Novel Drug Delivery System: Liquid Solid Compacts
Received: 27-Oct-2013 / Accepted Date: 19-Nov-2013 / Published Date: 29-Nov-2013 DOI: 10.4172/2329-9053.1000108
Abstract
The “Liquid-solid” technique is a novel and capable addition towards such an aims for solubility enhancement and dissolution improvement, thereby it increases the bioavailability. It contains liquid medications in powdered form. This technique is an efficient method for formulating water insoluble and water soluble drugs. This technique is based upon the admixture of drug loaded solutions with appropriate carrier and coating materials. The use of non-volatile solvent causes improved wettability and ensures molecular dispersion of drug in the formulation and leads to enhance solubility. By using hydrophobic carriers (non-volatile solvents) one can modify release (sustained release) of drugs by this technique. Liquid-solid system is characterized by flow behavior, wettability, powder bed hydrophilicity, saturation solubility, drug content, differential scanning calorimetry, Fourier transform infra red spectroscopy, powder X-ray diffraction, scanning electron microscopy, in vitro release and in vivo evaluation. By using this technique, solubility and dissolution rate can be improved, sustained drug delivery systems be developed for the water soluble drugs.
Keywords: Bioavailability; Solid dispersions; Oleophilic medication
1565Introduction
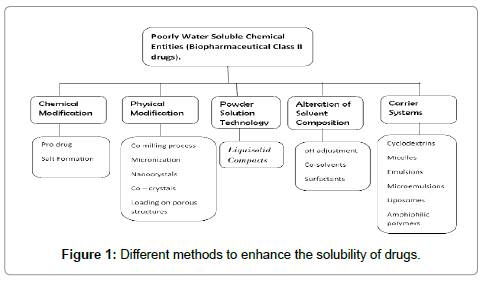
Solubilityof medicine could be a major consider the planning of pharmaceutical formulations result in variable oral bioavailability [1]. Dissolution is a very important issue for absorption of medicine particularly just in case of water insoluble or poorly soluble medicine one. The rate limiting step for many of the Pharmaceutical formulations is dissolution. Varied ways used to increase the solubility of poorly water soluble medicine are solid dispersions [2], inclusion complexes with β-cyclodextrins [3], micronization [4], mixture mixtures [5] and spray drying technique [6]. The new developed technique by Spireas [7], Liquid-solid system improves the dissolution properties of water insoluble or poorly soluble medicine. The term ‘Liquid-solid systems’ (LS) is a pulverized kind of liquid drug developed by changing liquid oleophilic drug or drug suspension or resolution of water-insoluble solid drug in appropriate non-volatile solvent systems, into dry trying, non-adherent, free-flowing and readily compressible pulverized mixtures by mixing with selected carrier and coating materials. Various grades of cellulose, starch, lactose, etc. square measure used because the carriers, whereas terribly fine oxide powder is employed because the coating (or covering) material [8]. The good flow and compression properties of Liquid-solid may be attributed because of massive area of oxide and fine particle size of avicel. Hence, Liquidsolid compacts containing water-insoluble medicine expected to show enhanced dissolution characteristics and consequently improved oral bioavailability. The in vitro drug dissolution rates of such preparations were compared to those of conventionally ready directly compressed tablets mistreatment a USP-II apparatus [9]. Liquid oleophilic medicine (e.g. Chlorpheniramine and Clofibrate) or solid medicine (e.g., prednisone, anti-inflammatory, hydrocortisone, Elixophyllin, Polythiazide and Spiranolactone) dissolved in non volatile, high-boiling purpose solvent systems (e.g., polythene and polypropene glycols, glycerin, N,N-dimethylacetamide, varied oils) have been developed in pulverized solutions by admixture with varied carriers (e.g., cellulose) and coating materials (e.g., silica). This method has been according to provide improved dissolution profiles as compared to the commercially obtainable products [10]. Shah [11] planned mathematical expressions for the calculation of the quantity of excipients required for pulverized solution formulations [12]. The foremost downside of this approach was that the ultimate product exhibited poor and erratic flow ability because of the inadequacy of the planned model to calculate the suitable quantity of excipients needed to produce powder admixtures of acceptable and consistent flow properties [13]. Mathematical model expressions supported powder properties and also the fundamentals principles and mechanisms of pulverized solutions square measure derived [14].
Advantages
1) It’s utilized in controlled drug delivery systems.
2) Drug may be molecularly distributed within the formulation.
3) Drug unleash may be changed exploitation appropriate formulation ingredients.
4) Capability of business production is additionally attainable.
5) Increased bioavailability may be obtained as compared to conventional tablets.
6) Differentiate the indefinite quantity kind by admixture of color into liquid vehicle.
7) To attenuate excipients in formulation compare with other formulations like solid dispersions.
8) Omit the method approaches like nanonisation, micronization techniques.
9) Immense variety of Bio-Pharmaceutical classification class II medicine with high porosity, slightly or terribly slightly water soluble and much insoluble liquids and solid medicine may be developed into Liquid-solid systems.
10) Improvement of bioavailability of an orally administered water insoluble medicine is achieved.
11) This principle governs or administers the mechanism of drug delivery from Liquid-solid systems of small-grained drug solutions and it’s primarily answerable for the improved dissolution profiles exhibited by this preparations.
12) During this technique, cost is low compared to soft gelatin capsules.
13) Drug is developed in a very pill type or encapsulated dosage type and is command in solubilized liquid state, which confers developed or improved drug wetting properties thereby rising drug dissolution profiles.
14) Bigger drug extent is exposed to the dissolution medium.
15) This Liquid-solid system is specifically for small-grained liquid medications.
16) These Liquid-solid systems formulate into immediate release or sustained unleash dose forms.
17) Optimized sustained unleash, Liquid-solid tablets or capsules of water insoluble medicine demonstrate constant dissolution rates (zero order release) [15].
Disadvantages
1) Formulation of high dose oleophilic medication the Liquidsolid pill is one in all the restrictions of this technique.
2) So as to attain acceptable flow ability and compatibility for Liquid-solid powder formulation, high levels of carrier material and coating materials should be adscititious. This may increase the burden of tablets to higher than one gram that makes them tough to swallow. Consequently, it’s not possible with conventional pill strategies to convert high dose to Liquidsolid pills with a tablet weight of but 50 mg. Dissolution profile sweetening happens within the presence of low levels of hydrophilic carrier, where coating material isn’t important.
Theory Of Liquid Solid Systems
A powder will retain solely restricted amounts of liquid whereas maintaining acceptable flow and compression properties. To calculate the desired amounts of powder excipients (carrier and coating materials) a mathematical approach for the formulation of Liquidsolid systems has been developed by Spireas [16]. This approach relies on the flow able (Ф- value) and compressible (Ψ-number) liquid retention potential introducing constants for every powder/liquid combination. The Ф-value of a powder represents the most quantity of a given non-volatile liquid which will be preserved within its bulk (w/w) whereas maintaining suitable flow ability. The flow ability could also be determined from the powder flow or by measurement of the angle of repose. The Ψ-number of a powder is outlined because the most quantity of liquid the powder will retain within its bulk (w/w) whereas maintaining acceptable compact ability leading to compacts of ample hardness with no liquid leaky out throughout compression [17]. The compact ability could also be determined by the alleged “pactisity” that describes the most (plateau) crushing strength of a onegram pill compacted at sufficiently high compression forces. The terms “acceptable flow and compression properties” imply the required and so preselected flow and compaction properties that should be met by the ultimate Liquid-solid formulation [18]. Depending on the excipient quantitative relation (R) of the powder substrate and so-so flowing associate degreed compressible Liquid-solid system can be obtained on condition that a most liquid load on the carrier material isn’t exceeded. This liquid/carrier quantitative relation is termed “liquid ratio low frequency (w/w) and is outlined because the weight ratio of the liquid formulation (W) and also the carrier material (Q) within the system:
Lf=W/Q------ (1)
‘R’ represents the quantitative relation between the weights of the carrier (Q) and also the coating (q) material gift within the formulation:
R=Q/q------ (2)
The liquid ratio that ensures acceptable flow ability (Lf ) is determined by:
Lf=Φ+ φ. (1/R) ----- (3)
Where Φ and φ ar the ?-values of the carrier and coating material, severally. Similarly, the liquid ratio for
production of Liquid-solid systems with acceptable compactability (ΨLf) is determined by:
ΨLf=Ψ+ψ.(1/R ------- (4)
Where Ψ and ψ ar the Ψ-numbers of the carrier and coating material, severally. In Table 1 samples of Liquid-solid formulation parameters of varied powder excipients with commonly used liquid vehicles are listed.
| Powder Excipient or System | Ф-values | Ψ-numbers | ||
|---|---|---|---|---|
| Propylene glycol | PEG-400 | Propylene glycol | PEG-400 | |
| Avicel pH 102 | 0.16 | 0.005 | 0.224 | 0.242 |
| Avicel pH 200 | 0.26 | 0.02 | 0.209 | 0.232 |
| Cab-O-Sil M5 (silica)* With Avicel pH 102 | 3.31 | 3.26 | 0.560 | 0.653 |
| Cab-OSilM5 (silica)* With Avicel pH 200 | 2.57 | 2.44 | 0.712 | 0.717 |
Table 1: Liquid-solid formulation parameters of various powder excipients with commonly used liquid vehicles.
Therefore, the optimum liquid ratio (Lo) needed to obtain so-so flowing and compressible Liquid-solid systems are adequate either ΦL for ΨLf, whichever represents the lower price. As shortly because the optimum liquid ratio is decided, the appropriate quantities of carrier (Qo) and coating (qo) material needed to convert a given quantity of liquid formulation (W) into associate degree so-so flowing and compressible Liquid-solid system could also be calculated as follows:
Q0 = W/Lo----- (5) And q0 = Q0/R------- (6)
The validity and relevancy of the on top of mentioned principles are tested and verified by manufacturing Liquid-solid compacts possessing acceptable flow and compaction properties [19].
Mechanisms Of Enhanced Drug Release From Liquid- Solid Systems
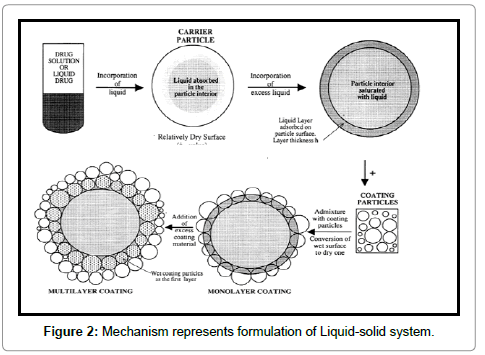
Several mechanisms of increased drug unleash are postulated for Liquid-solid systems. The 3 main instructed mechanisms embrace AN exaggerated extent of drug available for unleash, AN exaggerated binary compound solubility of the drug, and an improved wettability of the drug particles. Formation of a posh between the drug and excipients or any changes in crystallinity of the drug can be dominated out using DSC and XRPD measurements a exaggerated Drug extent If the drug at intervals the Liquid-solid system is totally dissolved within the liquid vehicle it’s placed within the powder substrate still in a very solubilized, molecularly distributed state. Therefore, the extent of drug out there for unleash is much bigger than that of drug particles at intervals directly compressed tablets. Exaggerated binary compound Solubility of the Drug In addition to the primary mechanism of drug unleash enhancement it’s expected that, the solubility of the drug, might be exaggerated with Liquid-solid systems. In fact, the relatively bit of liquid vehicle in a very Liquid-solid compact isn’t sufficient to extend the general solubility of the drug within the binary compound dissolution medium. However, at the solid/liquid interface between a personal Liquid-solid primary particle and also the unleash medium it’s potential that in this microenvironment the number of liquid vehicle diffusing out of one Liquid-solid particle beside the drug molecules can be sufficient to extend the binary compound solubility of the drug if the liquid vehicle acts as a cosolvent13.c. Improved Wetting Properties due to the very fact that the liquid vehicle will either act as surface active or incorporates a low physical phenomenon, wetting of the Liquid-solid primary particles is improved (Figure 1).
Wettability of those systems has been incontestable by measurement of contact angles and water rising times fifteen. Many poorly soluble medicines are developed as Liquid-solid systems showing increased drug unleash. Different liquid vehicles, carrier and coating materials were used to formulate these drug delivery systems.
Classification Of Liquid-Solid Systems
A. supported the kind of liquid medication contained in this liquidsolid systems could also be classified into 3 subgroups:
• Fine drug solutions
• Fine drug suspensions
• Fine liquid medicine
The first 2 could also be created from the conversion of drug solutions or (e.g. Prelone resolution in propylene glycol) or drug suspensions (e.g. medication suspension in Polysorbate 80), and also the latter from the formulation of liquid drugs (e.g. Clofibrate, liquid vitamins, etc.), into Liquid-solid systems. Since non-volatile solvents area unit accustomed prepare the drug resolution or suspension, the liquid vehicle doesn’t evaporate and so, the drug is carried at intervals the liquid system that successively is distributed throughout the ultimate product.
B. supported the formulation technique used Liquid-solid systems could also be classified into 2 categories:
• Liquid-solid compacts
• Liquid-solid Microsystems
Liquid-solid compacts area unit ready victimization the antecedently outlined methodology to provide tablets or capsules, whereas the Liquid-solid micro systems area unit supported a brand new conception that employs similar methodology combined with the inclusion of associate additive, e.g., Polyvinylpyrrolidone (PVP), in the liquid medication that is incorporated into the carrier and coating materials to provide an acceptably flowing admixture for encapsulation. The advantage stemming from this new technique is that the ensuing unit size of Liquid-solid micro systems could also be the maximum amount as 5 times but that of Liquid-solid compacts (Figure 2).
Preparation of Liquid Solid Compacts
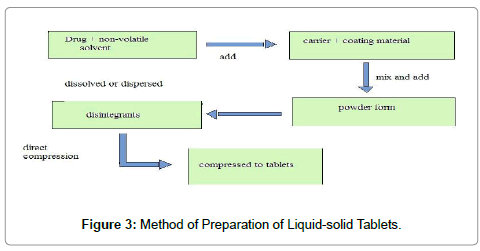
The liquid-solid tablet preparation method involves, first a mathematically calculated amount of pure drug weighed and dissolved in the suitable amount of solvent in a molecularly dispersed state. For attaining good flow properties trial and error methods were used i.e. changing the carrier: coating material ratio from 50:1 to 5:1 ratios according to new mathematical model expressions proposed by Liao. This liquid medication is poured on the suitable amount of carrier material. The liquid medication is absorbed into the carrier material internally and externally and then a suitable disintegrant was added to this material. Finally, coating material was added for dry looking, adherent to the carrier material for achieving good compression properties. Liquid medication is incorporated into carrier material which has a porous surface and closely matted fibers in its interior as cellulose. Both absorption and adsorption take place, i.e., the liquid absorbed into the interior of the particles is captured by its internal structure and after saturation of this process, adsorption of the liquid onto the internal and external surface of the porous carrier particles occurs. Excipients possessing fine and highly adsorptive particles such as various types of amorphous silicon dioxide (silica) are most suitable for this step. Before compression or encapsulation, various ingredients such as lubricants disintegrants or Polymers, and binders (Figure 3), may be mixed with the finished liquid-solid systems to produce liquidsolid compacts in the dosage form of tablets or capsules.
Formulation Parts
The major formulation parts of Liquid-solid compacts are:
Carrier material
These area unit compression-enhancing, comparatively massive, preferably porous particles possessing a comfortable absorption property that contributes in liquid absorption. E.g. varied grades of polyose, starch22, lactose25, sorbitol26 etc.
Coating material
These area unit flow-enhancing, terribly fine (10 nm to 5000 nm in diameter), extremely surface-assimilative coating particles (e.g., silica of various grades like Cab-O-Sil M5, Aerosil two hundred, Syloid
244FP etc.) Contributes in covering the wet carrier particles and displaying a dry-looking powder by take up any excess liquid twenty two [20].
Non-volatile solvents
Inert, high boiling purpose, ideally water-miscible and not highly viscous organic solvent systems e.g., propylene glycol, liquid synthetic resin glycols, polysorbates, glycerin, N, N-dimethylacetamide, fixed oils, etc. area unit best suited as vehicles.
Disintegrants
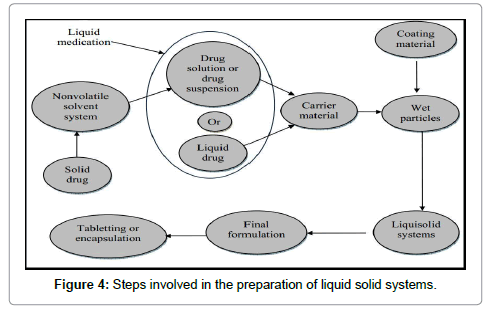
Most commonly used disintegrant is atomic number 11 starch glycolate (Explotab13, Pumogel, etc.). Approach to reinforce Dissolution of Drug unleash from its Immediate unleash Tablets In the Liquid-solid systems the drug can be in an exceedingly solid type, it is command at intervals the powder substrate in resolution, or in a solubilized, nearly molecularly distributed state, consequently, due to their considerably exaggerated wetting properties and surface of drug on the market for dissolution. Liquid-solid compacts of water- insoluble substances could also be expected to show enhanced drug unleash properties and at the same time improved bioavailability. Rofecoxib may be a much insoluble nonsteroidal anti inflammatory drug. The Liquid-solid tablets of Vioxx showed vital exaggerated in dissolution profiles compared to the industrial tablets. Naproxen may be a nonsteroidal anti-inflammatory (NSAID) commonly used for the reduction of fever, pain and inflammation. Liquid-solid compacts amendment the properties of naproxen particles by merely dispersing the drug particles in a non-volatile hydrophilic liquid vehicle that increase the wetting properties of drug particles, and enhances the dissolution rate and shows improved bioavailability of the drug. At present, NSAID is out there commercially in high dose tablets between 250 and five hundred mg; the liquid-solid formulations could facilitate in reduction of the dose conjointly. Bromhexine coordination compound (BXH) may be a mucolytic agent used in the treatment of metastasis disorders related to viscid or excessive mucous secretion. It’s a poor solubility that may be a major consider the planning of pharmaceutical formulations. Liquid-solid compacts of BXH were clearly higher compared to directly compressed tablets. It shows vital advantages of liquid-solid in increasing wetting properties and surface area of drug on the market for dissolution. Prednisolone, awfully slightly water soluble internal secretion, prepared in directly compressed tablets and liquid-solid compacts. in line with the ready technique of liquid-solid compacts, liquid medications like solutions or suspensions of water insoluble medicine in appropriate non-volatile liquid vehicles is reborn into soso flowing and compressible powders by mixing with selected powder excipients. It’s been speculated that such systems shows enhanced unleash profiles as a result of the exaggerated wetting properties and surface of drug available for dissolution. Several liquid-solid pill formulations were ready by applying a replacement mathematical model to calculate the appropriate quantities of powder and liquid ingredients required to provide so-so flowing and compressible admixtures. Liquid-solid compacts confirmed considerably higher drug release rates, in numerous dissolution media, compared to tablets ready by the direct compression technique. It was also ascertained that the drug dissolution rate from liquid-solid tablets were freelance of the degree of dissolution medium, in distinction to the plain tablets that expose declining drug unleash patterns with decreasing dissolution volumes. Piroxicam (PX) may be a category II drug in line with BCS because it possesses poor water solubility and extremely porousness. The rate of its oral absorption is usually controlled by the dissolution rate within the canal. The poor dissolution rate of water insoluble medicine remains a significant drawback confronting the pharmaceutical trade. There square measure many techniques to reinforce the dissolution of poorly soluble drugs. Among them, the technique of liquid-solid compacts may be a promising technique towards such a completely unique aim. During this study, the dissolution behavior of commissary from liquid-solid compacts was investigated in simulated stomachic fluid (SGF, pH1.2) and simulated internal organ fluid (SIF, pH 7.2). Many liquid-solid tablets formulations containing numerous ratios of drug: Tween 80 (10% to five hundredth w/w) was ready. The quantitative relation of MCC as a carrier to oxide as coating powder material was unbroken constant in all formulations. The results showed that liquidsolid compacts incontestible significantly higher drug unleash rates than those of conventionally created capsules and directly compressed tablets containing micronized commissary. The higher dissolution rates displayed by liquid-solid compacts may additionally simply increased oral bioavailability owing to the exaggerated wetting properties and surface of drug on the market for dissolution. This study reveals that, the liquid-solid compacts of commissary during which polysorbate eighty is that the liquid vehicle, in different drug concentrations in their liquid medications, exhibit drug dissolution rates that square measure directly proportional to the fraction of the molecularly distributed drug in their liquid medication it’s terminated that liquidsolid compacts technique is a promising different for the formulation of water-insoluble medicine. Carbamazepine (CBZ), 5H-dibenzazepine- 5-carboxamide, is an atomic number 11 channel blocker that has been in routine use within the treatment of brain disease and neuralgy for over forty years. CBZ is taken into account a primary line drug within the treatment of Epilepsy. It’s much insoluble in water. The oral absorption of CBZ is slow, erratic and unpredictable in humans as a result of slow dissolution. Several studies were done in trial to boost the bioavailability of CBZ. This drug conjointly belongs to category II, that its bioavailability is restricted by its poor dissolution rate in GI. In fact, its solubility and dissolution rate square measure key factors in its bioavailability. Different liquid-solid formulations of CBZ were consummate by dissolving the drug within the non-toxic hydrophilic liquids, and adsorbing the answer onto the surface of oxide. To reduce the amounts of carrier and aerosil in liquid-solid formulations, some specifically additives Polyvinylpyrrolidone (PVP), hydroxypropyl methylcellulose (HPMC) and synthetic resin glycol (PEG 35000) were additional to liquid medication to increase loading issue. The results of varied ratios of carrier to coating material, impact the aging and kind of the carrier on dissolution rate of liquid-solid compacts were studied. Cyclosporine (CS) Self Micro-Emulsifying pill (SME), the tablets were ready by the liquid-solid compaction technique. Formulation consists of oil, chemical agent and cosurfactant which were selected on the idea of solubility and emulsification ability for the SME formulation. During this study, the mixture of Lauroglycol FCC: Maisine 35-1 (1:1 w/w) was selected because the oil section, PEG-35 aperients was selected because the chemical agent and PEG-400 was selected because the co-surfactant. One to six was selected because the quantitative relation between the drug and therefore the mixture. Associate in Nursing Emulsion couldn’t be shaped in several oils, like Carpryol ninety, Lauroglyol ninety and Lauroglycol FCC even during which the caesium has sensible solubility. Due to the cyclic structure of CS-A, some excipients absorbed the drug and will not be selected as carrier material and coating material, e.g., oxide powders. The liquid-solid tablets were effective in enhancing dissolution of CS-A, a poorly soluble drug. The tablets exhibited good flow ability and compact ability. The results showed that the liquidsolid compaction technique may be a promising different technique to boost the solubility and therefore the dissolution rate, for poorly soluble medicine CS-A (Figure 4).
Limitations
• Not applicable for the formulation of high dose insoluble medicine.
• If additional quantity of carrier is additional to supply free flowing powder, the pill weight will increase to additional than one gram that is tough to swallow.
• Acceptable compression properties might not be achieved since throughout compression liquid drug could be squeezed out of the liquid-solid pill leading to tablets of unsatisfying hardness.
• Introduction of this methodology on industrial scale and to overcome the issues of blending small quantities of viscous liquid solutions onto giant amounts of carrier material might not be possible.
Conclusion
Liquid solid compacts are improved helpful technology to overcome the low bioavailability of the drug. The improvement within the dissolution characteristics of a liquid-solid technique changes the properties of drug unharness by simply spread the drug particles in a very non volatile liquid vehicle, that successively increase the wetting properties and surface area of drug particles, and thus improve the dissolution profiles and may well be oral bioavailability of the drug.
References
- Merisko-Liversidge E, Liversidge GG, Cooper ER (2003) Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci 2: 113-120.
- Jarowski CI, Rohera BD, Spireas S (1992) Powdered solution technology: principles and mechanism. Pharm Res 9: 1351-1358.
- Barzegar JM, Javadzadeh Y, Nokhodchi A, Siahi-Shadbad MR (2005) Enhancement of dissolution rate of piroxicam using liquisolidcompacts. II Farmaco 60: 361-365.
- Nokhodchi A, Hentzschel CM, Leopord CS (2011) Drug release from liquisolid system: speed it up, slow it down. Expert Opin Drug Del 8: 191-205.
- El-Houssieny BM, Wahman LF, Arafa NMS (2010) Bioavailability and biological activity of liquisolid compact formula of repaglinide and its effect on glucose tolerance in rabbits. Bio Sci Trends 4: 17-24.
- Khaled KA, Asiri YA, El-Sayed YM (2001) In-vivo evaluation of hydrochlorothiazide liquisolid tablet in beagles dogs. Int J Pharm 222: 1-6.
- Naseem A, Olliff CJ, Martini LG, Lloyd AW (2004) Effects of plasma irradiation on the wettability and dissolution of compacts of griseofulvin. Int J Pharm 269: 443-450.
- Setty CM, Prasad DVK, Gupta RM (2008) Development of fast dispersible aceclofenac tablet: effect of functionality of super disintegrants. Indian J Pharm Sci 70: 180-185.
- Yadav VB, Yadav AV (2009) Liquisolid granulation technique for tablet manufacturing: An overview. J Pharm Res 2: 670-674.
- Shah TJ, Amin AF, Parikh JR (2007) Process optimization and characterization of poloxamer solid dispersions of a poorly watersoluble drug. AAPS Pharm Sci Tech 8: E1-E7.
- Aguiar AJ, Kinkel AW, ZelmerAJ (1979) Deagglomeration behavior of relatively insoluble benzoic acid and its sodium salt. J Pharm Sci 56: 1243-1252.
- Finholt P, Solvang S (1968) Dissolution kinetics of drugs in human gastric juice the role of surface tension. J Pharm Sci 57: 1322-1326.
- Lin SL, Lachman L, Menig J (1968) Interdependence of physiological surfactant and drug particle size on the dissolution behavior of water insoluble drugs. J Pharm Sci 57: 2143-2146.
- Ayres JW, Kapsi SG (2001) Processing factors in development of solid solution formulation of Itraconazole for enhancement of drug dissolution and bioavailability. Int J Pharm 229: 193-203.
- Jafari NB, Javadzadeh Y, Nokhodchi A (2007)Liquisolid technique for dissolution rate enhancement of a high dose water-insoluble drug Carbamazepine. Int J Pharm 341: 26-34.
- Javadzadeh Y, Musaalrezaei L, Nokhodchi A (2008) Liquisolid technique as a new approach to sustained propranolol hydrochloride release from tablet matrices. Int J Pharm 362: 102-108.
- Kavitha K, Kotha NS, Raja L, Ganesh NS, Ramesh B (2011) Effect of dissolution rate by Liquisolid Compact Approach: An Overview. Der Pharma Lett 3: 71-83.
- Spiro S, Srinivas S (1998) Enhancement of Prednisolone dissolution properties using liquisolid compacts. Int J Pharm 66: 177-188.
- Pouton CW (2006) Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci 29: 278-287.
Citation: Peddi MG (2013) Novel Drug Delivery System: Liquid Solid Compacts J Mol Pharm Org Process Res 1: 108 DOI: 10.4172/2329-9053.1000108
Copyright: ©2013 Peddi MG. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 23345
- [From(publication date): 11-2013 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 18401
- PDF downloads: 4944