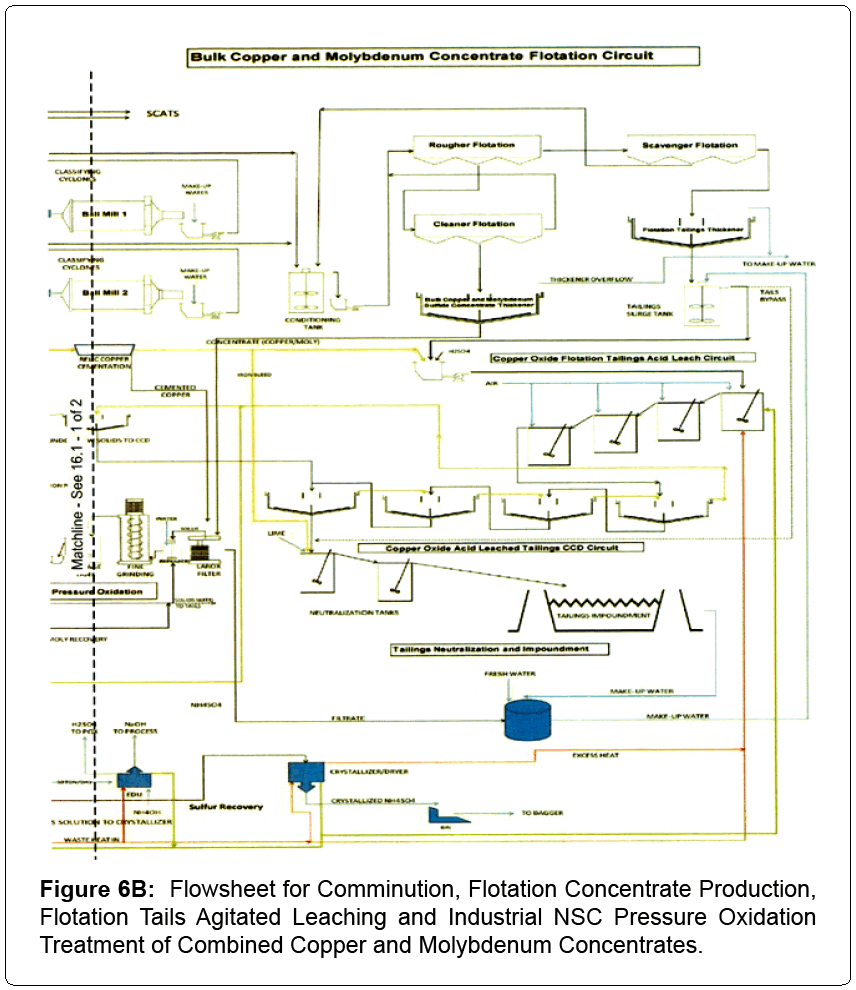
Based on industrial comminution, flotation and NSC pressure oxidation testing, industrial operating plant data and pertinent design criteria a comprehensive flowsheet was formulated for treating a copper and molybdenum ore body. The resultant flowsheet is split in half for better clarity and illustrated as Figures 6A and 6B. A detailed description of the process and its detailed development follows.
Based on a review of the available geologic resources and various contained mineral types, both an oxide and sulfide treatment scheme was required for the orebody. A literature review was undertaken of various copper deposits around the world with the intent of maximizing the copper recovery using the smallest footprint possible. The resource proposed to be treated is listed in Table 6. Illustrative Resource Proposed To Be Mined and Processed Via Open Pit Mining, Bulk Concentrate Flotation, Tailings Agitated Leaching and NSC Pressure Oxidation of Combined Copper and Molybdenum Concentrates.
| Circuit Configuration | 2 Extract +2 Strip |
| Organic | 30 v/o Acorga M5640 |
| PLS | 10 – 15 gpl Cu, Ph 1.8 |
| Lean Electrolyte | 25 gpl Cu, 185 gpl H2SO4 |
| O: A Ratio-Extract | 1.0 – 1.4:1 Strip 1.5: 1 |
| Stage Efficiency | Extract 95% Strip 95% |
Table 6: Vat Leaching Flowsheet Design Parameters.
A site visit identified an undetermined amount of sulfides located within the oxide portions of the ore body. Based on this find, an oxide heap leach scenario was dismissed as leaving an excessive amount of unleached sulfides on the pad that would potentially create environmental issues and ultimately not maximize the value of the deposit. Based on this, a flotation/tails leach/pressure leach scenario (i.e. a flowsheet similar to that of First Quantum Minerals – Kansanshi Operation in Africa) was developed. Based on information gleaned in part from the previous metallurgical testing, adjacent operations, and past experience, the following parameters were used to develop a realistic flowsheet based on proven industrial concepts.
Comminution
Previous work undertaken consisted of six Bond Ball Mill Work Indices, indicating a range of 13.4 to 16.2 kWh/t (14.8 to 17.8 kWh/t), and the results reflect a final screen size of 65% passing 200 mesh. Overall, the data, together with inspection of the drill cores, indicates that the ore is generally hard and highly competent. A detailed program is recommended to identify and confirm the range of ball mill work indices. This work should be expanded to include the testing of Rod Mill Work Indices, Unconfined Compressive Strength (UCS), Abrasion Indices, and JK Drop Weight Tests. Since no SAG Mill testing has been completed on the project, a review was performed to determine a basis point for the prefeasibility comminution design of a SAG Mill grinding circuit. The review included the investigation of the comminution circuits at both adjacent mills at as well as several other North American copper projects. The investigation examined grinding conditions with similar work indexes and rock types. Based on this review, the basic design of the Asarco South Mill (Pima) was chosen as a base case for the flow sheet development.
Sulfide flotation
Previous testwork undertaken has provided useful background data for the development of the current flowsheet. Key findings of the work include the following:
• Flotation of sulfide material is relatively simple and conventional with high recovery and good concentrate grades.
• Separate sands and slimes circuits do not appear to be necessary.
• Oxide and mixed ores respond poorly to flotation. As a consequence, oxide and mixed ores will be treated in a tails leach system with oxide ore by-passing the flotation circuit.
Most of the testwork was carried out on drill core with separate testing being completed on leaching and flotation, but not on both together. Further testwork will be required to confirm whether or not there is an effect on the leaching circuit due to excess organic carry over from the flotation circuit.
Oxide leaching
Preliminary bottle roll testwork undertaken identified the following:
• Potentially long residence times of 24 hours at a pH range of 1.0 to 1.2.
• Estimated recovery of 85 to 95% of the soluble copper in 24 hrs at 61 microns.
• Major improvement in extraction rate through decreasing grind size.
• Gangue acid consumption has varied between testwork programs and is dependent on carbonate content, but seems to be insensitive to grind size.
• Gangue acid consumption is estimated at 17 kg/t between samples and needs to be modeled across the orebody as a component of the mine schedule.
During testing of the vat leaching, an agitated leaching test of the 38 micron thickener underflow material was undertaken to identify why there were unleached fines. The testing of the material identified the material leached well at a pH of 1.9 for 48 hours and obtained a total extraction of approximately 73% of unleached fines.
Solvent extraction
Solvent extraction testwork was completed on resource material for the development of the vat leaching. The SX circuit consisted of twostage extraction and two-stage stripping. The organic phase employed in this circuit was 25% v/v ACORGA® M5774 (Cytec) extractant in Exxsol® D80 (Exxon Mobil aliphatic solvent) diluents matrix. Prior to introducing the PLS from the vat leach to the SX feed, the solution was filtered through 1-micron string-wound cartridge filters. The PLS was collected in batches using 1000 L carboys. Each batch was filtered, stirred to homogeneous, sampled, and then fed to the SX circuit. The SX circuit was fed PLS solution with a copper grade averaging 2.75 g/L Cu and it generated a raffinate bearing an average 0.038 g/L Cu during the final 2 d of operation (during steady-state conditions). The copper grade in the aqueous solution in the preceding two stages of extraction averaged 0.046 and 0.181 g/L, respectively. This equates to a calculated recovery of 93.4%, 98.3%, and 98.6% as the SX feed solution was sequentially processed through the 3-stage extraction circuit. The pH of the SX feed and raffinate solution during this same period averaged 1.74 and 1.50, respectively. The copper concentration in the loaded and stripped organic averaged 6.52 and 3.17 g/L. Some crud formation was observed in the extraction and strip mixer-settlers. During the short testing period, the crud did not appear to interfere with the SX operation. The strip solution employed at start-up was a 235 g/L sulfuric acid solution. When the strip solution reached 45 g/L copper the EW process was initiated. During EW operation, the copper grade in the rich and lean electrolyte averaged 47.5 and 35.4 g/L Cu, respectively. The free acid averaged 169 and 189 g/L H2SO4, respectively. Prior to starting the EW process, the strip solution was dosed with sufficient guar gum to achieve 10 mg/L in the strip solution inventory. Cobalt sulfate salt was also added to the strip solution, which yielded ~25 mg/L Co in solution. The target electrolyte temperature and current density were 40°C and 270 A/m2. The actual measured temperature of the electrolyte and current density were 31.1ºC and 313 A/m2. Three copper plates weighing a total of 35.2 kg were harvested. The deposit was dense and the appearance reflected typical cathode surface morphology. The nominal purity of the metal was 99.97%. This could be improved with the use of different anodes and fine tuning of reagent additions to the EW cell. Overall copper recovery from the circuit was 98%. The basic design parameters noted above were used in the flowsheet design along with a design review by Cytec below in Table 7.
| File | Org. Flow | PLS Flow | L.E. Flow | PLS | O:A Ext. | O:A Strip | M5640 | Raffinate | Rich E. | Recovery | Production | Production | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (gpm) | (gpm) | (gpm) | Cu | pH | (v/o) | (g/L) | (g/L) | (%) | (mt/d) | (mt/y) | |||
| CABC1 | 1,500 | 1,500 | 1,000 | 10.0 | 1.8 | 1.00 | 1.50 | 30 | 0.73 | 48.90 | 92.7 | 80.9 | 27,650 |
| 1A | 1,650 | 1,500 | 1,100 | 10.0 | 1.8 | 1.10 | 1.50 | 30 | 0.60 | 47.82 | 94.0 | 82.9 | 28,068 |
| 2 | 1,500 | 1,500 | 1,000 | 11.0 | 1.8 | 1.00 | 1.50 | 30 | 1.09 | 49.86 | 90.1 | 84.9 | 29,558 |
| 2A | 1,650 | 1,500 | 1,100 | 11.0 | 1.8 | 1.10 | 1.50 | 30 | 0.85 | 48.83 | 92.2 | 88.1 | 30,273 |
| 3 | 1,500 | 1,500 | 1,000 | 12.0 | 1.8 | 1.00 | 1.50 | 30 | 1.59 | 50.62 | 86.8 | 84.9 | 30,988 |
| 3A | 1,650 | 1,500 | 1,100 | 12.0 | 1.8 | 1.10 | 1.50 | 30 | 1.22 | 49.69 | 89.8 | 88.1 | 32,156 |
| 4 | 1,500 | 1,500 | 1,000 | 13.0 | 1.8 | 1.00 | 1.50 | 30 | 2.21 | 51.19 | 83.0 | 80.0 | 32,193 |
| 4A | 1,650 | 1,500 | 1,100 | 13.0 | 1.8 | 1.10 | 1.50 | 30 | 1.71 | 50.40 | 86.9 | 92.4 | 33,689 |
| 4B | 1,800 | 1,500 | 1,200 | 13.0 | 1.8 | 1.20 | 1.50 | 30 | 1.36 | 89.5 | 95.2 | 34,748 | |
| 5 | 1,500 | 1,500 | 1,000 | 14.0 | 1.8 | 1.00 | 1.50 | 30 | 2.93 | 51.60 | 79.1 | 90.5 | 33,033 |
| 5A | 1,650 | 1,500 | 1,100 | 14.0 | 1.8 | 1.10 | 1.50 | 30 | 2.30 | 50.95 | 83.5 | 93.8 | 34,237 |
| 5B | 1,800 | 1,500 | 1,200 | 14.0 | 1.8 | 1.20 | 1.50 | 30 | 1.84 | 50.20 | 86.9 | 99.5 | 36,301 |
| 5C | 1,950 | 1,500 | 1,300 | 14.0 | 1.8 | 1.30 | 1.50 | 30 | 1.50 | 49.41 | 89.3 | 102.2 | 37,295 |
| 6C | 1,950 | 1,500 | 1,300 | 15.0 | 1.8 | 1.30 | 1.50 | 30 | 1.98 | 50.03 | 86.8 | 106.5 | 38,852 |
| 6D | 2,100 | 1,500 | 1,500 | 15.0 | 1.8 | 1.40 | 1.50 | 30 | 1.66 | 49.30 | 89.0 | 109.1 | 39,822 |
Table 7: Cytec Vat Leaching Flowsheet Design Review.
No significant concerns were noted. A laboratory-scale pilot plant run will be required to confirm the operating parameters and examine the build-up of deleterious elements such as silica and crud under a flotation/leach setting. A relic copper cementation circuit will be setup on the raffinate to maximize copper recovery. The cementation process will be similar to that of Kennecott’s Spedden process and used to ensure minimal copper is sent back to the tails leach and potentially lost. The cemented copper product will be recycled back to the pressure oxidation circuit. Subsequent, testwork will be required to optimize the cementation circuit design, recovery, and economics.
Thickening
Limited thickening or rheological testing has been completed. Only the slimes from the Vat leaching tests had thickening and rheological testing. Industry averages were used to size the thickeners and other necessary settling equipment in the flotation and leaching circuits. The following design parameters were used in developing the thickener characteristics:
Thickener design criteria
Flotation Tails
Thickener U/F density - 50% solids
Flux rate t/h/m2 - 0.52
The current design uses conventional thickening and assumes a 50% underflow density. Further testing will be required to ensure that high compression thickening will not be required to make the necessary 50% underflow prior to leaching.
Post Leach
Thickener U/F density - 50% solids
Flux rate t/m2/h - 0.52
Flocculant may be required to meet the specific design criteria noted above.
The current design uses conventional thickening and assumes a 50% underflow density. A case study comparing filtration with countercurrent decantation (CCD) for the leach residue application will need to be reviewed. Based on the available data, CCD typically has slightly better overall economics, with significant performance advantage and reliability in the cleaning of the leach residues. The process design will need to review the use of high compression thickeners to maximize underflow densities and minimize losses of copper through the CCD thickeners.
NSC Pressure Oxidation (POX) Residue
Thickener U/F Density - 50% solids
Flux rate t/h/m2 – 1.0
Flocculent may be required to meet the specific design criteria noted above.
The current design uses conventional thickening and assumes a 50% underflow density. A case study comparing thickening to filtration will be required to optimize this part of the circuit. Further thickening and rheological testing will be required on all aspects of the operation.
Filtration
No vacuum and pressure filtration tests were carried out in conjunction with the Pregnant Feed Solution thickening tests. Industry averages for chalcopyrite concentrate filtration were used. The following design parameters were used in developing the filtration characteristics.
Filtration design criteria
Copper Concentrate (Includes concentrate thickener)
(Assumes 65% solids, product has a P80 of 325 mesh)
Flux rate t/h/m2 - 0.487
NSC POX Material (includes any vertical mill ground material to include leach residues)
(Assumes 65% solids, product has a P80 of 10 micron)
Flux rate t/h/m2 – 1.0
Further filtration test data will be required for the finalization of the flowsheet.
Mixed copper and molybdenum concentrate leaching
The sulfide flotation concentrate will be treated using industrially proven Nitrogen Species Catalyzed Pressure Oxidation Leaching (NSC). A vertical grinding mill will be fed concentrate containing a nominal 29% copper and approximately 0.9% molybdenum and ground to approximately 10 microns. The ground concentrate is then fed to one of two pressure oxidation autoclaves using the following leaching parameters listed in Table 8.
| Parameter | Unit | Value |
|---|---|---|
| Solids Flowrate | t/d | 202 |
| Slurry Solids Content | g/L | 50 |
| Initial Free Sulfuirc Acid | g/L | 25 |
| Reactor Working Pressure | kPag | 620 |
| Maximum Temperature | °C | 155 |
| Total Leach Time | min | 45 |
Table 8: NSC Pressure Oxidation Combined Concentrate Leaching Parameters
From the autoclave, a Cu/Mo bearing solution is recovered from the autoclave effluent via a high-rate thickener and polishing filtration, and then through a series of activated carbon columns for molybdenum recovery. Then an EcoTec acid purification unit (APU) is used to recover excess sulfuric acid which is recycled back to the autoclave feed system. After molybdenum and acid recovery, the copper-laden leach solution is sent to a conventional SX/EW circuit and the copper recovered as “electrowon” cathode.
From industrial operating data, the NSC process results in near total oxidation (+99%) of the sulfide concentrate and is estimated to convert a majority of the sulfide sulfur to sulfuric acid (60%) based on operating experience, and some to elemental sulfur (40%). The elemental sulfur is collected in the POX residue through thickening and filtration. The POX residue is then leached for the sulfur content with sodium hydroxide to make a soluble sodium sulfate and a final leach residue. The NSC POX residue leaching parameters are listed in Table 9. Approximately 50% of the POX residue will be recycled back to the NSC leach for enhanced copper and molybdenum recovery and to provide inert heat sink materials to the NSC POX system. This helps to optimize the concentrate pressure oxidation system by providing more heat capacity. The other estimated 50% of residue will be sent to tails. The amount recycled and sent to tails will be optimized to maximize throughput and metal recoveries. In the overall POX residue reaction, sodium sulfate is formed. After thickening and polishing filtration, the sodium sulfate solution is passed through a set of carbon columns to recover relic molybdenum. The purified sodium sulfate solution will be further processed via electrodialysis (EDU) and evaporation to recover a 70% sulfuric acid product and a 100 g/L sodium hydroxide product. These will be recycled back into the process plant. Any excess sodium sulfate will be sent to a multiple effect crystallizer, crystallized into flake sodium sulfate, filtered, dried, bagged, and sold. The relic molybdenum recovered will be processed as identified below. The molybdenumladen activated carbon is atmospherically stripped with ammonium hydroxide to make an ammonium molybdate solution. The pH of the stripped solution is then decreased to 2.0 using sulfuric acid causing ammonium molybdate to precipitate. The precipitated ammonium molybdate is then filtered with the clear solution being recycled back to the process. The filtered ammonium molybdate solid is then calcined at 5500C, converted to molybdenum trioxide, bagged, and sold. The ammonia driven off by the calcination process is scrubbed, collected, and reused in the system. The stripped carbon will be recycled back to the molybdenum recovery circuit. A portion of the carbon will also be reactivated in a conventional kiln operation. Both the NSC concentrate and leached residue pressure oxidation processes can provide significant heat. For efficient process, heat, or energy economy, the resultant heat will be used in the process plant to increase the atmospheric flotation tailings leach temperatures, thereby improving copper leaching kinetics and ultimately increasing copper recovery levels. This waste heat will also be utilized in the EDU sulfuric acid recovery and sodium sulfate multiple effect evaporation system.
| Parameter | Unit | Value |
|---|---|---|
| Solids Flowrate | t/d | 126 |
| Slurry Solids Content | g/L | 50 |
| Reactor Working Pressure | kPag | 620 |
| Maximum Temperature | °C | 130 |
| Total Leach Time | Min | 45 |
Table 9: NSC POX Residue Leaching Parameters
The production rate for the NSC concentrate pressure oxidation circuit is designed with excess capacity so outside copper and molybdenum containing concentrates can be processed through the plant on a toll or purchased basis. Further testing will be required to ensure the optimized parameters of the NSC pressure oxidation system are identified and quantified for concentrate treatment and for final sizing of the various items associated in the metal recovery systems.
Crushing and ore storage and reclaim
Initial ore to the plant is expected to be relatively easy to treat. A small portion is highly weathered and the remainder is highly competent with low moisture content values reported at approximately 3%. The proposed crushing circuit utilizes a C160 Nordberg Jaw Crusher that would crush run of mine rock to a nominal 152 mm. The jaw crusher feeds a live-surge capacity stockpile which is nominally designed for 24 hours of live capacity, and six reclaim feeders will feed the two proposed grinding circuits.
Grinding and classification
The two milling circuits have been designed on the basis of the use of a SAG mill and ball mill combination. The design is based on the Asarco South Mill (Pima Mill). The ore milling circuits have been designed using a SAG Mill Work Index of 5.58 kWh/t to 1,200 microns, and a ball mill work index of 16.5 kWh/t. The bond ball mill work Index for the ore is reported to vary from 15.4 to 17.6 kWh/t, and there is no significant difference between the sulfide and oxide material. On this basis, the installed power for the SAG mill grinding 5.2 Mt/y of ore is calculated at 8.5 MW.
The designed steel charge for the SAG Mill consists of a nominal ball load of 8% by volume, with a design load of 12%. Abrasion indexes are based on the nearby Highmont Mill which are considered moderate and have been incorporated into the operating costs. Values for the abrasion indices vary from 0.5 to 0.7. The design cyclone overflow product size is at a P65 of 74 micrometer for the downstream processes of leaching and/or flotation.
Flotation
Oxide and sulfide ores will be campaigned separately with all of the ores initially treated by flotation and leaching. As oxide ore is depleted, the leaching circuit will be bypassed. The flotation of the sulfide ore is based on a conventional rougher/scavenger and two-stage cleaning circuit. Rougher concentrate potentially will be reground to liberate sulfide copper minerals from gangue. Testing will be required to confirm the need for a regrind mill. The selected reagent regime is relatively simple, using only xanthate as collector combined with a frother. The plant has been designed to maximize recovery of sulfide mineralization.
Recovery of acid soluble copper mineralization is not critical as the flotation tailings will be acid leached. General reagent consumption and residence times have been based largely on recent testwork performed with flotation prior to leaching. Locked cycle bench testing has been performed on the different ore types and locations.
Pre-leach dewatering
The pre-leach dewatering (thickening) step is designed to maintain the solution balance in the leach SX/EW circuit. The leach circuit is designed for a 50% underflow.
Copper leach-oxide/mixed ore only
Testwork results indicate relatively slow copper leach kinetics for the flotation tail streams. The leach circuit design is based on a 24 hour residence time at 50% solids. Gangue acid consumption will be based on the mine schedule, with a total estimated acid consumption of 17 kg/t, allowing for losses and bleed streams.
Leach residue counter current decantation (CCD)
Literature data has been used to design a CCD circuit comprising four stages of 35 m diameter standard thickeners. Testing will be required to evaluate the CCD circuit design.
Solvent extraction: The mass balance indicates a maximum PLS flowrate of approximately 617 m3/h. The mixer-settlers have then been sized on the basis of residence time of two minutes in each of the two mixer stages, and a settler specific area of 4.5 m3/h/m2. The configuration of the solvent extraction circuit is comprised of two extract mixer/ settler stages, a wash stage, and a single strip stage.
Maximum flow will occur during mining of the predominantly oxide leach cap. Flow rates to the pregnant leach solution pond will consistently drop during the first 3 y of the project to approximately 143 m3/h as sulfides become the predominant species mined. The solvent extraction system is designed for maximum expected flows and copper grades.
Electrowinning: The tank house will use stainless steel cathode technology, with anodes consisting of a lead-calcium-tin alloy. Electrode sizes will be standard for copper recovery, with a submerged area of 1.1 m2, and the cathodes will be pulled on a 7 d stripping cycle. An average current density of 258 (maximum 275) A/m2 will be used and which should be readily achievable in the operating environment, resulting in a requirement for 149 cells of 45 cathodes each. This cell size results in a design current of 24,000 Amps divided between two rectifiers. The cells will be arranged in two blocks of 75 cells, each with its own rectifier. Cathode stripping will typically be manual with automatic presentation of the cathodes to the stripping station, and will operate on at least two shifts per day. Total design cathode copper production is 30 kt/y.
Concentrate handling: The concentrate handling system will consist of a Larox pressure filter and storage system. The Larox filter is designed for 37 m2 of filtration capacity. Dewatered material from the Larox filter will either be repulped and fed to the Nitrogen Species Catalyzed (NSC) concentrate leach system, or stored and shipped to a smelter. The NSC oxidative pressure leaching system consists of a 300 hp tower mill to grind to a P80 of 10 micron. From there, the material will be diluted to 50 g/L with recycled acidic process solution and pressure leached in one of two unlined grade 316 stainless steel pressure leach vessels. Pressure and temperature will be maintained accordingly for full leaching of the copper and molybdenum, while maintaining some of the sulfur as elemental sulfur.
Upon discharge from the leach vessels, the pulp will be flashed and the leach residue thickened and filtered. The pregnant leach solution (PLS) will pass through secondary filtration and on to molybdenum recovery. The filtered pressure leached residue, or POX residue, will report to sulfur recovery.
Molybdenum recovery: The PLS grade will be maintained at approximately 15 g/L copper and 0.28 g/L molybdenum. The PLS will be passed through a series of five 2 t carbon columns for molybdenum recovery. Based on industrial practices, approximately 99% of the molybdenum is expected to be recovered in the carbon columns. After molybdenum recovery, the PLS will pass through an industrial EcoTec acid purification unit to recover free acid from the PLS. The recovered sulfuric acid solution product will be recycled back to the NSC pressure oxidation system. The resultant PLS free acidity will be dropped from about 30 g/L to approximately 5 g/L. This molybdenum-free PLS solution is then sent to the PLS pond where the copper is recovered through a conventional SX/EW circuit. Upon loading the carbon with molybdenum, the carbon is transferred to an atmospheric strip vessel. The pH is raised with ammonium hydroxide to strip the molybdenum from the carbon, and a concentrated solution of ammonium molybdate is then formed. The ammonium molybdate solution is treated by pH adjustment to 2.0 using sulfuric acid, and then solid ammonium molybdate precipitates. The ammonium molybdate slurry is then filtered in a 3.7 m2 filter, dewatered, and fed to a dryer heated to 550 0C. The ammonium molybdate is calcined to form molybdenum trioxide. The ammonia driven off in the calcining process is captured in a water scrubber and reused in the process. The molybdenum trioxide product is dried, bagged and sold.
NSC POX leached residue sulfur recovery system: Due to the high cost of sulfur based products, another POX system will be implemented to recover sulfur from the NSC leached residue. The NSC POX residue is settled in a 35 m thickener and the underflow is filtered in a Larox filter. The POX material is stored until ready for sulfur recovery. During sulfur recovery, the POX residue is fed into a leach vessel and ultimately pressure leached with sodium hydroxide and oxygen. The sulfur is converted to sodium sulfate and a sulfur free POX residue is formed. The POX residue is thickened and approximately 50% of this residue is recycled back to NSC, with the remaining 50% of the material going to final tails. The sodium sulfate solution is filtered and polished, and then fed to a relic molybdenum recovery circuit similar to that discussed above. The clarified and purified sodium sulfate solution is then fed into an Electrodialysis Unit (EDU) and evaporator system. The EDU will convert approximately 50 t/d of sodium sulfate into about 25 t/d of caustic. The caustic grade will be 100 g/L of sodium hydroxide. Also, 35 t/d of sulfuric acid will be produced at a concentration of 70% sulfuric acid. These by-product solutions will be recycled and utilized in the process where required for leaching, sulfur recovery, and neutralization. Excess sodium sulfate solution from this process will also be treated in a conventional crystallizer system and solid sodium sulfate will be produced, bagged, and sold based on market prices and demand.
Order of magnitude operating and capital cost estimate: Annual production is based on mining activities operating at assumed 90% efficiency. The pre-production stripping numbers are also assumed and will be defined when the final geologic and mine reserve models have been completed. Haul road distances are also assumed and will be determined once a location for the processing plant has been selected and waste rock storage areas designated. The development and operational key process indicators are:
| • Pre-production Stripping (North Pit) | 300 ktonnes |
| • Pre-production Stripping (South Pit) | 200 ktonnes |
| • Haul Road Construction (North Pit) | 2.5 km |
| • Haul Road Construction (South Pit) | 2.5 km |
Initial Production:
| Ore Production | 25 ktonne/d |
| Waste Production | 50 konnet/d |
| Concentration Production | 325 tonne/d |
| Annual Production- Ore | 8.75 million tonnes |
| Annual Production- Waste | 17.5 million tonnes |
The milling plan estimates capital and operating costs assume a nominal 25 kt/d mining operation. The milling plan includes:
• All labor, material, supply and equipment operating costs for the mill and associated concentrate leach plant.
• Supervision, administration and on-site management.
• Benefits and employment taxes.
• All on-site development for start-up and production.
• Mill equipment and facilities purchase and installation or construction.
• Engineering and construction management fees.
Pre-production development, installation and construction of all equipment and facilities necessary to operate the mill at a nominal 25 kt/d are included. Costs associated with the following facilities and operations are included. However final locations and design details are pending:
• Crushing and conveyance of ore to the grinding circuit.
• Grinding and flotation.
• Copper and molybdenum concentrate pressure leaching.
• Molybdenum trioxide recovery.
• Copper solvent extraction and copper cathode electrowinning.
• Eco Tec APU sulfuric acid recovery.
• Sodium sulfate production.
• Electrodialysis salt splitting of sodium sulfate for caustic and sulfuric acid production.
• Tailings facility.
• Basic access roads, power lines, and pipelines.
• Construction, installation and operation of facilities and equipment necessary for equipment maintenance and repair, electrical system, fuel distribution, water storage and drainage, sanitation facilities, offices, labs, storage, and equipment parts and supply storage.
• The mill and metallurgical plan does not include:
• Permitting and environmental assessment costs.
• Home office overhead.
• Taxes (except sales taxes and employment taxes).
• Insurance.
• Depreciation.
• Off-site transportation of products.
• Incentive bonus premiums.
• Overtime labor costs.
• Sales expenses.
• Interest expenses.
• Start-up costs (except for working capital).
• Depletion rates.
• Environmental costs, including reclamation.
• Reclamation on-going assuming a 2 y start-up period.
Tables 10 and 11 summarize the updated order of magnitude capital and operating cost estimates done to an accuracy of +/- 30%.
| COST SUMMARY | |
|---|---|
| Category | $/tonne |
| Mine | $ 1.21 |
| Process Plant | $ 11.10 |
| Administration | $ 0.46 |
| Total Operating Cost | $ 12.66 |
Table 10: Updated Operating Cost Estimate US$ +/- 30%.
| COST SUMMARY | |
|---|---|
| Category | US$ |
| Mine | $55,000,000 |
| Mill & Hydromet Plant | $ 275,000,000 |
| Indirects (G&A, EPCM and Startup) | $ 60,000,000 |
| Contingency | $ 7,500,000 |
| Working Capital & Reclamation | $ 27,500,000 |
| Total Capital | $ 425,000,000 |
Table 11: Updated Capital Cost Estimate, US$ +/- 30 %.
Based on the above updated estimates, using a copper price of $ 3.00 USD/lb and assuming some minor historic by product credits, a before tax scoping level discounted cash flow analysis for an estimated mine life just over ten years was carried out. In summary, at a discount rate of 5.0 % the project NPV is estimated to be about $ 500,000,000 USD with an IRR of almost 30%, a present value ratio of 1.18 and a project capital investment payback period of about four years.
Summary
This paper has demonstrated the successful application of industrial NSC pressure oxidation to combined concentrates containing both copper and molybdenum as chalcopyrite and molybdenite respectively. Confirmatory optimized NSC pressure leaching was successfully undertaken to leach copper, molybdenum and rhenium. Finally, using this confirmatory test data along with industrial operating data and experience, design criteria, flow sheets and order of magnitude capital and operating costs estimates were produced.
Acknowledgement
Thank you to Dr. Paul J. Miranda for all the analysis and mineralogical assessment for the NSC confirmatory testing of the bulk molybdenite and chalcopyrite concentrates. In addition, as a courtesy to the reader, both pertinent references and a bibliography of related work are included in the paper.
References
- Anderson CG, Krys LE, Harrison KD (1992) Treatment of Metal Bearing Mineral Material. US Patent 5: 096-486.
- Anderson CG, Harrison KD, Krys LE (1996) Theoretical Considerations of Sodium Nitrite Oxidation and Fine Grinding in Refractory Precious Metals Concentrate Pressure Leaching. Minerals and Metallurgical Processing, AIME-SME 13.
- Baldwin SA, Van Weert GV (1996) On the Catalysis of Ferrous Sulfate Oxidation in Autoclaves by Nitrates and Nitrites. Hydrometallurgy, Elsevier Science 42.
- Gok OS (2009) On The Role of Low-Concentration Nitrite In Oxidative Leaching with Oxygen, PhD Thesis, Colorado School of Mines, USA.
- Peters E (1992) Hydrometallurgical Process Innovation. Hydrometallurgy 29: 431-459.
- Anderson CG (2003c) The Application and Economics of Industrial NSC Pressure Leaching to Copper Ores and Concentrates. COBRE 2003, Santiago, Chile, December.
- Peters E (1976) Direct Leaching of Sulfides: Chemistry and Applications. Metall. Trans 7B: 505.
- Kerfoot DGE, Stanley RW (1976) Hydrometallurgical Production of Technical Grade Molybdic Oxide from Molybdenite. U.S. Patent 3: 988-418.
- Weber FW (1925) Silver from Sulfides. US Patent 1,555,615. Chemical Abstracts 193: 473.
- Olsen D (2008) Kennecott to invest $ 270 million in molybdenum processing facility. The Enterprise.
- Freeport McMoRan Copper and Gold (2010) Bagdad Mine Molybdenum Concentrate Pressure Oxidation, Personal Communication.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 17928
- [From(publication date):
September-2013 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 13165
- PDF downloads : 4763