Novelty and Compliance of Oral Fast Dissolving Thin Film A Patient Friendly Dosage Form
Received: 18-Jan-2021 / Editor assigned: 01-Jan-1970 / Reviewed: 01-Jan-1970 / Revised: 01-Jan-1970 / Accepted Date: 25-Jan-2021 / Published Date: 01-Feb-2021
Abstract
The oral fast-dissolving film is an advanced, patient compliant and novel delivery system. The acceptance of this ingenious dosage form is increasing day by day due to its several comparative advantages of being cost-benefit, rapid dissolving without water aid, as well as greatly compliant to both geriatric and pediatric patients in emergency conditions and specific diseases. Moreover, it is a worthwhile dosage form for the drug experiencing pre-systemic metabolism and lesser bioavailability. This review article covers history, advantages, disadvantages, limitations, ideal properties, classification, formulation consideration, method of manufacturing, quality parameters both in-vitro and in-vivo, advancement in technology, commercial trends and literature review of the previous work for oral fast dissolving films. It can be concluded that it one of the fastest-growing dosage forms holding a lot of potentials especially for commercial use due to its unique characteristics, novel attributes, competitive standing, and cost-adequacy.
Keywords
Fast dissolving film; Patient compliance; Novel; Oral formulation
Introduction
The fast-dispersible film is defined as a highly advanced type of solid dosage form having greater compliance. It promotes the effectiveness of a drug component, dissolving rapidly in the oral cavity [1]. This delivery system offers convenience to patients, however, the product will be acknowledged truly after more formulations become accessible [2].
History of film formulation
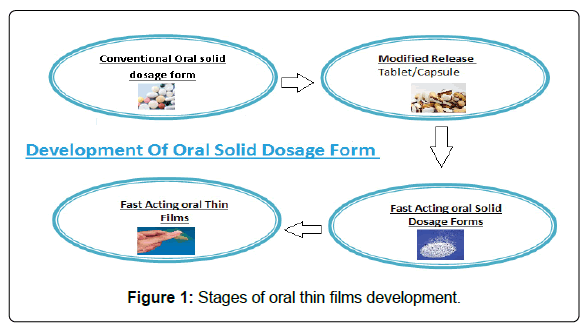
In the present era, researchers are dependably looking for current methods for developing advanced, contemporary dosage designs which are not just safe and efficacious, yet additionally savvy and simple to formulate. Dosage forms, holding patient compliance, are in demand these days. Among conventional dosage forms, orally administered medicines are considered as the most favourable and desired products [3]. Oral rapid dissolving tablets were introduced in the 19th century beginning to combat the issues related to liquid formulations, however, disadvantages associated with tablet form open the door for new advancement called oral dissolving films [4]. In comparison to other dosage forms, rapid dissolving oral films currently enjoy the centre of attention [5,6] the majorities of quick-dissolving pharmaceutical products are available in form of tablets and disintegrate rapidly. Oral fast-dissolving films disintegrate immediately upon placing over the tongue and do not require water or chewing. This innovative drug delivery system was initially formed in the late 1970s [7] given in Figure 1.
Merits
• Oral rapid dissolving thin films feature several advantages than the other oral preparations:
• The presence of an immense surface area promotes rapid disintegration as well as dissolution [8].
• Being flexible and handy, fast dissolving thin films provide ease during storage and transport in contradiction to orodispersible tablets [8] and do not need specific packaging as required by other products [9].
• Each film strip assures accurate dose administration [10].
• It can be administered without requiring water and the film containing drug dissolves quickly in the mouth [11].
• Any special practice of administration is not required in the case of oral thin films [12].
• The dosage form provides the best systemic absorption to drugs that experience the first effect [13], hence improve their bioavailability [14] therefore in this way promotes rapid therapeutic action [15].
• Due to the escape of first-pass metabolism, this delivery system can reduce dose and side effects [16].
• This dosage form is extremely patient compliant as it is non-invasive [17,18], suitable for paediatrics and geriatrics [19]uncooperative and unconscious patients with different illnesses [20], for cardiovascular patients [9], for people with respiratory ailments [5] and chemotherapy patients [16], patients having swallowing problems [21]
• It is exceptionally useful in cases where a local action [22] and an ultra-rapid drug onset are needed [5].
• It delivers a pleasant mouthfeel and also valued for masking the taste [23], moreover, well favourable for travelling patients [5].
• The oral bioavailability of drugs also gets enhanced in this dosage form because of lesser drug decomposition. [24]
• Fast dissolving oral thin film has longer and improvised stability. It has the advantage of both the solid and liquid dosage forms concerning solubility and bioavailability respectively [23]. The unique packaging of OFDTF enhances product stability [25].
• There is an immediate release of one or more drug ingredients, as these films disintegrate within seconds once taken orally [3].
• The dosage form is technically advantageous since its excipients include sugars and belong to the GRAS category [25]
• Business benefits concerning product differentiation and promotion, as well as patent extension, are also provided [8]. An unprecedented business structure is not required for industry [26].
Demerits
• Special moisture-resistant packaging is required as fast dissolving thin films are sensitive to moisture [12], and this particular packing is costly [26]
• From the technical aspect, dose uniformity in the strip is a serious risk [27]
• The dosage form is inappropriate for the active ingredients that show instability at buccal mucosa pH [28]
Limitations
• Drugs with unpleasant taste must be avoided [26] or inert substances needed to mask the taste of bitter API [27].
• There is a limitation in administration or incorporation of higher doses [15].
• Mucosal irritants shouldn’t be administered following this route [29].
• Saliva contains a proteolytic enzyme, inhibition of which is required in case of protein-based drugs, with the help of enzyme inhibitors [26].
Absolute characteristics
According to [3,23], fast dissolving films must possess the following properties:
• Adequate taste/ must not be bitter
• A pleasant sensation in the mouth.
• Having lesser friability and appropriate mechanical capacity to combat post-production handling.
• Good stability in natural conditions.
• The drug must not have a higher dose.
• No or minimum residue should be left in the mouth
• It should dissolve rapidly and release drug content instantly in the oral cavity.
• It should possess compatibility with other ingredients.
Classification
Fast dissolving thin films are classified into three types based on their properties [30,31]
• Flesh release wafer
• Mucoadhesive melt – away wafer
• Mucoadhesive and sustain release
Flesh release wafer: Thickness and area of the flesh release wafer are approximate 20-70μm and 2-8 cm2 respectively. Structurally, it is single-layered, contains hydrophilic polymers of high solubility and solid solution as drug phase. It takes not more than a minute for the strip to dissolve over the tongue, providing either local or systemic action.
Mucoadhesive melt – away wafer: Mucoadhesive melt away wafer acquires around 2-7cm2 area and 50-500μm thickness. The system may be single or multi-layered, with suspended drug particle or solid solution as a drug phase. Polymers used are hydrophilic and highly soluble. The strip, when placed inside the mouth, dissolves in a short period and forms a gel. The activity site could be local or systemic.
Mucoadhesive and sustain release: Mucoadhesive and sustain release film has about 2-4 cm2 and 50-250μm of area and thickness respectively. It is a multi-layered system, comprising of non-soluble or slightly soluble polymers. The phase of a drug may be a solid solution or suspension. The strip dissolves in maximal 8-10 hours after placing over the buccal or gingival region, with systemic or local activity.
Comparison
The comparison of fast dissolving oral thin film to that of the tablet as described by [8,32,33] are mentioned below Table 1.
| Oral Rapid Dissolving | ||
|---|---|---|
| Parameters | Thin Film | Tablet |
| Dissolution | Greater because of higher surface area | slower because of smaller surface area |
| Compliance | More compliance | Lesser than Film |
| Durability | Better than oral dissolving tablets | Lesser than Film |
| Dose | A lower dose can be added | A higher dose can be added |
| Choking | No danger of chocking | Risk of chocking |
Table 1: Difference between thin film and tablet dosage form.
Formulation consideration
The development of fast dissolving film requires special concern and effort as it tends to disintegrate rapidly in the mouth with no need of water. It should have particular attributes of appearance, taste, and mouthfeel. Generally, consist of the following composition Table 2 [34].
| Sr. No | Ingredient | Percentage amount% |
|---|---|---|
| 1 | Drug (API) | 1-30% |
| 2 | Polymer | Up to 50% |
| 3 | Plasticizer | 0-20% |
| 4 | Surfactant (Solubility Enhancer) |
q.s |
| 5 | Saliva stimulating agent | 2-6% |
| 6 | Sweetening agent | 3-6% |
| 7 | Flavouring agent | 0-10% |
| 8 | Colouring agent | q.s |
| 9 | Stabilizer or Thickening agent | 0-5% |
Table 2: Composition of oral fast dissolving films (API; active pharmaceutical ingredient).
Selection of API
The film composition usually contains a drug concentration of about 5-30 % w/w. Drugs having small doses are the ideal candidates for incorporation in mouth dissolving films and should possess good stability and permeability through oral mucosa [25].
Selection of excipients
Generally Recognized as Safe (GRAS-listed) and accepted excipients are used in the formulation of orodispersible films.
Polymers
For formulating thin films, multiple synthetic and natural polymers are available which can be used either solely or blended in a mixture, depending on desirability [35]. Fast dissolving films synthesized using natural polymers, such as mucilages and gums, possess greater value due to the ease in availability and administration as well as lesser side effects [36]. It is the most significant component and required not below 45% w/w of total dry film content [37].
Pullulan is considered more suitable because of its lower moisture and oxygen penetrability. It produces an exceptionally homogenous and clear film[38].
Modified starches when combined with pullulan, provides ease and reduces product cost[39]. A recent study utilized the chitosan/pullulan combination for preparing the oral fast dissolving thin films [13].
Polyvinyl pyrrolidone usually mixed with copovidone to achieve the film’s flexibility [40].
Microcrystalline Cellulose improves drug dissolution and disintegration properties [41]. In the formulation of a smooth and non-sticky piroxicam film, microcrystalline cellulose has been used along with maltodextrin [42].
HPMC Low-grade E3& E5 are among the preferred polymers to manufacture thin films because of providing rapid dissolution. The more viscous gel influences the disintegration time of the film, due to higher polymer concentration [43].
To make a hydrophilic flexible thin film, with pleasant mouth feel and good solubility, it is better to use a combination of polymers [40].
True attributes of polymers
Mentioned polymer’s ideal characteristics of as follows [44-46]
• Non-irritant and Non-toxic
• Absence of leachable impurities
• Must have sufficient wetting, spreading and penetration enhancing the quality
• Must possess adequate share and tensile strength
• Should be less expensive and easily available
• Shelf life must be appropriate
• Should not cause secondary infections
Plasticizer
The plasticizer is one of the primary ingredients of mouth dissolving films that makes the strip flexible and less fragile. It lowers the glass transition temperature of the polymer and enhances its strength [47]. The concentration of plasticizer used is 20%, as more than 30% leads to difficulty in drying while less than 10% makes the product less flexible.
Sweeting agent
Sweetening agents are an essential component of mouth dissolving formulations, to enhance product palatability [3]. Natural and artificial are two types of sweeteners, used either alone or as a part of a blend, in 3-6% w/w concentration [1].
Fructose, glucose, honey, mannitol, sorbitol, liquorice, and glycerol sucrose represent natural sweeteners while artificial sweeteners could be nutritive and non-nutritive.
Artificial Sweetener include nutritive include; maltose, fructose, and glucose, polyol; includes mannitol, sorbitol, maltitol, srythriol, xylitol, Non-Nutritive include; sucralose, saccharine, neotame and aspartame; Novel sweeteners include; trehalose and tagatose [48].
Saliva stimulants
Saliva stimulants aid in the rapid dissolution of the film by enhancing saliva production [49]. Citric acid, ascorbic acid, lactic acid, and tartaric acid are some of the examples of saliva stimulating agents, among which citric acid is more recommended [50]. These stimulants utilized in 2-6% w/w concentration, either combined or used alone [1].
Colouring agent
Colouring agents approved by FDA & C are employed for the development of mouth dissolving thin films. Titanium oxide is one of the examples. The concentration used must not be more than 1% w/w [51].
Stabilizing and thickening agent
Stabilizing and thickening agents behave as viscosity enhancers for dispersions before casting. The concentration of about 5% w/w is used. Emulsifiers, surface active agents and gums are generally used as thickeners and stabilizers. Carrageenan, xanthan gum, locust bean gum, and cellulose derivatives are commonly utilized gums [40].
Flavouring agent
The opinion of flavour differs among individuals and relies on personal choice, age, ethnic background and importantly, the formulation type [52]. Oleoresins, synthetic flavour oil and certain plant extracts can serve as a flavouring agent. A wide range of flavouring agents is available to be utilized in a formulation such as mint flavour, fruity flavour and confectionery [16]. Depending upon the type and strength of flavouring agent, up to 10%, w/w can be combined with the formulation, along with cooling agents to amplify mouth feel [53].
Permeation enhancer
The role of permeation enhancers is to promote drug absorption by improving permeability. Menthol, dextran sulfate, benzalkonium chloride, Apoprotin, sodium taurodexycholate, cyclodextrin, cetylpyridinium chloride, 23 Lauryl ether, azone, and sodium glycodeoxycholate are the commonly employed permeation enhancers [26].
Manufacturing Methods
Solvent casting method
Hydrophilic components are made soluble in water, forming a viscous solution, which is then mixed with another solution, containing dissolved active and inert ingredients in an appropriate solvent. The solution mix is then cast onto a base of the Petri plate [3]. Another technique utilizes a high shear processor to combine two solutions with the bulk, employing a vacuum to remove entrapped air. The resultant solution then used for forming the film by pouring in glass moulds, drying at 40-50°C and eventually cutting into suitable sizes [23].
Formation of a desirable uniform flexible film, with an ideal thickness of 12-100μm and enhanced physical qualities, are the certain advantages of this method while solubilizing polymer in a suitable solvent and forming viscosity are among the demerits [9,31].
Semisolid casting method
A solution of the film-forming hydrophilic polymer is made in water and incorporated next to an acid-insoluble polymer with the addition of plasticizer to achieve gel-like consistency. Heat controlled drums are used for casting the mixture into films or ribbon. 1:4 should be the proportion of acid-insoluble to a water-soluble polymer. The film diameter should be approximately 0.015 - 0.05 inches [54].
Hot-melt extrusion
A solid mix of components is formed providing controlled temperature and speed, followed by subjecting the mixture to extruder containing heaters, which results in the melting of the mixture. Films are then shaped utilizing dies and punch [55].
This method advances the bioavailability of less soluble drugs, without incorporating water or other solvents. Lesser unit operations decrease the production time and cost. Homogenous distribution, better stability, improved uniformity, and modified-release capability are among the advantages of this technique. Certain disadvantages are also associated with the method including unsuitable technique for temperature-sensitive products, access to polymer flow properties, non-volatility requirement for excipients, availability of limited polymers and need of high energy. Additionally, dealing with higher or lower MP binders can create problems during processing [9,31].
Solid dispersion extrusion
In this technique, before the formation of solid dispersions, immiscible ingredients are extruded with active components. Then in the final step, the shaping of solid dispersions is performed with the help of dies [9,23].
Rolling method
A drug solution, having particular rheological specifications, is made to roll onto a carrier utilizing water or solvent mixture. Drying of films is then carried out on a roller, followed by shaping into required measurements [9,12,31].
Quality Assessment Parameters
In Vitro Determination
Drug-excipients interaction analysis
The potential interaction of the drug with excipients can be assessed by employing infra-red spectroscopy, differential calorimetry scanning, X-ray diffraction and thin layer chromatographic techniques [25].
Thickness
Strip thickness (from corners and centre points) is measured with the help of verniercalliper (digital calibrated) or micrometre screw gauge [56]. The average thickness is determined because uniformity in thickness is essential for dose accuracy. 130 ± 3 μm is the normal range for film thickness [12,23,31].
Dryness test/tack
Dryness is the measurement of moisture content in the film while tack is the capacity of film to hold fast to paper or some other material that came in its contact during pressing. Firm to contact, dry to hold and touch, dust-free, dry to move, dry-firm and print free are the recognized edges of drying procedure of film formation [12,23,25,31].
Tensile strength
The ultimate pressure subjected to a strip, which results in its breakage is tensile strength [57]. It is calculated by using the below-mentioned formula:
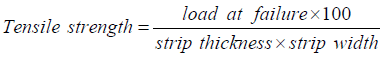
Typically, the tensile strength of a strip ranges as 1.80 ± 0.20 MPa [3,12,23,40]
Percent elongation
The strain is the stretching of the strip after stress application. The division of strip deformity to actual strip measurements gives the value of strain. Higher the plasticizer content higher will be the strip elongation. 322.4 ± 63.3% is the conventional value for % elongation [9,12,23,40]
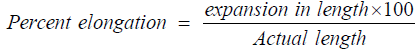
Young's modulus
Young's modulus, a relation between stress and strain, is the representation of film stiffness, having a typical value of 0.30 ± 0.07MPa, and measured as
Young’s modulus = Slope×100/Strip thickness×Cross‐head speed
Higher tensile strength and Young's modulus are shown by a hard and brittle film, with little elongation [3,12,23,40].
Folding endurance
Take the random sample of six films and observe folding endurance, by cleaving film and repeatedly folded at a similar place unless the film broke. The sum of attempts for folding the film at a constant point with no damage represents folding endurance, typically ranges between 100-150. After measuring the folding endurance calculate its mean and standard deviation [9,12,23,25,31,40].
Weight variation
Randomly take ten samples, weigh them individually and measure their mean weight as well. Now compare both the weights to check variation [58].
Tear resistance
It is an intricate ability of a film to oppose damage. The maximal force required for tearing the sample is valued as tear resistance (Newton/ pounds‐force) [3,23,40].
Swelling index
Simulated saliva is used for the conduct of this test. Weigh the film sample and place it on an already weighed wire sieve, submerge the system in 50 ml of medium i.e. simulated saliva. The rise in weight is measured at every interval unless a uniform value is achieved [9,23,25].
Uniformity of drug content/ assay
Content uniformity is analyzed by following the specified assay method as mentioned in Pharmacopeia and each film is tested for the acceptance criteria of 85-115% drug content [9,12,23,31].
Palatability test
It is a test for ranking the taste of the film as a strip must be palatable. Film batches are rated grades from A to C in descending order for taste acceptance. A product that receives a single A is regarded as average, two A grades make a formulation good while a very good formulation receives all three A grades [25].
Disintegration test
The time taken by a film to disintegrate after coming in contact with saliva or water is the disintegration time. The disintegration criteria for oro-dispersible tablets i.e. the 30s or less, as specified by CDER, are also employed to oral thin films. The disintegration apparatus described in pharmacopoeia can be utilized for analysis.
Slide frame approach: Place a water droplet on the film. Slide frames are used for clamping the film and arranged parallel on a Petri plate. The moment of appearance of a hole, once the film starts dissolving, is noted.
Petri dish approach: In a Petri plate, take two ml of water and place a film over it. Measure the time of complete dissolution of thinfilm [25]. Take the sample three times for each formulation [57].
Dissolution test
The conventional dissolution assembly mentioned in pharmacopoeia is generally employed for performing the dissolution test. The selection of medium depends on sink conditions and maximum drug dose. Mostly basket assembly is used, because the film can float in the medium in case of paddle apparatus, creating trouble during testing [56; 59] Dissolution studies performed in triplicate [60]
Permeation studies
Advanced diffusion cell and porcine oral mucosa is utilized for determination of permeation [25].
Stability study
Accelerated limitations of 35 °C and 65% relative humidity are applied for 90 days to evaluate product stability [9].
Surface PH
For measuring surface pH, place the film in an already moistened petri dish (with 0.5 ml-1ml distilled water) and keep it aside for 30 - 60 Seconds [21]. The pH meter is then used and its electrode is contacted with the film surface and let the equilibrium achieved. [61,62]. It can be measure by dissolving film in 4ml of distilled water by calibrated pH meter [63].
Moisture loss
For moisture uptake film along with anhydrous calcium chloride is placed in a desiccator for three days. After three days calculate the film. The percent moisture loss is then measured as follows:
Loss of moisture = [(initial weight – final weight) ÷ initial weight] ×100
In Vivo assessment
Clinical Safety Study
Safety evaluations of Quick-Dis TM formulation have been carried out on animal and human subjects to determine oral irritation. In a study utilizing cheek pouch of hamster, the formulation was administered to the subject two times daily up to four-and-a-half continuous days i.e. nine doses collectively. Quick- Dis TM was tested for irritation on the oral mucosa, employing healthy human subjects for clinical safety analysis [25].
Packaging
Valuable packing with a unique process and consideration is required for protecting an oro-dispersible thin film. For this, multiple packing materials are accessible, among which aluminium pouch is the most common option. Individual packing is compulsory for oral films. Rapid card, a packaging system patented by APR –Labtec, is specially designed for fast dissolving thin films, having a size equivalent to credit card and has a tendency of holding 3 strips on every side [64]. Characteristically, packaging material must;
• Have FDA approval
• Be non-toxic and non-reactive
• Protect the formulation from the environment.
• Satisfy tamper-resistance criteria.
Plastic, foil or paper pouches
The pliable pouch is thought to provide not only a tamper-resistant packaging but also high degree environmental protection [48].
Single pouch and aluminum pouch
Fast dissolving thin film pouch is peelable, with great preventive qualities, and has a clear presentation. 2 structure combination, having one side transparent and other low-priced foil lamination, can be used. The lamination should have zero transmission of air and moisture. Packaging can be used in both nutraceuticals as well as pharmaceutical applications. The single pouch keeps the product and formulation in intact form. It’s the most commonly used packaging [30,48].
Blister card with multiple units
Blister card comprises 2 parts i.e. a cavity for holding the product and a lid stock sealing it. The selection of blister type depends on how many degrees of protection is needed, as semi-flexible blister is also an option. Plastic is generally used for making the blister cavity while aluminium foil for the lid stock [30,64].
Barrier films
Many formulations are highly moisture susceptible and hence require efficient protective films. Various substances offer protection against moisture, for example; Polychlorotrifluoroethylene (PCTFE) film, Polypropylene, etc. Polypropylene has outstanding air and moisture barrier properties. It is flexible and does not stress crack in any situation. One problem is the absence of clarity [22].
Advancement in technology
Soluleaves™: This technology can be used to design the films that immediately dissolve when contact with saliva. This feature provides an outstanding delivery technique to multiple formulations that need a quick release. Soluleaves™ films can also use for designing the film that adheres to the mucous membrane and releases the API slowly over 15min. This can be employed to flavour release products like confectionaries, vitamin products, and mouth fresheners. This technique is capable of delivering the API to the buccal area effectively acceptably and conveniently [65].
Wafertab™: Wafertab™ is the system that contains API in a film adept of being ingested. It brings quick disintegration and rapid drug discharge from the film after interacting with saliva in the oral cavity. Wafertab™ system provides many opportunities for the advance and novel formulation designing, linking different films with varied drugs together. Wafertab™ formulated in a diverse range of shapes/sizes and is a perfect technique for the deliverance of medicine that needs rapid release or patients who have difficulty in swallowing [32].
Foamburst™: Foamburst™ is a specially modified capsule form of the Soluleaves™ technique, patented in 2004, formulated from the foamed capsule. This technique utilizes an inert gas during the development of film, resulting in a honeycomb designed film that dissolves quickly and provides innovative mouth feeling. To produce a specific test- burst characteristic, void in the film could be empty, occupied with gas or any other material. It is an attraction for food and confectionery[65,66].
X Gel™: X Gel™ is the soul of Meldex International's thoughtful creation. It can be employed in every film system and ingestible product technology. It is not animal drive and permitted on religious ground, reasonable for veggie lovers, does not contain GMI and its continuums formulation delivers monetary and challenging production benefits. Masking taste, colouring and, layering is possible in this technology.
With the help of X-Gel™ film systems encapsulated and dry oral dosage form can be made [67].
Minicab: In 2004, Micap Plc. consented to an arrangement to syndicate its competency in microencapsulation advancement, with Bioprogress water dissolvable films. The objective of the development is to deliver a new mechanism for the worldwide market of $1.4 billion for smoking cessation products [66].
Commercial trends of films
Listerine ® Pocket Packs are the first fast dissolving oral thin Films developed by Pfizer used as mouth freshener[1,40].
John Hopkin University in 2006 prepared thin films of Retrovirus vaccine which is stable at room temperature[1,40].
Oro-dispersible films are rapidly grown active delivery system, according to technology catalysts, its sale is around 850$ million in 2007 and possibly reached to 2$ billion in 2012 [1]. Contingent on the development of slants, its market likely amplified up to 13$ billion in 2015 [3]. Despite numerous challenges, promising research and advanced formulation strategies ensure a more rising future for oral thin films [68-73].
Following are the commercially available films [23,74-79]
• Klonopin Wafers (Generic: Clonazepam) Manufactured by Solvay pharmaceuticals
• Listerine Cool mint pocket packs (Generic: Cool Mint) Manufactured by Pfizer
• Benadryl (Generic: Diphenhydramine HCl ) Manufactured by Pfizer
• Listerine (Generic: cool mint) Manufactured by Pfizer
• Chloraseptic (Generic: Benzocaine/ Menthol) Manufactured by Prestige
• Gas-X Generic: Simethicone Manufactured by Novartis
• Sudafed PE (Generic: Phenylephrine) Manufactured by Wolters Kluwer Health Inc
• SupressR (Generic: Menthol) Manufactured by InnoZenR, Inc
• Triaminic (Generic: Diphenhydramine HCl ) Manufactured by Novartis
• Theraflu (Generic: Dextromethorphan HBR) Manufactured by Novartis
Recent research trends of films formulation
In the recent past oral dissolving films is one of the most trading topics in among researchers [80-85]. In Table 3 we have compile the few formulation studies conducted in previous years.
| Sr. No | Topic | Drug | Year | Reference |
|---|---|---|---|---|
| 1 | “Development, In Vitro and In Vivo Evaluation of Racecadotril Orodispersible Films for Pediatric Use’’ | Racecadotril | 2021 | [69] |
| 2 | “Development and Evaluation of Ginkgo biloba L. Extract Loaded into Carboxymethyl Cellulose Sublingual Films” | Ginkgo biloba L. Extract | 2021 | [70] |
| 3 | “Improved bioavailability of montelukast through a novel oral mucoadhesive film in humans and mice” | Montelukast | 2021 | [71] |
| 4 | “Optimization and evaluation of venlafaxine hydrochloride fast dissolving oral films” | Venlafaxine hydrochloride | 2020 | [72] |
| 5 | “Development and pharmaceutical evaluation of oral fast dissolving thin film of escitalopram: A patient-friendly dosage form” | Escitalopram | 2020 | [73] |
| 6 | “Formulation and Evaluation of Fast Dissolving Oral Films of Cetirizine Hydrochloride” | Cetirizine Hydrochloride | 2020 | [58] |
| 7 | “Preparation and Evaluation of Fast Dissolving Oral Film of Losartan Potassium” | Losartan Potassium | 2020 | [74] |
| 8 | “Fabrication of polyvinyl alcohol based fast dissolving oral strips of sumatriptan succinate and metoclopramide HCL” | sumatriptan succinate and metoclopramide HCL | 2020 | [75] |
| 9 | “A novel and innovative drug delivery system in fast dissolving oral film of glimepiride-betacyclodextrin inclusion complexes” | Glimepiride | 2020 | [76] |
| 10 | “Production of Itraconazole Nanocrystal-Based Polymeric Film Formulations for Immediate Drug Release” | Itraconazole | 2020 | [77] |
| 11 | “Designing orodispersible films containing everolimus for enhanced compliance and bioavailability” | Everolimus | 2020 | [78] |
| 12 | “Polymeric Nanosuspension Loaded Oral Thin Films of Flurbiprofen: Design, Development and In Vitro Evaluation” | Flurbiprofen | 2020 | [79] |
| 13 | “Development and characterization of orodispersible film containing cefixime trihydrate” | Cefixime trihydrate | 2020 | [80] |
| 14 | “Carvedilol-loaded polyvinylpyrrolidone electrospun nanofibers film for sublingual delivery” | Carvedilol | 2020 | [81] |
| 15 | “Technology of Orodispersible Polymer Films with Micronized Loratadine—Influence of Different Drug Loadings on Film Properties” | Loratadine | 2020 | [82] |
| 16 | “Improvement of mechanical properties of orodispersible hyaluronic acid film by carboxymethyl cellulose addition” | Hyaluronic acid | 2020 | [83] |
| 17 | “Designing fast-dissolving orodispersible films of amphotericin B for oropharyngeal candidiasis” | Amphotericin B | 2019 | [84] |
| 18 | “Self‐micro emulsifying oral fast dissolving films of vitamin D3 for infants: Preparation and characterization” | Vitamin D3 | 2019 | [85] |
| 19 | “Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets” | Diclofenac | 2019 | [60] |
| 20 | “Fast Dissolving Oral Film of Piroxicam: Solubility Enhancement by forming an Inclusion Complex with β-cyclodextrin, Formulation and Evaluation” | Piroxicam | 2019 | [4] |
| 21 | “Formulation Development and Evaluation of Taste Masked Oral Disintegrating Films of Atenolol by Using Natural Polymers” | Atenolol | 2019 | [36] |
| 22 | “Additive manufacturing of personalized orodispersible warfarin films” | Warfarin | 2019 | [21] |
| 23 | “Evaluation of the solid-state form of tadalafil in sub-micron thin films using nanomechanical infrared spectroscopy” | Tadalafil | 2019 | [18] |
| 24 | “Evaluation of self-nano emulsifying drug delivery systems using multivariate methods to optimize permeability of captopril oral films” | Captopril | 2019 | [59] |
| 25 | “Formulation and Characterization of Fast-Dissolving Sublingual Film of Iloperidone Using Box–Behnken Design for Enhancement of Oral Bioavailability” | Iloperidone | 2018 | [63] |
Table 3: Recent Research Trends of Films Formulation.
Conclusion
In the light of the above facts, it can be concluded that oral fast dissolving films are one of the patient compliant, rapid release with improve bioavailability and can be alternative to conventional dosage form due to its various advantages and low production cost. This dosage forms helpful in loading various type of potent medicine and increasing the patent of the old products in the pharmaceutical sector. Various research studies are published in the recent past which can be helpful in the industrialization of this unique and novel dosage form.
References
- Thakur N, Bansal M, Sharma N, Yadav G, Khare P (2013) Overview “A Novel Approach Of Fast Dissolving Films and Their Patients”. Adv Biol Res 7:50-58.
- Khadra I, Obeid MA, Dunn C, Watts S, Halbert G, et al. (2019) Characterisation and optimisation of diclofenac sodium orodispersible thin film formulation. IntJ Pharm 561:43-46.
- Mandeep K, Rana A, Nimrata S (2013) Fast Dissolving Films: An Innovative Drug Delivery System. Int J Pharma Res Allied Sci 2:14-24.
- Dharmasthala S, Shabaraya AR, Andrade GS, Shriram RG, Hebbar S, et al. (2019) Fast Dissolving Oral Film of Piroxicam: Solubility Enhancement by forming an Inclusion Complex with β-cyclodextrin, Formulation and Evaluation. J Young Pharm 11.
- Patil P, Shrivastava S (2012) Fast Dissolving Oral Films: An Innovative Drug Delivery System. Int J Sci Res 20:50-500.
- Barua S, Kim H, Jo K, Seo CW, Park TJ, et al. (2016) Drug delivery techniques for buccal route: formulation strategies and recent advances in dosage form design. J Pharm Investigation 46:593-613.
- Arya A, Chandra A, Sharma V, Pathak K (2010) Fast Dissolving Oral Films: An Innovative Drug Delivery System And Dosage Form. Int J Chem Tech Res 2:576-583.
- Bhyan B, Jangra S, Kaur M, Singh H (2011) Orally Fast Dissolving Films: Innovations In Formulation And Technology. Int J Pharma Sci Rev And Res 9:50-57.
- Kalyan S, Bansal M (2012) Recent Trends In The Development Of Oral Dissolving Film. Int J Pharm Tech Res 4:725-733.
- Niese S, BreitkreutzJ,Quodbach J (2019) Development of a dosing device for individualized dosing of orodispersible warfarin films. Int J Pharm 561:314-323.
- Solanki A, Gupta N, Jain S (2019) Formulation, development and evaluation of fast dissolving oral film of antipsychotic drug. J Drug Delivery Ther 9:181-185.
- Radhakisan UR, Chavan V, Tribhuvan N (2012) Mouth Dissolving Film And Their Patent: An Overview. Int Res J Pharm 3:39-42.
- Qin ZY, Jia XW, Liu Q, Kong BH,Wang H (2019) Fast dissolving oral films for drug delivery prepared from chitosan/pullulanelectrospinningnanofibers. Int J Biological Macromolecules 137:224-231.
- Montenegro-Nicolini M, Morales JOJ (2017) Overview and future potential of buccalmucoadhesive films as drug delivery systems for biologics. AAPS Pharm Sci Tech 18:3-14.
- Kumar RS, Yagnesh TNS (2019) Oral dissolving films: an effective tool for fast therapeutic action. J Drug Delivery Ther 9:492-500.
- Mahajan A, Chhabra N, Aggarwal G (2011) Formulation And Characterization Of Fast Dissolving Buccal Films: A Review. Der Pharmacia Lettre 3:152-165.
- Dnyaneshwar H, Wale K, SayyedS, Chaudhari S (2014) Orodispersible Film Dosage Form: A Review. World J Pharm Res 3:1093-1111.
- Samaeifar F, Casci CA, Bose GS, Hagner NL, Afifi A, et al. (2019) Evaluation of the solid state form of tadalafil in sub-micron thin films using nanomechanical infrared spectroscopy. Int J Pharm 565:227-232.
- Preis MJAP (2015) Orally disintegrating films and mini-tablets innovative dosage forms of choice for pediatric use. AAPS Pharm Sci Tech 16:234-241.
- Ghodake PP, Karande KM, Osmani RA, Bhosale RR, Harkare BR, et al. (2013) Mouth Dissolving Films: Innovative Vehicle For Oral Drug Delivery. Int J Pharm Res & Rev 2:41-47.
- Sjöholm E, Sandler N (2019) Additive manufacturing of personalized orodispersible warfarin films. IntJ Pharm 564:117-123.
- Heer D, Aggarwal G, Kumar S (2013) Recent Trends Of Fast Dissolving Drug Delivery System–An Overview Of Formulation Technology. Pharmacophore 4:1-9.
- Saini P, Kumar A, Sharma P,Visht S (2012) Fast Disintegrating Oral Films: A Recent Trend of Drug Delivery. Int J Drug Dev & Res 4:80-94.
- Singh S, Virmani T, Virmani R, Kumar P,Mahlawat GJUJPR (2018) Fast dissolving drug delivery systems: formulation, preparation techniques and evaluation. Univ J Pharma Res 3:60-69.
- Vaidya MM, Khutle NM, Gide PS (2013) Oral Fast Dissolving Drug Delivery System: A Modern Approach for Patient Compliance. World J Pharm Res 2:558-577.
- Siddiqui MN, Garg G, Sharma PK (2011) A Short Review on-A Novel Approach in Oral Fast Dissolving Drug Delivery System and Their Patents. Adv Biol Res 5:291-303.
- Keshari A, Sharma PK, Parvez N (2014) Fast Dissolving Oral Film: A Novel and Innovative Drug Delivery system. Int J Pharm Sci Res 5:92-95.
- Ye X, Patil H, Feng X, Tiwari RV, Lu J, et al. (2016) Conjugation of Hot-Melt Extrusion with High-Pressure Homogenization: a Novel Method of Continuously Preparing Nanocrystal Solid Dispersions. AAPS Pharm Sci Tech 17:78-88.
- Patel Jitendra C, Patel K, Patel N (2013) Advanced Pharmaceutics. Int JAdv Pharm 3:44-50.
- Jangra PK, Sharma S,Bala R (2014) Fast Dissolving Oral Films: Novel Way For Oral Drug Delivery. Int J Univ Pharm Bio Sci 3:6-29.
- Khatoon N, Rao NR, Reddy BM (2013) Overview on Fast Dissolving Oral Films. Int J Chem Pharm Sci 1:63-75.
- Bhura N, Sanghvi K, Patel U, Parmar B, Patel D (2012) A Review On Fast Dissolving Film. IJPRBS 1:66-89.
- Dhere M, Patwekar S (2011) Review on Preparation and Evaluation of Oral Disintegrating Films. IJPT 3:1572-1585.
- Metkari V, Kulkarni L, Patil P, Jadhav P, JadhavP,et al. (2014) Fast Dissolving Film: Novel Drug Delivery System. J Curr Pharm Res 4:1225-1230.
- Corniello C (2006) Quick Dissolving Strips: From Concept to Commercialization. Drug Del Technol 6:68-71.
- Shukla KV, Rai A, Pathak R (2019) Formulation Development and Evaluation of Taste Masked Oral Disintegrating Films of Atenolol by Using Natural Polymers. J Drug Delivery Ther 9:711-714.
- Fankhauser C, Slominski G, Meyer S (2007) Disintegrable Oral Films. Google Patents.
- Scott R, Cade D, He X (2005) Pullulan Film Compositions. Google Patents.
- Kulkarni N, Kumar L, Sorg A (2003) Fast Dissolving Orally Consumable Films Containing An Antitussive And A Mucosa Coating Agent. Google Patents.
- DixitR,Puthli S (2009) Oral Strip Technology: Overview And Future Potential. JControlled Release 139:94-107.
- Nishimura M, Matsuura K, Tsukioka T, Yamashita H, Inagaki N, et al. (2009) In Vitro And In Vivo Characteristics of Prochlorperazine Oral Disintegrating Film. Int J Pharm 368:98-102.
- Cilurzo F, Cupone IE, Minghetti P, Selmin F, Montanari L (2008) Fast Dissolving Films Made Of Maltodextrins. European J Pharm Biopharm 70:895-900.
- Chauhan SS, Lin S, Madan P (2012) Preparation And Evaluation Of Nicotine Hydrogen Tartrate Fast Dissolving Films For Smoking Cessation. Asian J Pharm Sci 7:181-192.
- Nagar P, Chauhan I, Yasir M (2011) Insights Into Polymers: Film Formers In Mouth Dissolving Films. Drug Invention Today 3:280-289.
- Perumal V, Lutchman D, MackrajI,Govender T (2008) Formulation of Monolayered Films With Drug And Polymers of Opposing Solubilities. Int J Pharm 358:184-191.
- Salamat-Miller N, Chittchang M, Johnston TP (2005) The Use of Mucoadhesive Polymers in Buccal Drug Delivery. Adv Drug Delivery Rev 57:1666-1691.
- Sakellariou P, Rowe R (1995) Interactions in Cellulose Derivative Films For Oral Drug Delivery. Prog Polym Sci 20:889-942.
- Jadhav YG, Galgatte UC, Chaudhari PD (2013) Challenges in formulation development of fast dissolving oral films. J Pharm Res 3:6391-6407.
- Desu PK, Brahmaiah B, Nagalakshmi A, Neelima K, NamaS,et al. (2013) An Overview On Rapid Dissolving Films. Asian J Pharm Res 3:15-23
- JaiswalH (2014) Oral Strip Technology: A Review. Indian J Pharm Biol Res 2:130-143.
- Maibach T (2008) Film Comprising Nitroglycerin. Google Patents.
- Brown D (2003) Orally Disintegrating Tablets-Taste over Speed. Drug Del Tech 3:58-61.
- Leung SHS, Leone RS, Kumar LD, Kulkarni N, Sorg AF (2009) Fast dissolving orally consumable films. Google Patents.
- KaurP,Garg R (2018) Oral dissolving film: present and future aspects. J Drug Delivery Ther 8:373-377.
- Nair VS, Saudagar R, Gondkar S (2015) A Review on Fast Dissolving Sublingual Films for Systemic Drug Delivery. World J Pharm Pharmaceut Sci 4:342-361.
- Speer I, Preis M, Breitkreutz J (2019) Novel Dissolution Method for Oral Film Preparations with Modified Release Properties. AAPS Pharm Sci Tec 20:7.
- Takeuchi Y, Nishimatsu T, Tahara K, Takeuchi H (2019) Novel use of insoluble particles as disintegration enhancers for orally disintegrating films. J Drug Delivery Sci Tech 54:101310.
- Baniya DP, Pandey G, Bajaracharya M, Dhungana BRJMS (2020) Formulation and Evaluation of Fast Dissolving Oral Films of Cetirizine Hydrochloride. Europasian J Med Sci 2:1-11.
- Talekar SD, Haware RV, Dave RH (2019) Evaluation of self-nanoemulsifying drug delivery systems using multivariate methods to optimize permeability of captopril oral films. Eur J Pharm Sci 130:215-224.
- Speer I, Lenhart V, Preis M, Breitkreutz J (2019) Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets. Int J Pharm 554:149-160.
- Prabhu P, Malli R, Koland M, Vijaynarayana K, D’Souza U, et al. (2011) Formulation And Evaluation Of Fast Dissolving Films Of Levocitirizine Di Hydrochloride. Int J Pharm Invest 1:99-104.
- Yasmeen BR, Firoz S, Mouli YC, Vikram A, Mahitha B, et al. (2012) Preparation And Evaluation Of Oral Fast Dissolving Films Of Citalopram Hydrobromide. Int J Biopharm 3:103-106.
- LondheV,Shirsat R (2018) Formulation and Characterization of Fast-Dissolving Sublingual Film of Iloperidone Using Box–Behnken Design for Enhancement of Oral Bioavailability. AAPS Pharm Sci Tech 19:1392-1400.
- Vollmer U, Galfetti P (2006) Rapid Film: Oral Thin Films As An Innovative Drug Delivery System and Dosage Form. Drug Dev Report 5:64-67.
- Kushwaha V, Akhtar J, Usmani S, Singh SP (2015) A Review On Fast Dissolving Formulation Technologies.
- Panda B, Dey N, Rao M (2012) Development Of Innovative Orally Fast Disintegrating Film Dosage Forms: A Review. Int J Pharm Sci Nanotech 5:1666-1674.
- Bala R, Pawar P, Khanna S, Arora S (2013) Orally Dissolving Strips: A New Approach To Oral Drug Delivery System. Int J Pharm Invest 3:67-76.
- Godbole A, Joshi R, Sontakke M (2018) Oral Thin Film Technology-Current Challenges and Future Scope. Int J Adv Res Eng Appl Sci 7.
- Wang B, Yang L, Luo C, Wang Y, Wang H, et al. (2021) Development, In Vitro and In Vivo Evaluation of Racecadotril Orodispersible Films for Pediatric Use. AAPS Pharm Sci Tech 22:1-13.
- Rimkiene L, Baranauskaite J, Marksa M, Jarukas L, Ivanauskas L (2021) Development and Evaluation of Ginkgo biloba L. Extract Loaded into Carboxymethyl Cellulose Sublingual Films. Appl Sci 11:270.
- Michael J, Bessa de Sousa D, Conway J, Gonzalez-Labrada E, Obeid R, et al. (2021) Improved bioavailability of montelukast through a novel oral mucoadhesive film in humans and mice. Pharmaceutics 13:12.
- Al-Mogherah AI, Ibrahim MA, Hassan MA (2020) Optimization and evaluation of venlafaxine hydrochloride fast dissolving oral films. Saudi Pharm J 28:1374-1382.
- Mushtaque M, Muhammad IN, Hassan SMF, Ali A, Masood R (2020) Development and pharmaceutical evaluation of oral fast dissolving thin film of escitalopram: A patient friendly dosage form. Pak J Pharm Sci 33.
- Sadique S, Sri SR (2020) Preparation and Evaluation of Fast Dissolving Oral Film of Losartan Potassium. Res J Pharm Dosage Forms Tech 12:13-16.
- Zaman M, Hassan R, Razzaq S, Mahmood A, Amjad MW, et al. (2020) Fabrication of polyvinyl alcohol based fast dissolving oral strips of sumatriptan succinate and metoclopramide HCL. Sci Prog 103:0036850420964302.
- Darusman F, Soewondo B, Alatas S (2020) A novel and innovative drug delivery system in fast dissolving oral film of glimepiride-betacyclodextrin inclusion complexes. JPhCS 1469:012021.
- Karagianni A, Peltonen L (2020) Production of Itraconazole Nanocrystal-Based Polymeric Film Formulations for Immediate Drug Release. Pharmaceutics 12:960.
- Ma Y, Guan R, Gao S, Song W, Liu Y, et al. (2020) Designing orodispersible films containing everolimus for enhanced compliance and bioavailability. Expert Opin Drug Delivery 17:1499-1508.
- JadhavPA,Yadav AV (2020) Polymeric Nanosuspension Loaded Oral Thin Films of Flurbiprofen: Design, Development and In Vitro Evaluation. Res J Pharm Tech 13:1907-1912.
- Khan QUA, Siddique MI, Rasool F, Naeem M, Usman M, et al. (2020) Development and characterization of orodispersible film containing cefiximetrihydrate. Drug Dev Ind Pharm 1-11.
- Li J, Pan H, Ye Q, Shi C, Zhang X, et al. (2020) Carvedilol-loaded polyvinylpyrrolidoneelectrospunnano fibers film for sublingual delivery. J Drug Delivery SciTech 101726.
- Centkowska K, Ławrecka E, Sznitowska M (2020) Technology of Orodispersible Polymer Films with Micronized Loratadine Influence of Different Drug Loadings on Film Properties. Pharmaceutics 12:250.
- Kim S, Cho DH, Kweon DK, Jang EH, Hong JY, et al. (2020) Improvement of mechanical properties of orodispersible hyaluronic acid film by carboxymethyl cellulose addition. Food Sci Biotech 29:1233-1239.
- Serrano DR, Fernandez-Garcia R, Mele M, Healy AM, Lalatsa A (2019) Designing fast-dissolving orodispersible films of amphotericin B for oropharyngeal candidiasis. Pharmaceutics 11:369.
- Zhang M, Zhang T, Zou Y, Han P, Liu K (2019) Self‐microemulsifying oral fast dissolving films of vitamin D3 for infants: Preparation and characterization. Food Sci Nutr 7:2577-2583.
Citation: Mushtaque M, Muhmmad IN, Kanwal S, Khalid F, Masood R, et al. (2021) Novelty and Compliance of Oral Fast Dissolving Thin Film – A Patient Friendly Dosage Form. Clin Pharmacol Biopharm 10: 211.
Copyright: © 2021 Mushtaque M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3771
- [From(publication date): 0-2021 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 2937
- PDF downloads: 834