Nosocomial Contamination and Bacterial Resistance in Intensive Care Units after Disinfection: An Emerging Challenge under Biofilm and Planktonic Growth Condition
Received: 03-Jul-2022 / Manuscript No. jcidp-22-68378 / Editor assigned: 05-Jul-2022 / PreQC No. jcidp-22-68378 / Reviewed: 18-Jul-2022 / QC No. jcidp-22-68378 / Revised: 25-Jul-2022 / Manuscript No. jcidp-22-68378 (R) / Published Date: 29-Jul-2022 DOI: 10.4172/2476-213X.1000153
Abstract
Disinfectants are widely used in healthcare facilities to prevent the occurrence of hospital associated infections (HAIs), especially in intensive care units (ICUs). Microorganisms adhere to different surfaces and become physically structured in biofilms by secreting extracellular polymeric substances (EPSs) which act as an external shield for the microorganisms, reducing the penetration and diffusion of biocidal substances. This study analyzed the presence of phenotypically resistant Gram-negative rods in ICUs and investigated whether biofilms are responsible for the persistence of multidrug-resistant bacteria in these specialist hospital wards. Multidrug-resistant Gram-negative rods were isolated after ICU disinfection and evaluated for susceptibility to three disinfectants [benzalkonium chloride and biguanide (BCB), sodium hypochlorite (NaClO), and hydrogen peroxide (H2O2)] under two different conditions: when they are in a planktonic state (unicellular-life phase) and after induction to form biofilms (multicellular-life phase). We compared the efficacy of these chemical disinfectants in removing monospecies biofilms by quantifying the mature biofilm biomass using the crystal violet technique, and through evaluation using scanning electron microscopy (SEM).All disinfectants tested showed bactericidal activity against bacteria growing in suspension. After inducing the bacteria to form a biofilm, we observed that BCB was unable to reduce biofilm biomass, while H2O2 was only weakly effective against A. baumannii, A. calcoaceticus complexes ACB (p<0.05) and E. coli ATCC 25922 (p<0.05). SEM images corroborated crystal violet data regarding the pronounced NaClO activity observed against all bacteria (p<0.0001) and allowed a better evaluation of the action of BCB, demonstrating variation in sensitivity to this disinfectant for each species evaluated. Multidrug-resistant bacteria isolated from ICU inanimate surfaces did not show the same sensitivity to the assessed disinfectants when they were in a biofilm as when they were in a single cell state, and it appeared that biofilms are able to reduce the activity of disinfectants and contribute to the spread of bacteria involved in the occurrence of HAIs.
Keywords
Healthcare-associated infections, Biofilms, Disinfection, Antimicrobial Resistance, Intensive care units.
Introduction
The survival of pathogenic microorganisms in hospital settings plays an important role in healthcare-associated infections (HAIs) [1]. Among hospitalized patients, the individuals most susceptible to HAIs are those admitted to ICUs, to hospital wards which specialize in the treatment of severe burn injuries, to organ transplant and neonatology units [2], with the highest incidence observed in patients admitted to the ICU [3]. Good hygiene and disinfection of inanimate areas and objects in the nosocomial environment may be able to overcome this situation, probably by reducing cross-infection [4].
Disinfectants normally used in hospitals can eliminate most environmental contaminants [5]. However, some microorganisms may survive and contribute to the constantly growing multitude of antimicrobial-resistant microorganisms causing substantial problems to public health, including significant morbidity and mortality, in addition to the direct and indirect costs burdening the already limited financial resources allocated to healthcare [6, 7]. In fact, the World hospital settings [9]. The increased resistance of these microorganisms to disinfectants has hampered their eradication [10-12].
The resistance of microorganisms to disinfection is often associated with the presence of biofilms on surfaces [5, 13, 14]. Biofilms are widely disseminated in nature and constitute an important strategy implemented by microorganisms to adapt, evolve, and survive under adverse environmental conditions [15, 16]. According to Dincer, Uslu, and Delik [15], "biofilm growth confers several advantages to bacteria, including protection against hostile environmental conditions such as osmotic stress, metal toxicity, and antibiotic exposure"
Bacteria growing in biofilms are intrinsically more resistant to antibiotic treatments than non-adherent planktonic (free-living) cells of the same strain. In fact, a subset of biofilm bacterial cells can survive in the presence of concentrations of bactericidal antibiotics up to 1,000 times higher than their minimum inhibitory concentration (MIC), thus contributing to the establishment, maintenance, and spread of infections [17,18]. In addition, the multicellular behavior of bacteria growing in biofilms is one of the main mechanisms of resistance, enabling the microorganisms to protect themselves from host defense, disinfectants, and antimicrobials [15, 19, 20].
It is known that the anti-biofilm effectiveness of a cleaning agent depends on its ability to separate biofilms from a surface (21]. The failure of disinfectants in eliminating biofilms is a major factor resulting in outbreaks of infections and diseases, due to the persistence of microorganisms in the environment, which generates several contamination problems [22-24]. It is necessary to take into account the specific characteristics, either of biofilm-forming microorganisms or of biofilms themselves that limit both the diffusion and the biocidal activity of antimicrobial agents in the context of biofilms, in order to formulate effective disinfectants [23,25].
Disinfection protocols, as well as the correct execution of aseptic techniques, are important procedures for the control and prevention of infections and require careful and constant evaluation of their antimicrobial effectiveness. In this scenario, we investigated whether the multidrug-resistant Gram-negative bacteria which, after disinfection with 1% BCB, were still isolated from inanimate surfaces in the ICU, can generate biofilms.
Materials and Methods
Study Setting and Design
This study was carried out in the ICU of a medium-sized hospital in the city of Ilhéus (14°47′20′′ S, 39°02′56′′ W), Bahia state, Brazil, from February 2019 to February 2020, before the start of the COVID-19 pandemic in this country. The 104 m2 ICU houses critically ill patients of all medical specialties. It accommodates 20 electric beds and consists of 4 sections sharing the same location in the hospital building, the same administrative area, and other facilities (e.g., patients’ bathroom, pantry). Visitors are permitted from 2 pm to 4 pm, and no limit to the number of visitors is stipulated.
Disinfection was performed three times a day and consisted of cleaning with 1% BCB solution using a mop on the floor and wet wipes on furniture surfaces and devices. Samples were collected in the morning before and immediately after one of the sanitizing procedures, when there was no more humidity over the area. During sampling, all ICU employees and facilities were fully operational. Collections were performed twice a week. Samples of inanimate surfaces were collected from different areas of the ICU, including occupied beds (n=8), administrative area, bathroom, pantry, sink, and pharmacy.
Bacterial Collection and Identification
Samples from occupied beds, including cardiac monitors and infusion pumps, serving table, refrigerator, microwave oven, tiles, sinks, dispensary, and patients’ bathroom walls were obtained. Sampling was performed by rubbing each surface with individual sterile swabs (Absorb, China) embedded in Stuart medium, applying firm movements to ensure full contact with the area (400 cm2 ). Each sample was individually immersed in a tube containing brain heart infusion (BHI) broth (HiMedia, India) and then the liquid was serially diluted.
The procedure was aseptically performed in a level 2 biosafety laboratory. After 24 h of incubation at 37°C in aerobiosis, samples were subcultured in selective media: eosin methylene blue agar (EMB) (Merck, Germany), nutrient agar (KASVI, Brazil), and blood agar (Micro Med, Brazil). After incubation for 24 h at 37°C in aerobiosis, the isolated strains were subjected to standard tests for biochemicalphysiological identification and evaluation of their morphotintorial aspect using Gram stain. Bacteria were frozen at –20°C in Luria Bertani Miller broth (KASVI, Brazil) with 20% glycerol. Samples from the floor were collected using a sterile Scott Duramax sheet (Kimberly- Clark, Brazil) moistened with sterile distilled water and conditioned in sterile Zip Lock plastic bags (Talge, Brazil). A total of 1 mL of sterile distilled water was added to the Zip Lock bags containing the collected material and then transferred to a tube containing 9 mL of sterile distilled water for serial dilution. Phenotypic identification and automated antimicrobial susceptibility tests were performed using the VITEK®2 system (bioMérieux, France). The following antimicrobials were tested for Enterobacter cloacae and Klebsiella pneumoniae: amoxicillin/clavulanic acid, piperacillin/tazobactam, cephalothin, cefuroxime, cefuroxime axetil, ceftriaxone, cefepime, ertapenem, meropenem, amikacin, gentamicin, nalidixic acid, ciprofloxacin, norfloxacin, nitrofurantoin, and trimethoprim/sulfamethoxazole. For Acinetobacter baumanii, Acinetobacter calcoaceticus complexes ACB, the antimicrobials were: ampicillin, amoxicillin/clavulanic acid, piperacillin/tazobactam, cefuroxime, cefuroxime axetil, ceftriaxone, cefepime, meropenem, amikacin, gentamicin, nalidixic acid, ciprofloxacin, norfloxacin, nitrofurantoin, and trimethoprim/ sulfamethoxazole.
For all tests, the isolated bacteria, and the standard strain Escherichia coli (Migula) Castellani and Chalmers (ATCC 25922) were reactivated in Müeller Hinton (MH) broth (KASVI, Brazil) at 37°C for 24 h in aerobiosis, and just before the test, adjusted to 108 unit forming colony (UFC)/mL and optical density (OD) = 0.6, using a Bio Drop WPA Bio wave (Nova-Analítica, Brazil) at 600 nm.
Disinfectant Solutions
Three disinfectant solutions were used to test bacterial and biofilm susceptibility: benzalkonium chloride and biguanide (BCB; Profilatica, Brazil), sodium hypochlorite (NaCIO; Start, Brazil), and hydrogen peroxide (H2O2; Infarma, Brazil) solutions. BCB was diluted in sterile distilled water to 1.0% (same dilution used in hospital facilities), 0.5, 0.3, and 0.1%; NaClO was diluted to 2.0, 1.0, 0.5, and 0.3%; and H2O2 was diluted to 3.0, 1.5, 0.5, and 0.3%.
Determination of Minimal Inhibitory and Bactericidal Concentration
The minimal inhibitory concentration (MIC) and the minimal bactericidal concentration (MBC) was determined using the broth micro dilution technique with adaptation. Briefly, 96 well microplates (TPP, Switzerland) were filled with 20 μL of the disinfectants and gentamicin (antibiotic positive control), each at their respective concentrations as defined for testing, 70 μL of MH broth, and 10 μL of each bacterial inoculum. Microplates were incubated at 37°C. After 24 h, 10 μL of the mix were transferred from each well to a plate containing MH agar (KASVI, Spain), and the MBC was determined by the lowest concentration of tested substances that prevented visible bacterial growth in the subculture. Gentamicin sulfate (Fagron, Brazil), at 0.004, 0.003, 0.002, and 0.001% concentration in sterile distilled water, was also tested. Controls consisted of substances (disinfectants and antibiotics) without bacteria, culture medium only, and bacterial strain only. Two independent experiments were performed in quadruplicate.
Only Gram-negative bacteria were chosen for this study because they are frequently isolated from patients and exhibit multidrug resistance. Enterobacter cloacae, Klebsiella pneumoniae, and Acinetobacter baumannii complex isolates with the best biofilm-forming results (data not shown) were chosen for the MBC test, for biomass quantification, and for scanning electron microscopy (SEM) imaging. Escherichia coli (Migula) Castellani and Chalmers (ATCC 25922) reference strain was used as a control for biofilm-forming capacity.
Biofilm Biomass Quantification
The effect of disinfectants on biofilm biomass was determined using the quantification method described by Santos et al. (2018). Three bacterial strains isolated from the hospital settings (E. cloacae, K. pneumoniae, A. baumannii complex) and the control strain E. coli (ATCC 25922) were used. In 96 well microplates, 200 μL of bacterial inoculum was cultivated for 48 h at 37°C in aerobiosis to form a mature biofilm. After the incubation period, the supernatant was removed, and the cells were carefully washed with sterile distilled water to remove planktonic cells. Then, the mature biofilm was treated with disinfectants at their MBC and incubated for 24 h at 37°C in aerobiosis. Controls consisted of gentamicin sulfate at MBC and phosphate buffer solution (PBS) (Thermo Fisher, USA). The supernatant was removed, and the adhered cells were carefully washed with sterile distilled water and fixed with methanol (15 min). Adhered cells were stained with 1% crystal violet (5 min), washed in sterile distilled water, and solubilized with 95% ethanol solution for reading at 570 nm in a VERSAMAX (Molecular Devices, USA) microplate reader. The biofilm biomass is directly proportional to the OD obtained for each well. All tests were performed in quadruplicate and in two independent experiments. The percentage of biofilm inhibition was calculated using the following equation:

Scanning Electron Microscopy
The effects of disinfectants on biofilms were evaluated using SEM. E. cloacae, K. pneumoniae, A. baumannii complex, and E. coli (ATCC 25922) were seeded on 13 mm diameter coverslips in 12-well plates (KASVI, China) to obtain biofilm biomass and to apply different antimicrobial treatments. After 48 h at 37°C in aerobiosis, bacteria were treated with BCB, NaCIO, or H2O2 at the MBC established for each disinfectant. A 0.003% gentamicin sulfate solution and PBS were used as controls. Samples were fixed in 2% glutaraldehyde (0.1M pH 7.2 cacodylate buffer) for two hours, followed by three washes with cacodylate buffer, dehydrated in an ethanol series ( 30, 50, 70, 80, 90, and 100%) and subjected to critical point in the EM CPD300 instrument (Leica, Germany). Posteriorly, the samples were mounted on aluminum stubs with double-sided adhesive tape and gold-coated in the SCD-050 Sputter Coater instrument (Bal-Tec, Liechtenstein). Images were obtained using a JEOL JSM-6390LV scanning electron microscope (Tokyo, Japan).
Statistical Analysis
Statistical analyses were performed using Graph Pad Prism software (version 8.0; San Diego, USA). The Kolmogorov-Smirnov test was used to examine the normal distribution of variables in all experiments before statistical analysis. Overall differences between treatments were assessed using the Kruskal-Wallis test followed by Dunn's posttest. Data are represented as mean ± standard deviation (error bars) to compare control and treated groups at a significance level of p < 0.05.
Results
The three species of Gram-negative rods used in this study were isolated from several areas in the hospital's ICU after disinfection with 1% BCB: E. cloacae, K. pneumoniae, and A. baumannii complex. Bacteria were recovered from the floor of the pantry from eight cardiac monitors and from the accessory preparation table (Table 1).
| Bacteria | Minimal Growth Dilution | Resistance profile | Isolation place in the ICU |
|---|---|---|---|
| Enterobacter cloacae | 10-2 | ESBL | The floor of the pantry |
| Klebsiella pneumoniae | 10-1 | ESBL | Cardiac monitor |
| Acinetobacter baumannii complex | 10-7 | KPC | Medication table |
| ESBL: Extended Spectrum Beta lactamases; KPC: Klebsiella pneumoniae Carbapenemase; ICU: Intensive care unit. | |||
Table 1: Gram-negative rods isolated from the ICU settings.
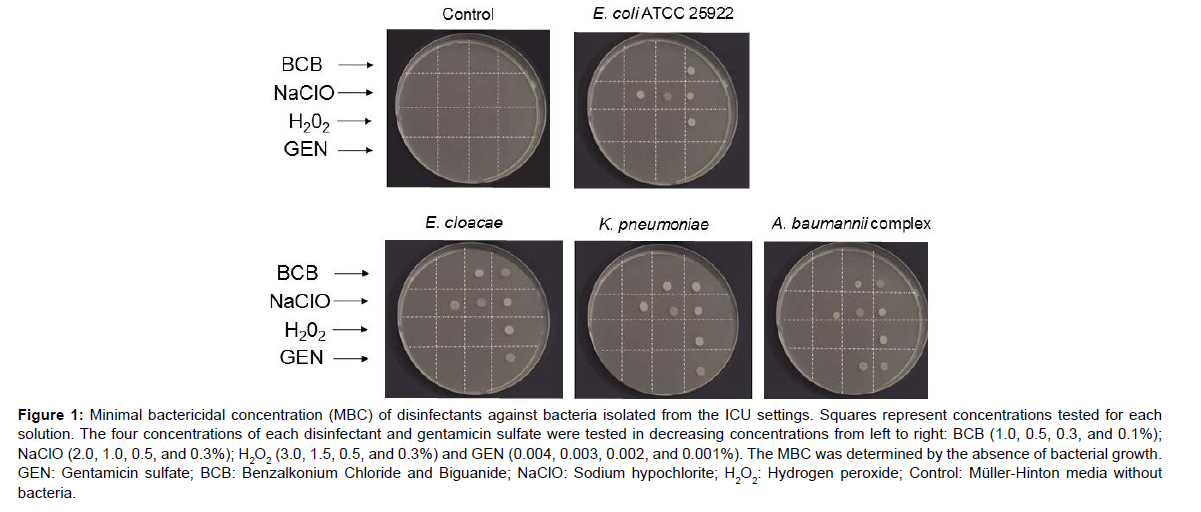
E. cloacae and K. pneumoniae were resistant to all beta-lactam antimicrobials, and A. baumannii was resistant to all carbapenem antimicrobials used. The susceptibility test performed with different disinfectant solutions showed that 0.5% BCB was effective against E. cloacae, K. pneumoniae, and A. baumannii complex, while a lower concentration of 0.3% BCB was able to kill E. coli (ATCC 25922) (Figure 1).Figure 1: Minimal bactericidal concentration (MBC) of disinfectants against bacteria isolated from the ICU settings. Squares represent concentrations tested for each solution. The four concentrations of each disinfectant and gentamicin sulfate were tested in decreasing concentrations from left to right: BCB (1.0, 0.5, 0.3, and 0.1%); NaClO (2.0, 1.0, 0.5, and 0.3%); H2O2 (3.0, 1.5, 0.5, and 0.3%) and GEN (0.004, 0.003, 0.002, and 0.001%). The MBC was determined by the absence of bacterial growth. GEN: Gentamicin sulfate; BCB: Benzalkonium Chloride and Biguanide; NaClO: Sodium hypochlorite; H2O2: Hydrogen peroxide; Control: Müller-Hinton media without bacteria.
| Biocides Bacteria |
BCB (%) | NaClO (%) | H2O2 (%) | GEN (%) |
|---|---|---|---|---|
| E. cloacae | 0.5 | 2.0 | 0.5 | 0.002 |
| K. pneumoniae | 0.5 | 2.0 | 0.5 | 0.002 |
| A. baumannii complex | 0.5 | 2.0 | 0.5 | 0.003 |
| E. coli ATCC 25922 | 0.3 | 2.0 | 0.5 | > 0.001 |
| MBC: Minimal bactericidal concentration. GEN: Gentamicin sulfate; BCB: Benzalkonium Chloride and Biguanide; NaClO: Sodium hypochlorite; H2O2: Hydrogen peroxide. | ||||
Table 2. Minimal bactericidal concentration (MBC) of disinfectant and antibiotic solutions against nosocomial isolates and Escherichia coli (ATCC 25922).
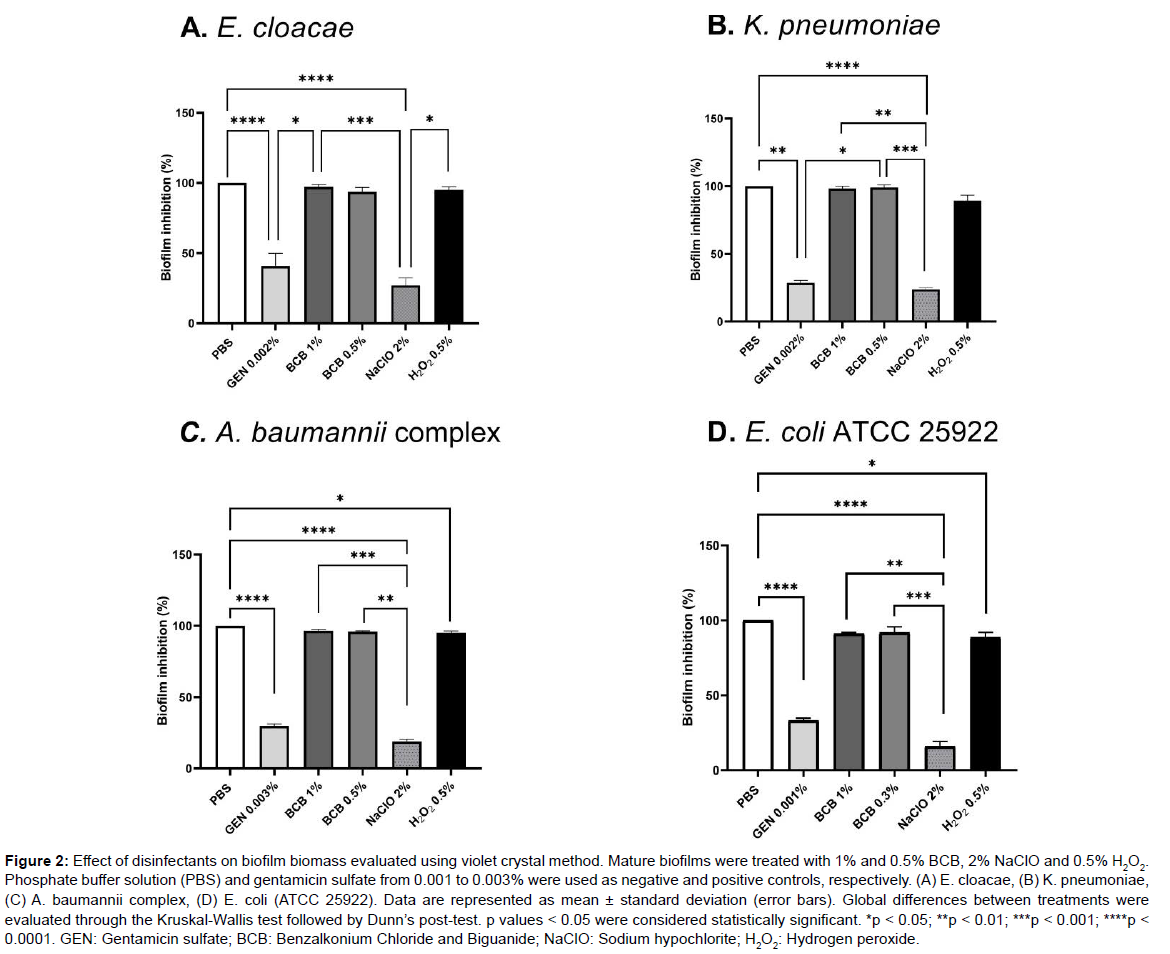
The ability of the disinfectant to eliminate mature biofilms and the sensitivity of environmental isolates to these substances at MBC concentrations are shown in (Figure 2).
Figure 2: Effect of disinfectants on biofilm biomass evaluated using violet crystal method. Mature biofilms were treated with 1% and 0.5% BCB, 2% NaClO and 0.5% H2O2. Phosphate buffer solution (PBS) and gentamicin sulfate from 0.001 to 0.003% were used as negative and positive controls, respectively. (A) E. cloacae, (B) K. pneumoniae, (C) A. baumannii complex, (D) E. coli (ATCC 25922). Data are represented as mean ± standard deviation (error bars). Global differences between treatments were evaluated through the Kruskal-Wallis test followed by Dunn’s post-test. p values < 0.05 were considered statistically significant. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. GEN: Gentamicin sulfate; BCB: Benzalkonium Chloride and Biguanide; NaClO: Sodium hypochlorite; H2O2: Hydrogen peroxide.
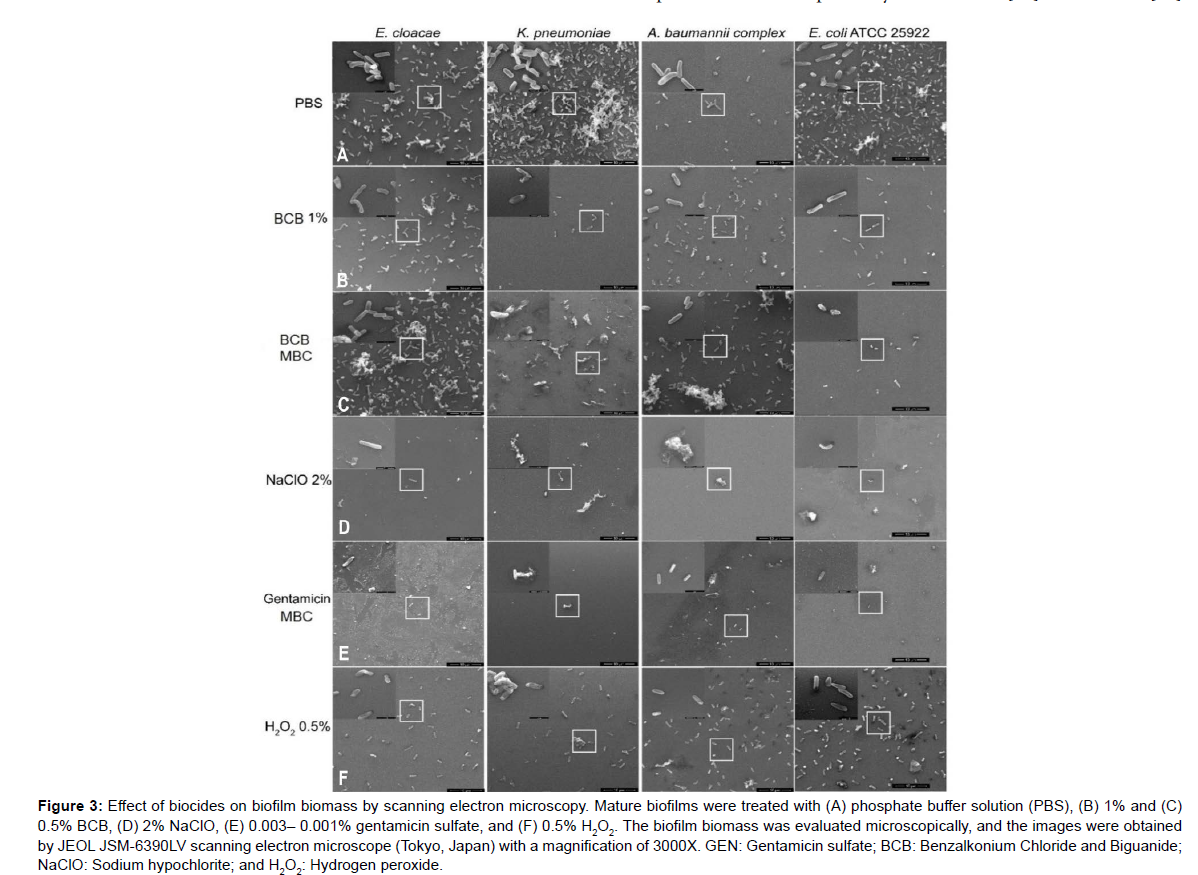
However, although the biomass quantification data did not show antibiofilm activity for BCB, in the SEM images it was possible to visualize the bactericidal effect of BCB (Figure 3B-C) compared to the negative control (Figure 3A). A morphological change with cell deformation and damage to the cell wall was observed. Furthermore, for K. pneumoniae and E. coli (ATCC 25922), SEM images suggest a decrease in cell adhesion after treatment with BCB (Figure 3B-C). In the case of NaClO, it was also possible to visualize only a few bacterial cells and what appeared to be remnants of the matrix on the surface, suggesting loss of cell adhesion (Figure 3D). Especially for E. cloacae and K. pneumoniae, it seems that H2O2 caused a reduction in cell adherence (Figure 3F).
Figure 3: Effect of biocides on biofilm biomass by scanning electron microscopy. Mature biofilms were treated with (A) phosphate buffer solution (PBS), (B) 1% and (C) 0.5% BCB, (D) 2% NaClO, (E) 0.003– 0.001% gentamicin sulfate, and (F) 0.5% H2O2. The biofilm biomass was evaluated microscopically, and the images were obtained by JEOL JSM-6390LV scanning electron microscope (Tokyo, Japan) with a magnification of 3000X. GEN: Gentamicin sulfate; BCB: Benzalkonium Chloride and Biguanide; NaClO: Sodium hypochlorite; and H2O2: Hydrogen peroxide.
Discussion
Currently, the prevention and treatment of biofilms is a point of concern in the hospital environment [26, 27]. On many surfaces, pathogenic microorganisms exist as biofilms, which means they secrete an extracellular matrix also known as extracellular polymeric substance (EPS) that protects them from the antimicrobial effects of disinfectants [28]. The biofilm extracellular matrix may be responsible for the increase in resistance to biocides as it acts as a diffusion barrier [5], and it may contribute to HAI cases by maintaining viable multidrugresistant microorganisms in the environment even after the usual disinfection.
The exposure of bacteria to sub lethal concentrations of disinfecting agents can activate adaptive stress response mechanisms, favoring survival in inhospitable environmental conditions [29, 30]. In this context, biofilms can function as a source of infections by periodically releasing multidrug-resistant bacteria into the ICU environment, even when the disinfection is performed as thoroughly as recommended by Vickery et al. [31].
In the present study, we confirmed the persistence of bacteria in the nosocomial environment even after disinfection, by isolating three bacterial strains from the ICU, E. cloacae, K. pneumoniae, and A. baumannii complex. It is noteworthy that the samples were collected before and after disinfection, as described in Materials and Methods. These species, isolated by us in the ICU, are also frequently found among the bacterial species causing HAIs [32] and, therefore, their persistence in the environment after disinfection could cause hospital infections, as reported by Bouzada et al. [33]. Ribeiro et al. [34]
isolated and identified Bacillus, Staphylococcus, and Pseudomonas as the most abundant genera (47% of the total reads) on ICU surfaces. They demonstrated that different bacterial strains were quite stable after cleaning and disinfection with 1% BCB, suggesting that these microorganisms adapt to the ICU environment and act as potential sources of HAIs. These authors reported that, given that the microbiota may vary across different ICUs, the HAI-related microbial signatures within these units remain underexplored [34].
It is important to note that the increasing prevalence of infections associated with Gram-negative bacteria, especially multidrug-resistant bacteria, poses a serious threat to public health because of the lack of available treatment options and the slow pace of development of new antimicrobial agents, which translates into a significant burden on health systems [35, 36].
The ESBL and KPC resistance profiles, that we detected for the bacteria isolated in the ICU environment, arouse high clinical interest because lactamases and carbapenemases confer resistance to classes of antimicrobials that are widely employed in the treatment of a wide range of serious infections [37]. ESBLs are enzymes capable of hydrolyzing all β-lactam antimicrobials except cephamycins and carbapenems, and carbapenemases related to the KPC profile are capable of hydrolyzing penicillins, cephalosporins, monobactams, and carbapenems.
Established biofilms represent a critical issue in the hospital environment because not all disinfectants can remove it [21, 38]. In the present study, among the products tested, 2% NaOCl showed the best efficacy on biofilm biomass of E. cloacae, K. pneumoniae, A. baumannii complex, and E. coli (ATCC 25922). The other solutions, 1% BCB and 0.5% H2O2, were not capable to considerably reduce the biomass of biofilms as measured using the crystal violet quantification assay. Nevertheless, this technique alone cannot tell whether the cells present in the biofilm are alive or dead, or even if the cells have undergone some morphological changes after treatment with biocides [39].
The BCB solution was made of 5.2% alkyl dimethyl benzyl ammonium chloride (benzalkonium chloride), 3.5% polyhexamethylene biguanide (PHMB), nonionic surfactant, solvent, and water. Benzalkonium chloride is a quaternary ammonium compound that exerts its bactericidal effect by inactivating enzymes responsible for energy transformation processes, by denaturing cellular proteins, and by disrupting the cell membrane [40, 41]. PHMB is a polymer used as a disinfectant and antiseptic with a broad antimicrobial spectrum, that acts by disrupting the bacterial cells. Its molecules exert their bactericidal effect through aggregation mechanisms, which are mediated by its cationic biguanide nuclei [42, 43].
The dual chemical composition of BCB alters the membrane of bacteria to achieve consequent cell disruption [40-43]. This may explain the bacterial morphology observed after treatment with BCB, including cell deformation and cell wall damage, as shown in (Figure 3).
Furthermore, quaternary ammonium and biguanide compounds are known to be cationic, and their interaction with a negatively charged biofilm matrix could inhibit their bactericidal efficacy [46]. In addition, the bactericidal efficacy of quaternary ammonium compounds may be subject to decay with time, as they have been shown to be biodegrade under aerobic conditions [41], which may explain why bacteria could be recovered from the ICU environment even after the 1% BCB cleaning procedure.
From the disinfectant chemical composition viewpoint, Lineback et al. [28] demonstrated that sodium hypochlorite- and hydrogen peroxide-based disinfectants are more effective against bacterial biofilms than quaternary ammonium compounds, such as BCB. Additionally, Boyce et al. [47] concluded that the risk of HAI incidence was lower with hydrogen peroxide-based disinfectants than with quaternary ammonium compounds.
Sodium hypochlorite disinfectant products irreversibly kill bacterial cells in biofilms by denaturing proteins in the biofilm matrix and inhibiting key enzymatic functions in bacterial cells [48]. As noted in Figure 3, this information corroborates the data described here and is consistent with the efficacy observed for NaOCl solution against biofilms (Figure 2).
The mechanism of action of H2O2 involves attacking the bacterial lipid membrane, DNA, and other cell components with the toxic free radicals produced by the peroxide [49]. H2O2 is also recognized as a highly efficient alternative for disinfecting medical devices [50]. Overall, the ability of biofilm matrices to interfere with the contact between disinfectant products and bacterial cells is complex [51]. In addition, biofilm cells are genetically primed to tolerate disinfectant products better than planktonic cells [51, 52]. These features prevent the diffusion of disinfectants and limit the efficacy of their actions [53].
Based on our data, we can also infer that in the environment evaluated, the bacteria isolated, E. cloacae, K. pneumoniae, and A. baumannii complex formed a physiologically integrated bacterial community, which in turn reduced the activity of the disinfectant product that was used and kept them viable in the environment. This resistance is likely associated with the low penetration of antimicrobial and disinfectant compounds in biofilms and their phenotypically protected state induced by the bacteria that compose it [54, 55].
The microbial community also functions as a source for the dissemination of the microorganisms that constitute it and may give rise to infections, which is worrisome because they can be found on almost any type of surface. When detached from biofilms, the single cells can migrate within the environment [24] and contribute to the occurrence and persistence of HAIs within the hospital setting, since the biofilm lifestyle represents an endless cycle [56].
Conclusion
We showed that the multidrug-resistant bacteria isolated from inanimate surfaces in the ICU after sanitizing procedures are resistant in the biofilm biomass tested up to 1% BCB (same concentration used in the sanitizing process) even though this biocide was able to kill them in their bacterial planktonic state. We found that in a biofilm situation, concentrations of disinfectants that are bactericidal for different bacteria in the planktonic state do not maintain the same disinfectant activity and do not completely remove different bacterial species from surfaces. The results presented here for 1% BCB raise some questions: 1) how many variables within the cleaning service, including the presence of biological materials, disinfection and mechanical clearance, exposure time to the product, and other factors interfere with bacterial elimination?, and 2) Can BCB-based products be effective against contamination caused by secretion that contain a massive load of bacteria, as wound secretion, or urine spillages?It is extremely important to guarantee the effectiveness of the disinfectants authorized for use in in the nosocomial context since the development of resistance to biocides contributes to the persistence of microorganisms in the hospital environment and to the occurrence of HAIs.
Author Contributions
Paloma Ohana S. Abreu: Conceptualization, Investigation, Methodology, Visualization, Formal analysis, Writing – original draft,preparation. Julio L. D. Guzman, Heitor P. P. Filho, and Ana Paula M.L. de Lemos: Provision of study, laboratory samples, or other analysis tools. Camila Pacheco S. M. da Mata: Provision of study materials, reagents, materials, or other analysis tools. Cid Edson Mendonça Póvoas: formal analysis. Raynah N. da Hora: Methodology and Visualization. Ana Carolina M. Apolônio: Provision of study materials, reagents, materials, instrumentation, computing resources, or other analysis tools. Vinicius N. R. Guerra: Provision of study materials, reagents, materials, instrumentation, computing resources, or other analysis tools. Valeria F. Fernandes: Provision of study materials, reagents, materials, laboratory samples, instrumentation, computing resources, or other analysis tools. Uener R. dos Santos: Visualization, Writing – preparation, review, & editing. Aline Silva: Provision of study materials, reagents, materials, laboratory samples, instrumentation, computing resources, and other analysis tools. Renato Fontana: Development or design of methodology; creation of models. João Luciano Andrioli: Development or design of methodology; creation of models. Maria Silvana Alves: Visualization, Writing – preparation, review, & editing. Rachel Passos Rezende: Aline O. da Conceição: Carla Cristina Romano, Guilherme R. G. Queiroz, João Carlos Teixeira Dias, Ana Paula T. Uetanabaro: visualization, Writing – preparation, review & editing. Luciana Debortoli de Carvalho: Conceptualization, Investigation, Supervision, Writing – original draft, preparation, and funding acquisition.
Funding
This project was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and UESC. CAPES promoted the master’s degree and UESC promoted the acquisition of material for the development of this article.
Acknowledgments
The authors would like to thank the Microscopy Center of the State University of Santa Cruz and the Federal University of Juiz de Fora for the support and realization of the microscopy experiments.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Bravo Z, Chapartegui-González I, Lázaro-Díez M, Ramos-Vivas J (2018) Acinetobacter pittii biofilm formation on inanimate surfaces after long-term desiccation. J Hosp Infect. 98:74-82.
- Khan HA, Baig FK, Mehboob R (2017) Nosocomial infections: Epidemiology, prevention, control, and surveillance. Asian Pac J Trop Biomed. 7:478-82.
- Baviskar AS, Khatib KI, Rajpal D, Dongare HC (2019) Nosocomial infections in surgical intensive care unit: A retrospective single-center study. Int J Crit Illn Inj Sci. 9:16-20.
- Han JH, Sullivan N, Leas BF, Pegues A, Kaczmarek JL et al. (2015) Cleaning Hospital room surface to prevent healthcare-associated infections. Ann Intern Med. 163:598-607.
- Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F (2011) Resistance of bacterial biofilms to disinfectants : a review. Biofouling. 27:1017-1032.
- Protano C, Cammalleri V, Romano Spica V, Valeriani F, Vitali M (2019) Hospital environment as a reservoir for cross-transmission: Cleaning and disinfection procedures. Ann di Ig Med Prev e di Comunita. 31:436-448.
- Reveals A (2012) Healthcare-associated infections: A public health problem. Niger Med J.53:59-64.
- WHO (2020) Antimicrobial resistance.
- Cepas V, López Y, Muñoz E, Rolo D, Ardanuy C et al. (2019) Relationship between Biofilm Formation and Antimicrobial Resistance in Gram-Negative Bacteria. Microb Drug Resist. 25: 72-79.
- Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 35:322-332.
- La Forgia C, Franke J, Hacek DM, Thomson RB, Robicsek A (2010) Management of a multidrug-resistant Acinetobacter baumannii outbreak in an intensive care unit using novel environmental disinfection: A 38-month report. Am J Infect Control 38:259-263.
- Swan JS, Deasy EC, Boyle MA, Russell RJ, O’Donnell MJ (2016) Elimination of biofilm and microbial contamination reservoirs in hospital washbasin U-bends by automated cleaning and disinfection with electrochemically activated solutions. J Hosp Infect. 94:169-174.
- Bressler D, Balzer M, Dannehl A, Flemming HC, Wingender J (2009) Persistence of Pseudomonas aeruginosa in drinking-water biofilms on elastomeric material. Water Sci Technol Water Supply. 9: 81-87.
- Vestby LK, Møretrø T, Langsrud S, Heir E, Nesse LL (2009) Biofilm forming abilities of Salmonella are correlated with persistence in a fish meal- and feed factories. BMC Vet Res.5: 1-6.
- Dincer S, Uslu FM, Delik A (2020) Antibiotic Resistance in Biofilm. In: Bacterial Biofilms. In Bacterial biofilms. IntechOpen.7: 1-14.
- Lewenza S (2013) Extracellular DNA-induced antimicrobial peptide resistance mechanisms in Pseudomonas aeruginosa. Front Microbiol. 4:1-6.
- Howlin RP, Brayford MJ, Webb JS, Cooper JJ, Aiken SS (2015) Antibiotic loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother. 59: 111-120.
- Souza C De, Mota HF, Faria YV, Cabral FDO, Oliveira DR De et al. (2020) Resistance to Antiseptics and Disinfectants of Planktonic and BiofilmAssociated Forms of Corynebacterium striatum. Microb Drug Resist. 26:1546-1558.
- Ilknur D, Yasemin O, Nuri K (2012) Effect of disinfectants on biofilm development by five species of Candida. African J Microbiol Res.6: 2380-2386.
- Kalia VC (2013) Quorum sensing inhibitors: An overview. Biotechnol Adv. 31: 224-245.
- Marion K, Freney J, James G, Bergeron E, Renaud FNR et al.(2006) Using an efficient biofilm detaching agent: an essential step for the improvement of endoscope reprocessing protocols. J Hosp Infect. 64:136-142.
- Bryers JD (2008) Medical biofilms. Biotechnol Bioeng.100:1-18.
- Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: A common cause of persistent infections. Science 284: 1318-1322.
- Srey S, Jahid IK, Ha S Do (2013) Biofilm formation in food industries: A food safety concern. Food Control. 31:572-585.
- Hathroubi S, Mekni MA, Domenico P, Nguyen D, Jacques M (2017) Biofilms: Microbial Shelters Against Antibiotics. Microb Drug Resist. 23:147-156.
- Quinn MM, Henneberger PK, Braun B, Delclos GL, Fagan K et al.(2015) Cleaning and disinfecting environmental surfaces in health care: Toward an integrated framework for infection and occupational illness prevention. Am J Infect Control. 43:424-434.
- Rutala WA, Weber DJ (2015) Disinfection, sterilization, and control of hospital waste. In: Mandell, Douglas, And Bennett's Principles And Practice Of Infectious Diseases. 48:3294-309.
- Lineback CB, Nkemngong CA, Wu ST, Li X, Teska PJ (2018) Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob Resist Infect Control. 7: 1-7.
- Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M et al.(2018) Bacterial biofilm and associated infections. J Chinese Med Assoc. 81:7-11.
- Kramer A, Schwebke I, Kampf G (2006) How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 6:1-8.
- Vickery K, Deva A, Jacombs A, Allan J, Valente P et al. (2012) Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J Hosp Infect. 80:52-55.
- Weber KL, Lesassier DS, Kappell AD, Schulte KQ, Westfall N et al.(2020) Simulating transmission of ESKAPE pathogens plus C. difficile in relevant clinical scenarios. BMC Infect Dis.20:1-15.
- Bouzada MLM, Silva VL, Sa Moreira FA, Silva GA, Diniz CG (2010) Antimicrobial resistance and disinfectants susceptibility of persistent bacteria in a tertiary care hospital. J Microbiol Antimicrob. 2:105-112.
- Ribeiro LF, Lopes EM, Kishi LT, Ribeiro LFC, Menegueti MG et al. (2019) Microbial community profiling in intensive care units exposes limitations in current sanitary standards. Front Public Heal. 7:1-14.
- Cerceo E, Deitelzweig SB, Sherman BM, Amin AN (2016) Multidrug-Resistant GramNegative Bacterial Infections in the Hospital Setting: Overview, Implications for Clinical Practice, and Emerging Treatment Options. Microb Drug Resist. 22:412-431.
- Patil A, Banerji R, Kanojiya P, Koratkar S (2021) Bacteriophages for ESKAPE: role in pathogenicity and measures of control. Expert Rev Anti Infect Ther. 19:845-865.
- Rello J, Kalwaje Eshwara V, Lagunes L, Alves J, Wunderink RG et al. (2019) A global priority list of the TOp TEn resistant Microorganisms (TOTEM) study at intensive care: a prioritization exercise based on multi-criteria decision analysis. Eur J Clin Microbiol Infect Dis.38: 319-323.
- Pajkos A, Vickery K, Cossart Y (2004) Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J Hosp Infect. 58:224-229.
- Li X, Yan Z, Xu J (2003) Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology.149: 353-362.
- Pereira BMP, Tagkopoulos I (2019) Benzalkonium Chlorides : Uses, Regulatory Status, and Microbial Resistance. Appl Environ Microbiol.85:1-13.
- Tezel U, Pavlostathis SG (2015) Quaternary ammonium disinfectants: Microbial adaptation, Degradation, and ecology. Curr Opin Biotechnol 33:296-304.
- Hübner NO, Kramer A (2010) Review on the efficacy, safety, and clinical applications of polihexanide, a modern wound antiseptic. Skin Pharmacol Physiol. 23:17-27.
- Sowlati-Hashjin S, Sowlati-Hashjin S, Carbone P, Karttunen M, Karttunen M, et al. (2020) Insights into the Polyhexamethylene Biguanide (PHMB) Mechanism of Action on Bacterial Membrane and DNA: A Molecular Dynamics Study. J Phys Chem B.124:4487-4497.
- Nasioudis A, Joyce WF, Velde JWV, Heeren RMA, Brink OFV Den, et al. (2010) Formation of low charge state ions of synthetic polymers using quaternary ammonium compounds. Anal Chem. 82: 5735-5742.
- Velpandian T, Nirmal J, Arora B, Ravi AK, Kotnala A (2012) Understanding the Charge Issues in Mono and di-Quaternary Ammonium Compounds for Their Determination by LC/ESI-MS/MS. Anal Lett. 45: 2367-2376.
- Tseng BS, Zhang W, Harrison JJ, Quach TP, Song JL et al. (2013) The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ Microbiol. 15:2865-2878.
- Boyce JM, Guercia KA, Sullivan L, Havill NL, Fekieta R et al.(2017) Prospective cluster controlled crossover trial to compare the impact of an improved hydrogen peroxide disinfectant and a quaternary ammonium-based disinfectant on surface contamination and health care outcomes. Am J Infect Control 45: 1006-1110.
- Tiwari S, Rajak S, Mondal DP, Biswas D (2018) Sodium hypochlorite is more effective than 70% ethanol against biofilms of clinical isolates of Staphylococcus aureus. Am J Infect Control 46:37-42.
- Rutala WA (1996) APIC guideline for selection and use of disinfectants. Am J Infect Control. 24: 313-342.
- Alfa MJ, Jackson M (2001) A new hydrogen peroxide-based medical-device detergent with germicidal properties: Comparison with enzymatic cleaners. Am J Infect Control. 29: 168-177.
- Mitchell KF, Zarnowski R, Sanchez H, Edward JA, Reinicke EL et al. (2015) Community participation in biofilm matrix assembly and function. Proc Natl Acad Sci U S A.112: 4092-4097.
- Al-Jailawi MH, Ameen RS, Al-Jeboori MR (2013) Effect of Disinfectants on Antibiotics Susceptibility of Pseudomonas aeruginosa. J Appl Biotechnol.1: 54-63.
- Gilbert P, Das JR, Jones M V, Allison DG (2001) Assessment of resistance towards biocides following the attachment of micro-organisms to, and growth on, surfaces. J Appl Microbiol. 91: 248-254.
- Fish KE, Osborn AM, Boxall J (2016) Characterising and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systems. Environ Sci Water Res Technol. 2: 614–630.
- Hall CW, Mah TF (2017) Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev.41: 276-301.
- Yin W, Wang Y, Liu L, He J (2019) Biofilms: The microbial “protective clothing” in extreme environments. Int J Mol Sci. 20: 1-18.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Abreu POS, Guzman JLD, Mata CPSM, Povoas CEM, Apolonio ACM, et al. (2022) Nosocomial Contamination and Bacterial Resistance in Intensive Care Units after Disinfection: An Emerging Challenge under Biofilm and Planktonic Growth Condition. J Clin Infect Dis Pract, 7: 153. DOI: 10.4172/2476-213X.1000153
Copyright: © 2022 Abreu POS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1721
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1348
- PDF downloads: 373