Review Article Open Access
Novel Strategies to Combat Antimicrobial Resistance
Inderpal Kaur*
Department of Pharmacology, GMC Amritsar, Punjab, India
- *Corresponding Author:
- Inderpal Kaur
Associate Professor
Department of Pharmacology
GMC Amritsar, Punjab
India
Tel: +917814405642
E-mail: inderpalpharma@gmail.com
Received date: June 09, 2016; Accepted date: August 04, 2016; Published date: August 08, 2016
Citation: Kaur I (2016) Novel Strategies to Combat Antimicrobial Resistance. J Infect Dis Ther 4: 292. doi:10.4172/2332-0877.1000292
Copyright: © 2016 Kaur I. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Infectious diseases form the major health-care burden for the developing world and antimicrobials prove to be the magical drugs to combat this. The discovery of antimicrobial agents was boon for the global health-care system and the wonderful cure by antimicrobials shifted the disease trends from infectious to life-style diseases in the developed world. Sudden appearance of the antimicrobial resistance hampered the whole success; and this situation is further complicated by the dry pipeline of antimicrobial development. Now, this is heading the world towards the “preantibiotic” era. The development of new antimicrobials is not able to match pace with the speedily growing antimicrobial resistance. Development of new active pharmaceutical principles is a difficult and costly practice. The other approach to achieve the same is by rejuvenating the existing antimicrobials. These contemporary novel approaches include bacteriophage therapy, fecal microbiota transplantation, antimicrobial peptides, combination drug therapy and antimicrobial adjuvants to combat antimicrobial resistance forms the main stay of discussion of this article.
Keywords
Antimicrobial resistance; Bacteriophage therapy; Fecal transplantation; Antimicrobial adjuvants
Introduction
Infectious diseases form the major health-care burden for the developing world and antimicrobials proved to be the magical drugs to combat this. The discovery of antimicrobial agents was boon for the global health-care system. The wonderful cure by antimicrobials shifted the disease trends from infectious to life-style diseases in the developed world. But this magic didn’t last long due to appearance of antimicrobial resistance, which is not a new phenomenon as thought by many, but a natural evolutionary process which started even before the development of antimicrobials due to ongoing continuous mutations. Evidence of this can be found in studies that show that resistance genes are prevalent in 30,000-year-old permafrost samples, and in bacteria living in a cave, sealed from the surface 4 million years ago [1,2]. Data from several developed nations has been submitted with WHO but is still lacking from the South-East Asian Region (SEAR-WHO) because of inadequate information of antimicrobial use and poor surveillance monitoring agencies [3]. The recent trends in development of antimicrobial resistance are more towards Gram Negative bacteria, although significant resistance is seen with Gram positive bacteria (MRSA, Clostridium difficle ) also. Similar reports are seen with Viruses (HIV), parasites (Plasmodium falciparum ) and several antifungals.
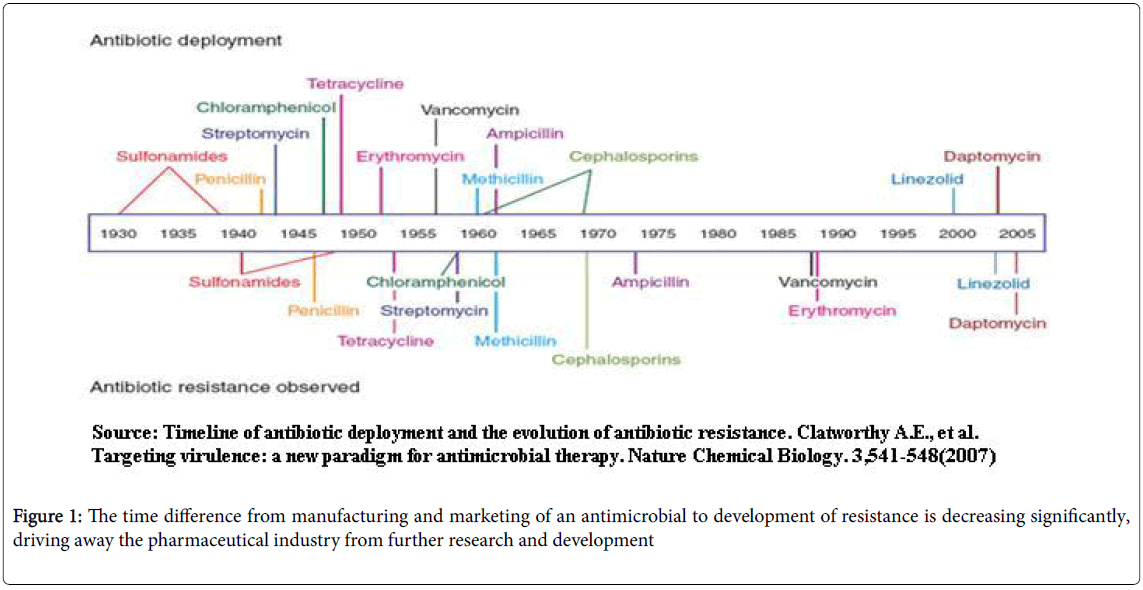
The main contributing factors for the development of resistance are irrational antibiotic prescribing practices (misuse, overuse), easy availability as non-prescription drugs in the developing world and lack of regulatory policies concerning the use of antimicrobials in humans, veterinary and agriculture. The time difference from manufacturing and marketing of an antimicrobial to development of resistance is decreasing significantly, driving away the pharmaceutical industry from further research and development (Figure 1).
Resistance to single antimicrobial became prominent in organisms that encountered the first commercially produced antibiotics. The most notable example is resistance to penicillin among staphylococci, specified by an enzyme (penicillinase) that degraded the antibiotic. Over the years, continued selective pressure by different drugs has resulted in organisms bearing additional kind of resistance mechanisms that led to multidrug resistance (MDR). Some of the most problematic MDR organisms that are encountered currently include Pseudomonas aeruginosa , Acinetobacter baumannii , Escherichia coli and Klebsiella pneumoniae bearing extended-spectrum β-lactamases (ESBL), vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant MRSA, and extensively drug- resistant (XDR) Mycobacterium tuberculosis [4].
The recent spread of Gram-negative Enterobacteriaceae with resistance to carbapenem due to New Delhi metallo-β-lactamase 1 (NDM-1) enzyme, a potential global threat, found among E.Coli and Klebisella pneumonia (KPC, Klebsiella pneumonia carbapenamase) is a matter of concern [5]. This conveys that we are near to the “preantibiotoc” era as antibiotics of last resort are also not spared of resistance.
Broadly, two approaches can be used to tackle this problem. Either look out for new antimicrobials or work on the existing ones to make them useful. Development of new active pharmaceutical principles is a difficult and costly practice, and usually doesn’t have common financial goals with pharmaceutical companies. The other approach to achieve the same is by rejuvenating the existing antimicrobials, and this forms the main stay of discussion of this review article
These contemporary novel approaches include:
a) Combination drug therapy
b) Bacteriophage therapy
c) Fecal microbiota transplantation
d) Antimicrobial adjuvants to combat antimicrobial resistance
e) Antimicrobial peptides
Combination Drug Therapy
In context of antimicrobials, this strategy is about treating the infections with set of drugs rather than individual therapy (monotherapy). This approach is currently being used in many drug regimens where the causative organisms are more prone to develop resistance (Mycobacterium tuberculosis, Human Immunodeficiency Viral, Plasmodium parasitic infections). The principle to rationalize its use is minimizing the probability of primary resistance to the combination regimen by many folds. Let us consider that organism X has primary resistance to drug A and B as 10-6 and 10-7 respectively. It is provided that both drugs lack cross-resistance and does not interfere with each other’s metabolism and pharmacokinetic properties. The new possibility of primary resistance of organism X to drug combination AB is 10-6 × 10-7=10-13 [6].
These combination drug regimens usually act by one of the three ways:
a) Combination drugs acting on different targets in different pathways.
b) Combination drugs acting on different targets in same pathway.
c) Combination drugs acting on single target, but in different dimensions.
Combination drugs acting on different targets in different pathways
Classical example is treatment modality used against Mycobacterium tuberculosis infections currently prevalent in many developing nations like India. Four first line drugs are used in this regimen: Rifampicin(R), Isoniazid (H), Ethambutol (E) and Pyrazinamide (Z). Their targets are: Rifampicin (RNA polymerase inhibitor), Isoniazid (enoylreductase subunit of fatty acid synthase), Ethambutol (an inhibitor of arabinosyl transferases involved in cell wall biosynthesis) and Pyrazinamide (mechanism of action poorly understood) [7,8]. Assuming that the bacterium got a chance to develop resistance by changing one target, this combination regimen will still be effective against at least other two pathways, minimizing the chances of bacterial propagation.
Similar is the case with various other combination therapeutic strategies such as Mycobacterium leprae infection, Human Immunodeficiency Virus and Plasmodium falciparum malaria treatment strategies. The efficacy of combination regimens is well appreciated by policy makers and incorporated into several national guidelines for treatment of infectious diseases.
Combination drugs acting on different targets in same pathways
Classical example using this strategy is combination of β-lactam antibiotic (Amoxicillin) with β-lactamase enzyme inhibitor (Clavulanic Acid) [9]. Many gram positive bacteria produces β- lactamase enzyme which opens up the β- lactam, making it ineffective. Adding β-lactamase enzyme inhibitor clavulanic acid degrades the enzyme, permitting the drug to act on these organisms. Other inhibitors include salbactum and tazobactum.
Combination drugs acting on single target, but in different dimensions
Streptogramins, a class of newer generation antibiotics, is a mixture of two active molecules. These two molecules – one a nonribosomal peptide and the other a polypeptide-nonribosomal peptide hybrid – bind in adjacent sites in the 50S subunit, near the peptidyl transferase center [10,11]. They are 10-100-fold more potent as a combination than either molecule, if used alone, as a single agent [12].
Bacteriophage therapy
Bacteriophage therapy, also known as phage therapy or viral phage therapy is no less than a magical cure for many antimicrobial resistant infections. Bacteriophage is a virus that infects and replicates within a bacterium. These are composed of proteins that encapsulate a DNA or RNA genome and replicate within the bacterium following the injection of their genome into its cytoplasm. This property can be used to kill the bacteriophage occupied bacterial cells, forming the principle of this therapy. Originally, developed by Frederick Twort and Felix d'Hérelle in 1915 and 1917, phage therapy was immediately recognized as an important tool for treating bacterial infections [13]. In 1896, the British bacteriologist Ernest Hankin reported antibacterial activity against Vibrio cholerae , which he observed in the Ganges and Jumna rivers in India. He suggested that an unidentified substance was responsible for this phenomenon and for limiting the spread of cholera epidemics [14]. This unidentified substance is now recognized as Bacteriophage. Much of the knowledge about this therapy remained hidden from the world, possibly due to publishing of scientific literature in non-english journals.
These bacteriophages have a high specificity in killing particular bacteria, leaving the other useful bacteria unharmed. This property is especially useful while killing the pathogens, without altering gut flora. Antibiotics being non-specific in their action destroy commensals in gut as commonly seen with fluoroquinolone, leading to superinfections with Clostridium difficle [15].
There are several advantages seen with bacteriophage therapy over antibiotics. Small doses of bacteriophages are required to treat bacterial infections as they self-replicate in vivo . This also provides an additional advantage of being less immunogenic as less dose of foreign substance is administered in the body. The concept of sub-lethal dose, as seen with antimicrobials holds no place in bacteriophage therapy as single bacteriophage is sufficient to kill single bacterium. As phages also continue to participate in evolution, they keep on adapting themselves at-par with the mutational changes occurring in bacteria, leaving less chances of development of resistant bacteria [16]. Bacteriophage therapy also has high therapeutic index. Being specific to bacterial species, development of cross-resistance is infrequent.
There are certain limitations to this therapy, not enough to limit their applications. Being highly specific for bacteria, in case of mixed bacterial infections (as commonly observed in clinical practice), we will need cocktail of bacteriophages, prepared and stored with the phage-banks. These bacteriophage cocktails are available at Tbilisi Institute, Georgia, a pioneer institute in bacteriophage research. There are other concerns like rapid clearance of bacteriophages from the body, but this can be taken care of by altering bacteriophage structural properties [17]. The negative public perception of viruses may also play a role in the reluctance to embrace phage therapy [18].
Enzybiotics, an experimental antibiotic approach employing enzymes to combat pathogenic bacterial infections, is also used for isolating bacteriophage enzymes in their pure form and using them as an independent therapeutic approach [19].
There are several regulatory issues regarding the implementation of this therapy. The need for phage-bank makes the regulatory testing more hard and expensive. There is a problem regarding approval of usage of phage therapy for humans in western world, mainly due to difficulty in establishing safety with self-replicating entity. But in 2006, US FDA has approved it for its use as a food additive targeted against Listeria Monocytogenes as a spraying agent for meat. This suggests that phage-therapy can also get approval for human use but there are several patent concerns regarding this which leads to difficulty in distribution of rights to various pharmaceutical companies. There is an ample scope in this field to overcome antibiotic resistance but further scientific research is needed to establish the safety and effectiveness.
Fecal Microbiota Transplantation
Fecal Micro biota Transplantation (FMT), also known as fecal bacteriotherapy or stool transplantation is a promising approach recognized recently. This process involves the transplantation of feces from a healthy donor to a recipient [20]. The active principle of this therapy aims at the total restoration of the gut commensals by infusion of the stools from healthy donor using various methods including enema, nasogastric, nasoduodenal and colonoscopic routes. Clostridium difficle infection (CDI) is the leading cause of antibiotic and health care-related diarrhea [21]. The first description of FMT was published in 1958 by Ben Eiseman and colleagues, who treated four cases of critically ill patients with fulminant pseudomemberanous colitis using fecal enemas [22].
Antibiotic targets being non-specific, kills both the pathogenic as well as gut flora. Antimicrobial action on gut flora kills all the organisms leaving behind few resistant strains of Clostridium difficle bacteria. These strains grow unchecked, which are refractory to most commonly used antimicrobials leading to pseudomemberanous colitis. This therapy transplants gut flora (from healthy donor’s feces) and restores it in the recipient colon, displacing the selective growth of virulent strains of Clostridium difficle bacteria, decreasing their number ultimately breaking the cycle of recurrent CDI. This minimizes repeated antibiotic use, which in turn reduces the risk of antibiotic associated resistance.
Fluoroquinolones are the most common antibiotics leading to this condition, clindamycin and β- lactam antibiotics are also the contributing agents. Current guidelines from the Infectious Disease Society of America focus on vancomycin and metronidazole based regimens, but therapeutic effects are limited by high recurrence rates because of development of resistance [21]. Search for newer therapeutic options is the need of the hour. In the last few years, this therapy has interested many researchers. A review article in 2011 found 22 reports of FMT, they included 239 patients. Resolution of symptoms was successful in 87% (145/166) of the patients described to have fulminant or refractory CDI [23]. In 2010 and 2011, 11 studies and case reports were done in different centers; the success rate of FMT was very high approaching 92% [24].
A randomized study published in the New England Medical Journal in January 2013 reported a 94% cure rate of pseudomemberanous colitis caused by Clostridium difficle , by administering fecal microbiota transplant compared to just 31% with Vancomycin. The study was stopped prematurely as it was considered unethical not to offer the FMT to all participants of the study due to the outstanding results [25,26]. In 2012, a team of researchers at Massachusetts Institute of Technology (MIT) founded OpenBiome, the first public stool bank in the US.
There are several regulatory and ethical issues regarding the safety and efficacy of this therapy. Further research and clinical trials are needed, and more data is required for its approval by US-FDA. This therapy is a new hope to save the humanity from menace of antimicrobial resistance.
Antimicrobial Adjuvants
The discovery of new and effective antimicrobials is the ideal approach to combat the issue of antimicrobial resistance. It seems a fair solution for the deficiencies in the existing antimicrobials for resistance-development point of view. But the development and marketing approval of these drugs by US-FDA had not matched the pace of development of antimicrobial resistance. So, the best practical strategy is to modify the existing drugs to make them more useful and potentiate their effectiveness, and this process is more economical. [27,28]. Using antimicrobial adjuvants is such an approach that aims to improve the efficacy of existing antibiotics and for suppressing the emergence of resistant strains. These are the compounds that make bacteria more susceptible to antibiotics [29]. Screening of molecules acting as antibiotic adjuvants has attracted many researchers. These compounds typically don’t possess any intrinsic antimicrobial activity, but those with such an activity can also be considered as adjuvants. The latter case can be justified as usage of two synergistically acting antimicrobials is also considered as adjuvants. Adjuvants acts by reversing the mechanisms adopted by microbes to develop antimicrobial resistance. The generic mechanisms of actions of adjuvants act includes:
Inhibition of antimicrobial resistance elements: Classical example includes addition of β-lactamase inhibitor to a β-lactam antibiotic, thus preventing the distortion of the β-lactam ring and maintaining its efficacy against gram-positive bacteria. Another notable example includes addition of Cilastatin, dehydropeptidase, an enzyme that degrades the β-lactam Imipenam. These adjuvants supplement the actions of these compounds.
Enhancing the uptake of antimicrobial in target cell: Colistin (Polymyxin B), a poly-cationic molecule with both lipophilic and hydrophilic ends, is an antibiotic whose use itself is limited due to nephrotoxicity. But its action as a membrane detergent can be used as an adjuvant principle in low doses. This limits the toxicity, and facilitates penetration of concurrently administered hydrophilic antibiotics such as rifampicin, carbapenams, glycopeptides and tetracyclines [30]. This principle can be further exploited to target lipopolysacchride layer of gram-negative bacteria.
Nullifying the effect of efflux pumps: The main mechanism for development of resistance to tetracyclines in by its expulsion from bacterial cells by efflux pumps [31]. The approach to achieve this effect is by co-administering structurally similar compound which can compete with tetracycline for efflux pumps, minimizing the efflux of tetracyclines. Hence, bringing down the development of resistance [32]. This same mechanism can be used even for quinolones and aminoglycosides.
By promoting bacterial oxidative stress: Tellurium oxyanion tellurite (tellurite) is known to cause high levels of oxidative stress in bacterial cells. Antibiotics acting by interfering with cell wall (ampicillin, cefotaxime) or protein synthesis (tetracycline, chloramphenicol, gentamicin), when used with sub-lethal concentrations of tellurite, their killing action is potentiated many folds. The possible mechanisms other than Reactive oxygen species generation [34], are damage to metabolic enzymes [35,36], glutathione depletion [37] or lipid peroxidation [38].
Antimicrobial Peptides
Antimicrobial peptides, also known as host-defense peptides are part of the innate immune response found among many organisms. They present significant antibacterial, antifungal, antiparasitic, and antiviral activity [39]. Usually these molecules are composed of 10-50 amino-acid residues, and arranged in different groups depending on the amino-acid composition, size, and conformation [40,41]. These protein molecules have weak intrinsic antimicrobial activity but potent and broad immune modulatory activity when the host cells are invaded by bacteria or viruses and this demonstrate their potential as novel antimicrobial agents. They have very specific properties including their amphipathic secondary structure, small size, positively charged and bind rapidly to the biological membranes [42,43]. These are very fast in killing microbes, a property that doesn’t allow development of resistance against these compounds, although few case of resistance has been documented. This represents a class with broad spectrum activity against bacteria (both gram positive and negative), mycobacterial (including Mycobacterium tuberculosis), enveloped viruses, fungi and even transformed or cancerous cells [44].
Antimicrobal peptides are produced by all known species, including peptides from bacteria, from fungi, from hydra, insects (mastoparan, poneratoxin, cecropin, moricin, melittin and others), [45] frogs (magainin, dermaseptin and others), [46] and mammals (for example, cathelicidins, defensins and protegrins).
The mode of action of antimicrobial peptides is not very clear, but they mainly act by targeting either cell membrane/cell wall or intracytoplasmic molecules. Additionally, they also act by neutralizing lipopolysacchride, prevent immune mediated response and further development of septic shock [47].
Antimicrobial peptides act by multiple modalities. These includes [48]:
a) Lipopolysacchride neutralization or disaggregation
b) Induction of membrane permeability
c) Inhibition of cytoplasmic proteins related to cell division or survival
d) Inhibition of macromolecular synthesis through interaction with nucleic acids
e) Anti-biofilm Activity of antimicrobial peptides against biofilm of multi-drug resistant bacteria.
They act synergistically with currently available conventional antibiotics by enhancing permeability of antimicrobials by their membranolytic effect, reducing dose and thus toxicity. This strategy is presently in infancy stages, more studies are needed to validate them for their usage in clinical practice. These can be newer generation antimicrobials for combating multi-drug resistant microbes and preventing biofilm production.
Conclusion
It is worth mentioning that rational use of antibiotics enjoys its own place, as this brings down the burden of rapidly developing antimicrobial resistance. To conclude, we feel that rejuvenating the already existing antimicrobials is more practical and better approach than to look for newer molecules from the beginning. The various modalities listed above seem to be quite promising, although more research into these is need of the hour. The combination therapy helps in overcoming the vulnerabilities of the existing antimicrobials by supplementing them with the missing links in their natural lytic pathway. Bacteriophage therapy is confined only to certain parts of the globe, this need to be highlighted to the entire scientific community for better understanding and newer applications. Fecal microbiota transplantation is a recently employed approach promoting the growth of commensals to outnumber the pathogenic resistant bacteria. Certain newer potential application to this approach is under research. Antibiotic adjuvants act like combination therapy, but they target more metabolic and other pathways vital in the survival of microbes and works by boosting the action of antimicrobials, even for the molecules with zero intrinsic antimicrobial activity. Antimicrobial peptides are ultra-fast acting broad spectrum proteins which mimic the natural innate immune system for clearing microbes. This class of proteins is expanding with the newer research. These modalities form the hope for the future to curb the development of antimicrobial resistance.
References
- Bhullar K, Waglechner N, Pawlowski A, Koteva K, Banks ED, et al. (2012) Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS One 7:e34953.
- D’Costa VM, King CE, Kalan L, Morar M, Sung WW, et al. (2011) Antibiotic resitance is ancient. Nature477:457-461.
- World Health Organization. Prevention and containment of antimicrobial resistance. Report of a regional meeting Chiang Mai,Thailand, 8th to 11th of June 2010.
- Alekshun MN, Levy SB (2007) Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell 128:1037-1050.
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, et al. (2010) Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis10: 597–602.
- Cottarel G, WierzbowskiJ (2007) Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol 25:547–555.
- Ginsberg AM, SpigelmanM (2007) Challenges in tuberculosis drug research and development. Nat Med 13: 290-294.
- Handbook of anti-tuberculosis agents (2008) Introduction. Tuberculosis (Edinb) 88:85–86.
- Lee N, Yuen KY, KumanaCR (2003) Clinical role of beta-lactam/beta-lactamase inhibitor combinations.Drugs 63: 1511-1524.
- Hansen JL, Moore PB, SteitzTA (2003) Structures of five antibiotics bound at the peptidyltransferase center of the large ribosomal subunit. J MolBiol330:1061-1075.
- Tu D, Blaha G, Moore PB, SteitzTA (2005) Structures of MLSBK antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell 121:257–270.
- CocitoC (1979) Antibiotics of the virginiamycin family, inhibitors which contain synergistic components. Microbiol Rev 43: 145-192.
- D’hérelleF(1922) The bacteriophage: its role in immunity.Williams and Wilkens Co.Waverly Press, Baltimore, USA.
- Van HelvoortT (1992) Bacteriological and physiological research styles in the early controversy on the nature of the bacteriophage phenomenon. MedHist 3: 243-270.
- KeenEC(2012) Phage Therapy: Concept to Cure. Frontiers in Microbiology 3.
- Abedon ST (2012) Salutary contributions of viruses to medicine and public health. In: Witzany G (ed). Viruses: Essential Agents of Life Springer389-405.
- MerrilC, Biswas B, Carlton R, Jensen NC, Creed GJ, et al. (1996) Long-circulating bacteriophage as antibacterial agents. ProcNatlAcadSci USA 93:3188-2192.
- Verbeken G, De Vos D, Vaneechoutte M, Merabishvili M, Zizi M, et al. (2007) European regulatory conundrum of phage therapy. Future Microbiol 2: 485-491.
- Veiga-CrespoP, VillaTG (2009) Phylogeny of Enzybiotics, in Enzybiotics: Antibiotic Enzymes as Drugs and Therapeutics. John Wiley and Sons, Inc, Hoboken, NJ, USA.
- Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC (2011) Treating Clostridium difficile Infection With Fecal MicrobiotaTransplantation. Clinical Gastroenterology and Hepatology9: 1044-1049.
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, et al. (2010) Society for Healthcare Epidemiology of America. Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious disease society of America (IDSA). Infect Control HospEpidemiol5:431-455.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ(1958) Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44: 854–859.
- Landy J, ALâÂ?Â?Hassi HO, McLaughlin SD, Walker AW, Ciclitira PJ, et al. (2011) Reviewarticle:Fecaltransplantationtherapyforgastrointestinaldisease.AlimentPharmacolTher34:409âÂ?Â?415.
- Karadsheh Z, SuleS (2013) Fecal transplantation for the treatment of recurrent Clostridium difficile infection. North Am J Med Sci 5:339-343.
- Van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, et al. (2013) Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N Engl J Med368:407-415.
- Kelly CP (2013) Fecal Microbiota Transplantation - An Old Therapy Comes of Age. N Engl J Med368:474-475.
- Ejim L, Farha MA, Falconer SB, Wildenhain J, Coombes BK, et al. (2011) Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat ChemBiol 7: 348–350
- Kalan L, Wright GD (2011) Antibiotic adjuvants: multicomponent anti-infectivestrategies. Expert Rev Mol Med 13: e511–e517.
- Cottarel G, Wierzbowski J (2007) Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol25:547-555.
- Conrad RS, GalanosC (1989) Fatty acid alterations and polymyxin B binding by lipopolysaccharides from Pseudomonas aeruginosa adapted to polymyxin B resistance. Antimicrob Agents Chemother33:1724-1728.
- Speer BS, Shoemaker NB, Salyers AA (1992) Bacterial resistance to tetracycline:mechanisms, transfer, and clinical significance.ClinMicrobiolRev 5:387.
- Van BambekeF, Pagès JM, Lee VJ (2006) Inhibitors of bacterial efflux pumps as adjuvants in antibiotic treatments and diagnostic tools for detection of resistance by efflux. Recent Pat Antiinfect Drug Discov1:157-175.
- Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, et al. (2010) D-amino acids trigger biofilm disassembly.Science 328: 627-629.
- Pérez J, Calderón I, Arenas F, Fuentes D, Pradenas GA, et al. (2007) Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS One 2:e211.
- Calderón I, Elías A, Fuentes E, Pradenas G, Castro M, et al. (2009) Tellurite-mediated disabling of [4Fe-4S] clusters of Escherichia coli dehydratases. Microbiology 155: 1840-1846.
- Castro M, Molina R, Díaz WA, Pradenas GA, VásquezCC (2009) Expression of Aeromonascaviae ST pyruvate dehydrogenase complex components mediate tellurite resistance in Escherichia coli. BiochemBiophys Res Commun 380: 148–152.
- Turner RJ, Aharonowitz Y, Weiner J, Taylor DE (2001) Glutathione is a target in tellurite toxicity and is protected by tellurite resistance determinants in Escherichia coli. Can J Microbiol 47: 33–40.
- Pérez J, Arenas F, Pradenas G, Sandoval J, VásquezCC (2008) Escherichia coli YqhDexhibits aldehyde reductase activity and protects from the harmful effect of lipid peroxidation-derived aldehydes. J BiolChem 283: 7346–7353.
- Jenssen H, Hamill P, Hancock REW (2006) Peptide antimicrobial agents. ClinMicrobiol Rev 19:491–511.
- Lai Y, Gallo RL (2009)AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol30: 131–141.
- Nakatsuji T, Gallo RL (2012) Antimicrobial peptides: old molecules with new ideas. J Invest Dermatol132: 887–895.
- YeamanMR,YountNY (2003) Mechanisms of antimicrobial peptide action and resistance.Pharmacol. Rev 55:27-55.
- BrogdenKA(2005) Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat RevMicrobiol 3: 238–250.
- Reddy KV, Yedery RD, Aranha C(2004)Antimicrobialpeptides:premisesand promises. Int J Antimicrob Agents 24: 536–547.
- Superfamily 3.1.021. Insect antimicrobial peptides (9 families) - Orientations of Proteins in Membranes (OPM) database.
- Superfamily 3.1.028. Amphibian antimicrobial peptides (7 families) - Orientations of Proteins in Membranes (OPM) database.
- GiacomettiA,CirioniO,GhiselliR,Mocchegiani F, Del PreteMS, et al.(2002) Potential therapeutic role of cationic peptides in three experimental models of septic shock. Antimicrob. Agents Chemother 46: 2132–2136.
- Park SC, Park Y, HahmKS (2011) The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation.Int J MolSci 12:5971-5992.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 18759
- [From(publication date):
August-2016 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 17074
- PDF downloads : 1685