Mini Review Open Access
Novel Concepts for Neurology and Medicine from the Interaction between Signalling Pathways Mediated by Ca2+ and cAMP: An Intriguing History
Leandro BB* and Afonso C
Department of Pharmacology, Escola Paulista de Medicina, Universidade Federal de São Paulo, Brazil
- *Corresponding Author:
- Leandro BB
Department of Pharmacology
Escola Paulista de Medicina
Universidade Federal de São Paulo, Brazil
Tel: 55 11 5576-4973
Email: leanbio39@yahoo.com.br
Received Date: April 12, 2017; Accepted Date: April 24, 2017; Published Date: May 04, 2017
Citation: Leandro BB and Afonso C (2017) Novel Concepts for Neurology and Medicine from the Interaction between Signalling Pathways Mediated by Ca2+ and cAMP: An Intriguing History. J Pediatr Neurol Disord 3: 112. doi:10.4172/2572-5203.1000112
Copyright: © 2017 Leandro BB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pediatric Neurological Disorders
Abstract
It is now well-established that the signalling pathways mediated by Ca2+ and cAMP can interact (Ca2+/cAMP signalling interaction), thus playing a vital role in cellular processes of mammalians. In the neurology and medicine, it has opened novel opportunities for the development of pharmaceuticals more efficient, and safer, for treating neurodegenerative diseases. The solution for the so-called “calcium paradox” has been revealed 4 years ago, when we demonstrated the involvement of the Ca2+/cAMP signalling interaction in this enigma. The “calcium paradox” emerged 4 decades ago, when numerous clinical studies have concluded that prescription of L-type Ca2+ channel blockers (CCBs) for hypertensive patients decreased arterial pressure, but produced stimulation of sympathetic hyperactivity. Indeed, initially these adverse effects of CCBs have been attributed to adjust reflex of arterial pressure, but this conclusion remained not completely satisfactory. The year of 2013 would change this history forever! Through an original experiment, we revealed that the "calcium paradox" phenomenon came from increased transmitter release from sympathetic neurons stimulated by CCBs due to its handling on the Ca2+/cAMP signalling interaction. Then, the manipulation of Ca2+/cAMP signalling interaction could improve therapeutic strategies for stimulating synaptic transmission compromised by transmitter release deficit, and attenuating death of neurons.
Keywords
Ca2+/cAMP signalling interaction; Paradoxical effects produced by CCBs; Neurology
Introduction
From the past years, it has been shown that the signalling pathways mediated by Ca2+ and cAMP can interact (Ca2+/cAMP signalling interaction), thus playing a vital role in cellular processes of mammalians. In the neurology and medicine, it could improve therapeutic strategies for stimulating synaptic transmission compromised by transmitter release deficit, and attenuating death of neurons.
It has been almost 4 years since we revealed the involvement of the Ca2+/cAMP signalling interaction in the enigma of the so-called “calcium paradox”. For understanding the “calcium paradox”, we should return to the past. Indeed, the concept of stimulus-secretion to elucidate neurotransmitters release has been achieved from creative experiments made by Douglas, et al. [1]. By their concepts, in 1970´s Baker and Knight [2] showed that an increase in the cytosolic Ca2+ concentration ([Ca2+]c) is a fundamental requirement to start transmitter release. In addition, the unquestionable result showing a correlation between neurotransmitter release and elevation in [Ca2+]c came from the interesting experiments made by the Nobel laureate Erwin Neher [3]. Thus, by reducing extracellular Ca2+ through blocking Ca2+ channels, we should have a reducing in the neurotransmitter release. Nonetheless, many reports have demonstrated that L-type Ca2+ channel blockers (CCBs), in concentrations below 1 μmol/L, could induce neurotransmitter release, a “paradox” [4-6]. In addition, many reports have demonstrated that cAMP enhances neurotransmitter release at several synapses in autonomic nervous system of mammalians [7]. Recently, we demonstrated that Ca2+/cAMP signalling interaction is implicated in the modulation of transmitters release from sympathetic neurons, and adrenal chromaffin cells [8-11].
The interaction between Ca2+ and cAMP signalling pathways as a classical concept: an intriguing history
It is well established that the interaction between Ca2+ and cAMP signalling pathways is as a vital cellular process in mammalians [8-11]. This classical concept assumes that these signalling pathways virtually exist in all mammalian cells, modulated by adenylyl cyclases (ACs) and phosphodiesterases (PDEs) [8-11]. In addition, endoplasmic reticulum (ER) Ca2+ channels have particularly been a forefront for the interaction between Ca2+ and cAMP signalling pathways field, such as Ca2+ channels modulated by ryanodine receptors (RyR) [8-11]. We reinforced the idea that the interaction between Ca2+ and cAMP signalling pathways plays a fundamental participation in the modulation of neurotransmitter release from neurons and neuroendocrine cells [8-11]. Then, the interaction of Ca2+ and cAMP signalling pathways could be a new therapeutic goal for pharmaceuticals.
The interaction between Ca2+ and cAMP signalling pathways and neurology
The prescription of L-type CCBs in hypertensive patients has been reported to decrease arterial pressure, but also produces sympathetic hyperactivity [12]. Initially, these adverse effects of CCBs have been attributed to adjust reflex of arterial pressure, but this conclusion remained not completely satisfactory. The year of 2013 would change this history forever! Through a creative experiment, we revealed that the solution for this so-called "calcium paradox" phenomenon was due to the increase of transmitter release from sympathetic neurons achieved by CCBs due to its handling on the interaction between Ca2+ and cAMP signalling pathways [9]. We demonstrated that contractions of the smooth muscle (vas deferens) were completely inhibited by Ltype CCBs in high concentrations (>1 μmol/L), but puzzlingly increased in concentrations below 1 μmol/L, thus defined as sympathetic hyperactivity promoted by CCBs [4-6,9]. Our studies clearly established that the contradictory sympathetic hyperactivity is due to an augmentation of transmitter release from sympathetic neurons achieved by L-type CCBs due to its interfering on the interaction between Ca2+ and cAMP signalling pathways.
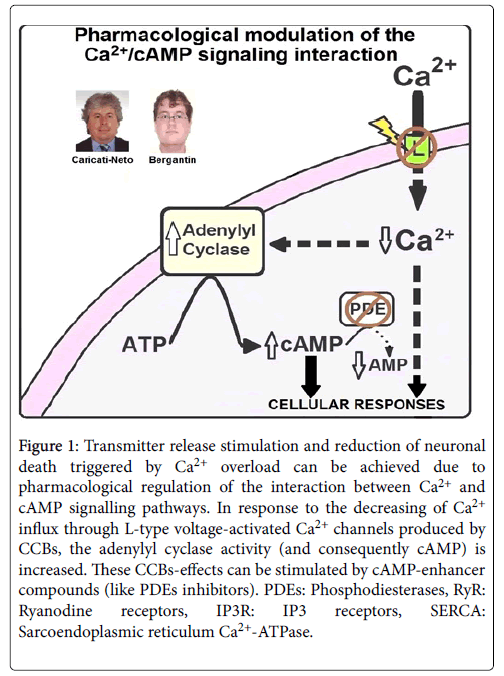
In fact, many reports have shown that elevation of cytosolic cAMP concentration ([cAMP]c) reduces neuronal death resulted from cytosolic Ca2+ overload, stimulating neuroprotective effect [13,14]. As mentioned above, the L-type CCBs increase transmitter release due to its handling on the interaction between Ca2+ and cAMP signalling pathways. This interference activates ACs, causing elevation of [cAMP]c that, in turn, induces Ca2+ release from ER that stimulates transmitter release [8-11]. In addition, this elevation of [cAMP]c produces neuroprotective effects mediated by the Ca2+ and cAMP signalling pathways [8-11]. It was proposed that this neuroprotective effect results from activation by cAMP on the cellular survival pathways mediated by PKA/CREB [8-11,13,14]. Then, the pharmacological interfering of the Ca2+/cAMP signalling interaction from the combined use of the L-type CCBs prescribed in the antihypertensive therapy, and [cAMP]c-enhancer compounds prescribed in the anti-depressive therapy like rolipram, could be a novel pharmacological goal for increasing neurotransmission in neurological and psychiatric disorders resulted from deficit of neurotransmitter release, and neuronal death [8-11]. 14 illustrates how the pharmacological handling of the interaction between Ca2+ and cAMP signalling pathways could produce increase of neurotransmitter release, and attenuation of neuronal death.
Figure 1: Transmitter release stimulation and reduction of neuronal death triggered by Ca2+ overload can be achieved due to pharmacological regulation of the interaction between Ca2+ and cAMP signalling pathways. In response to the decreasing of Ca2+ influx through L-type voltage-activated Ca2+ channels produced by CCBs, the adenylyl cyclase activity (and consequently cAMP) is increased. These CCBs-effects can be stimulated by cAMP-enhancer compounds (like PDEs inhibitors). PDEs: Phosphodiesterases, RyR: Ryanodine receptors, IP3R: IP3 receptors, SERCA: Sarcoendoplasmic reticulum Ca2+-ATPase.
In fact, it was showed that the prescription of L-type CCBs is able to reduce motor symptoms, and reduces the continued neuronal death in animal model of Parkinson´s disease, indicating that L-type CCBs are potentially workable neuroprotective pharmaceuticals [15]. Intriguingly, a 1-decade study involving thousands senile hypertensive patients demonstrated that prescription of L-type CCBs can reduce blood pressure, and incidence of dementia in hypertensive patients, indicating that these pharmaceuticals could be used to treat neurodegenerative diseases in clinics [16]. These results for the effects related to neuroprotection of CCBs have been reinvestigated in thousands elderly hypertensive patients with dysfunction of memory abilities [17]. These studies concluded that patients who have taken CCBs had their risk of cognitive dysfunction decreased, such as Alzheimer´s disease [17]. These findings reinforce the concept that Ltype CCBs can reduce cytosolic Ca2+ overload produced due to blocking of Ca2+ influx, and thus could be an alternative pharmacological goal to reduce, or prevent, death of neurons resulted from neurodegenerative diseases.
Based on these findings, we have anticipated that the pharmacological regulation of the Ca2+/cAMP signalling interaction by combined use of the L-type CCBs and [cAMP]c-enhancer compounds could be a novel therapeutic goal for increasing neurotransmission in neurological, and psychiatric disorders, resulted from neurotransmitter release deficit and neuronal death [8-11]. This pharmacological strategy opens a novel pathway for the drug development more efficient for the treatment of Alzheimer´s and other neurodegenerative diseases [18-24].
Conclusion
In conclusion, pharmacological interfering of the interaction between Ca2+ and cAMP signalling pathways could be a more efficient therapeutic approach for enhancing neurotransmission resulted from neurotransmitter release deficit, and reducing neuronal death. These findings could dramatically impact in neurology and medicine.
Disclosure Statement
Caricati-Neto and Bergantin thank the continued financial support from CAPES, CNPq and FAPESP (Bergantin´s Postdoctoral Fellowship FAPESP #2014/10274-3). The authors also thank Elsevier - “author use”.
References
- Douglas WW, Rubin RP (1961) The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol 159: 40-57.
- Baker PF, Knight DE (1978) Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature 276: 620-622.
- Neher E, Zucker RS (1993) Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron 10: 21-30.
- Kreye VA, Luth JB (1975) Proceedings: verapamil-induced phasic contractions of the isolated rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol 287 Suppl: R43.
- French AM, Scott NC (1981) A comparison of the effects of nifedipine and verapamil on rat vas deferens. Br J Pharmacol 73: 321-323.
- Moritoki H, Iwamoto T, Kanaya J, Maeshiba Y, Ishida Y, et al. (1987) Verapamil enhances the non-adrenergic twitch response of rat vas deferens. Eur J Pharmacol 140: 75-83.
- Chern YJ, Kim KT, Slakey LL, Westhead EW (1988) Adenosine receptors activate adenylate cyclase and enhance secretion from bovine adrenal chromaffin cells in the presence of forskolin. J Neurochem 50: 1484-1493.
- Caricati-Neto A, García AG, Bergantin LB (2015) Pharmacological implications of the Ca2+/cAMP signalling interaction: from risk for antihypertensive therapy to potential beneficial for neurological and psychiatric disorders. Pharmacol Res Perspect 3: e00181.
- Bergantin LB, Souza CF, Ferreira RM, Smaili SS, Jurkiewicz NH, et al. (2013) Novel model for “calcium paradox” in sympathetic transmission of smooth muscles: role of cyclic AMP pathway. Cell Calcium 54: 202-212.
- Bergantin LB, Jurkiewicz A, García AG, Caricati-Neto A (2015) A Calcium Paradox in the Context of Neurotransmission. Journal of Pharmacy and Pharmacology 3: 253-261.
- Bergantin LB, Caricati-Neto A (2016) Challenges for the pharmacological treatment of neurological and psychiatric disorders: Implications of the Ca2+/cAMP intracellular signalling interaction. Eur J Pharmacol 788: 255-260.
- Grossman E, Messerli FH (1998) Effect of calcium antagonists on sympathetic activity. Eur Heart J. F: F27-F31.
- Sommer N, Loschmann PA, Northoff GH, Weller M, Steinbrecher A, et al. (1995) The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat Med 1: 244-248.
- Xiao L, O'Callaghan JP, O'Donnell JM (2011) Effects of repeated treatment with phosphodiesterase-4 inhibitors on cAMP signaling, hippocampal cell proliferation, and behavior in the forced-swim test. J Pharmacol Exp Ther 338: 641-647.
- Ilijic E, Guzman JN, Surmeier DJ (2011) The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson's disease. Neurobiol Dis 43: 364-71.
- Wu CL, Wen SH (2016) A 10-year follow-up study of the association between calcium channel blocker use and the risk of dementia in elderly hypertensive patients. Medicine (Baltimore) 95: e4593.
- Hanon O, Pequignot R, Seux ML, Lenoir H (2006) Relationship between antihypertensive drug therapy and cognitive function in elderly hypertensive patients with memory complaints. J Hypertension 24: 2101-2107.
- Bergantin LB, Caricati-Neto A (2016) Insight from “Calcium Paradox” due to Ca2+/cAMP Interaction: Novel Pharmacological Strategies for the Treatment of Depression. Int Arch Clin Pharmacol 2: 007.
- Bergantin LB, Caricati-Neto A (2016) Novel Insights for Therapy of Parkinson’s disease: Pharmacological Modulation of the Ca2+/cAMP Signalling Interaction. Austin Neurol & Neurosci 1: 1009.
- Bergantin LB, Caricati-Neto A (2016) Recent advances in pharmacotherapy of neurological and psychiatric disorders promoted by discovery of the role of Ca2+/cAMP signaling interaction in the neurotransmission and neuroprotection. Adv Pharmac J 1: 66.
- Bergantin LB, Caricati-Neto A (2016) From discovering “calcium paradox” to Ca2+/cAMP interaction: Impact in human health and disease. Scholars Press, p: 108.
- Caricati-Neto A, Bergantin LB (2016) New therapeutic strategy of Alzheimer´s and Parkinson´s diseases: Pharmacological modulation of neural Ca2+/cAMP intracellular signaling interaction. Asian Journal of Pharmacy and Pharmacology 2: 136-143.
- Bergantin LB, Caricati-Neto A (2016) Impact of interaction of Ca2+/cAMP Intracellular Signalling Pathways in Clinical Pharmacology and Translational Medicine. Clinical Pharmacology and Translational Medicine 1-4.
- Bergantin LB, Caricati-Neto A (2016) Challenges for the Pharmacological Treatment of Dementia: Implications of the Ca2+/cAMP Intracellular Signalling Interaction. Avidscience 2-25.
Relevant Topics
- Behavioral Psychology
- Chiari malformation
- Chronic Traumatic Encephalopathy
- Congenital Brain Defects
- Duchenne Muscular Dystrophy
- Epilepsy and Seizures
- Genetic and Metabolic Disorders
- Genetic Epilepsies
- Headaches and Migraines
- Movement Disorders
- Neonatal encephalopathy
- Neurodevelopmental Disorders
- Neurogenetic Disorders
- Neurological Complications of AIDS
- Neuromuscular Disease
- Pediatric Brain Tumour
- Pediatric Sleep Disorders
- Stroke and Perinatal Injuries
Recommended Journals
Article Tools
Article Usage
- Total views: 2448
- [From(publication date):
December-2017 - Apr 19, 2025] - Breakdown by view type
- HTML page views : 1622
- PDF downloads : 826