Short Communication Open Access
N,N,N-Trimethyl Chitosan and Its Potential Bactericidal Activity: Current Aspects and Technological Applications
Débora P Facchi1, Suelen P Facchi1 and Alessandro F Martins1,2*1Environmental Engineering Program, Federal University of Technology, Paraná, Brazil
2Materials Science & Engineering Program, Federal University of Technology, Paraná, Brazil
- *Corresponding Author:
- Alessandro F Martins
Postgraduate in Materials Science & Engineering Program
Federal University of Technology-Paraná (UTFPR-LD)
CEP 86036-370 Londrina-PR, Brazil
Tel: 55-43 96543830
E-mail: afmartins@utfpr.edu.br
Received date: July 19, 2016; Accepted date: August 04, 2016; Published date: August 08, 2016
Citation: Facchi DP, Facchi PF, Martins AF (2016) N,N,N-Trimethyl Chitosan and Its Potential Bactericidal Activity: Current Aspects and Technological Applications. J Infect Dis Ther 4:291. doi:10.4172/2332-0877.1000291
Copyright: © 2016 Facchi DP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
N,N,N-Trimethyl chitosan (TMC) is one of most important chitosan derivative with stronger bactericidal property. Currently, the TMC has been attracted considerable attention, because it contains quaternized ammonium moieties (-+N(CH3)3) in its network. Its cationic property leads to a renowned bactericidal power. Besides, TMC has appropriate biocompatibility, biodegradability, mucoadhesive capacity and greater solubility at pH close to physiological condition regarding chitosan. In this way, there are not sources regarding revision papers toward TMC bactericidal action. So, this revision work reports the TMC potential to be applied in many fields, depicting its bactericidal performance, as well as, its bactericidal mechanism and some current technological aspects that must be considered.
Keywords
N,N,N-Trimethyl chitosan; Bactericidal activity; Applications
Introduction
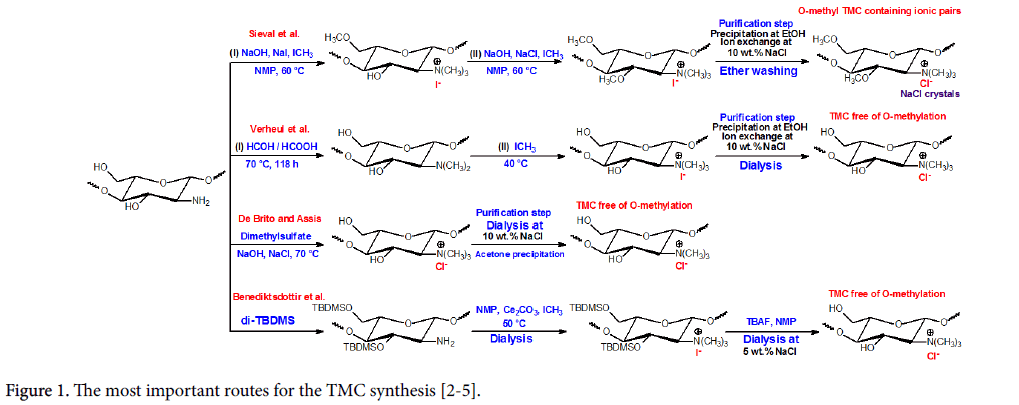
Chitosan (a chitin derivative) is water-insoluble (at physiological pH condition) and in most organic solvents, except in organic acids, which substantially limits its usefulness [1]. On the other hand, the N,N,NTrimethyl chitosan is the most famous chitosan derivative, obtained from reductive methylation of amino moieties. Several methodologies concerning the TMC synthesis are depicted in the (Figure 1). Among these, the highlighted methodologies were proposed by Sieval et al., Verheul et al., De Brito and Assis, and Benediktsdottir et al. (Figure 1) [2-5].
The most commonly used synthesis is based on the amino groups’ reduction (from two steps) in strong base, sodium iodide and iodomethane presence, using N-methyl-2-pyrrolidinone (NMP) as solvent [2]. However, this pathway provides the –OH/-NH2 uncontrolled methylation, being its reproducibility a negative factor (Figure 1). Among these procedures, the most important and simple synthesis methodology has been described [3]. This route offers lower cost and exhibits excellent reproducibility and such pathway occurs from two steps. In the first step, the N,N-dimethyl chitosan (DMC) is performed from formic acid-formaldehyde mixture (Eschweiler- Clarke) at 70°C (Figure 1). Then, the fully DMC N-dimethylated is quaternizaed, using only iodomethane under NMP presence and sodium iodide and sodium hydroxide absences (Figure 1). This methodology leads to TMC free of O-methylation.
De Brito and Assis [4] developed another methodology using the dimethylsulfate as reductor agent instead iodomethane (Figure 1). On the other hand, Bendiktsdottir et al. [5] synthesized a fully quaternized TMC from protection strategies toward –OH groups (using the tertbutyldimethylsilyl - TBDMS protection group and tetrabutylammonium fluoride (TBAF) as desprotetor agent), avoiding O-methylation (Figure 1). The Figure 1 will be further discussed in the following sections.
TMC has received great attention in the last 15 years, due to its excellent properties of biodegradability, biocompatibility, mucoadhesion, excellent water-solubility, and especially for its antimicrobial potential [6]. TMC may exhibit bactericidal activity up to 700 times greater than the neat chitosan. This property is intrinsically linked to -+N(CH3)3 quaternary groups existence in its network and some studies show that larger N-quaternized fraction, better bactericidal performance is reached i.e., the higher TMC quaternization degree (DQ) promotes higher bactericidal power [7]. Factors as, molar weight [8], specie types toward -+N(CH3)3 sites (counterion types) [9], synthesis methodology, as well as, the adopted purification process [10], and culture assay conditions (temperature and ionic strength) [8] can change the TMC antimicrobial capacity. Some of these factors have been extensively studied and they are not reported in this opportunity. The effects promoted by these factors regarding bactericidal activity were recently revised in a work published by Hosseinnejad and Jafari [8], they will not be discussed again.
Recent studies reinforce that TMC can be applied in pharmaceutical and medical field [11-13], as well as, in food industry [1]. TMC basedmaterials (thin films, beads, thermosensitive hydrogels, polyeletrolyte complexes, nanoparticles, etc.,) may be designed as drug delivery matrices [13], as building devices coated with bactericidal and cell growth potentials [12,14] and developing biodegradable membranes [15,16] with bactericidal action for use in food industry, among others. This paper highlights the latest issues concerning TMC antimicrobial potential, as well as, some TMC-based technological applications.
Probably TMC Bactericidal Mechanism
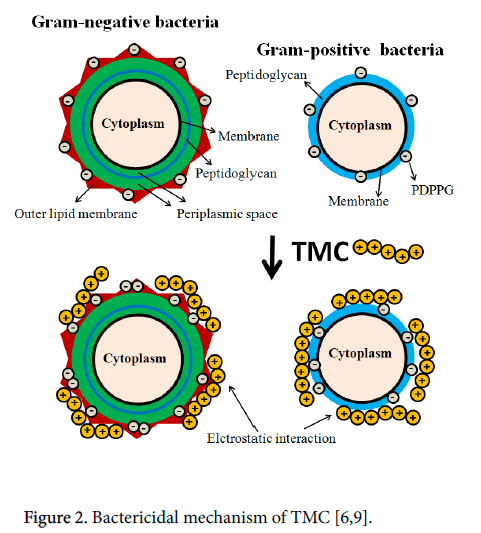
TMC bactericidal activity is the most targeted parameter toward the pharmacy and medicine fields. TMC showed potential to kill several bacteria types, such as Escherichia coli [9,10], Staphylococcus aureus [9], Pseudomonas aeruginosa [17], Listeria innocua [18] and Enterococcus facialis [17], i.e., it has antimicrobial activity on gramnegative and gram-positive bacteria. On the other hand, the precise TMC bactericidal mechanism is difficult to identify, because such activity depends on many parameters, as previously reported section [6,8-10]. Studies indicate that TMC has potential against Gramnegative and Gram-positive bacteria, and its action can be better understood from analysis as shown Figure 2.
Gram-negative cells are composed by a multilayer cell surface containing an outer membrane, a peptidoglycan layer between periplasmic space and an internal cytoplasmic membrane (Figure 1) [6,9]. On the other hand, gram-positive membrane cells are constituted by a broad dense wall that consists of 15–40 interconnecting peptidoglycans layers and an internal membrane cell [6,9]. Anionic phospholipid dipalmitoylphosphatidylglycerol (PDPPG) is the major component of both membrane cells (gram-negative and gram-positive bacteria) (Figure 1) [6]. Besides, outer membrane (OM) on gramnegative bacteria contains polyanionic lipopolysaccharides (PLPS), which are stabilized by Mg2+ and Ca2+ divalent ions [6]. Such species bind on out membrane, maintaining them stables, being essential to OM integrity. Therefore, gram-negative membrane cells have more negative charge densities than gram-positive cells.
Cationic TMC polymer has higher positive charge densities that can interact with the cell walls (Figure 1) [9,10]. This interaction occurs among cationic N-quaternized moieties (-+N(CH3)3) on TMC networks and anionic molecules, such as PDPPG on bacteria cells (Figure 1). It leads to a large increase on membrane permeability, causing structural changes (distortion-disruption), as well as, a severe leakage of cytoplasm constituents and eventually results in the death of bacteria [19]. TMC has greater adsorption capacity onto bacteria cell surface, especially on gram-negative bacteria cell, due its higher negative charge property, regarding the gram-positive bacteria [6].
Current Aspects Concerning the Bactericidal Potential of TMC
Some important points regarding the TMC bactericidal potential cannot be unnoticed. A recently paper showed that adopted purification process on TMC synthesis significantly influence its bactericidal activity [10]. TMCs are obtained as iodide salts and then they are dissolved in a 10% wt. sodium chloride solution for ion exchange (Figure 1). TMC chloride is more water-soluble than TMC iodide due to smaller chloride ionic radius concerning the iodide [2]. Then, the TMC chloride aqueous solution is precipitated in ether and then it is centrifuged and washed/purified. However, TMC chloride can be purified by two ways (Figure 1).
i) After centrifugation, the TMC chloride is washed several times with ether solvent and dried at 40°C to finally be obtained as a white crystalline powder [10];
ii) After centrifugation The TMC can be dispersed again in water and dialyzed (for 3 to 4 days), frozen and lyophilized [10];
The second process is more efficient and it has low cost, because it is not necessary carry out the TMC washing, using organic solvent. In the TMC chloride precipitation process (in EtOH), it attracts to its structure sodium chloride crystals, an insoluble salt in ethanol and ether solvents. Upon precipitation, sodium chloride crystals are directed to the -+N(CH3)3Cl- group, leading the ‘ionic pairs’ formation (Figure 1) [10]. The ionic pairs between TMC chloride/NaCl crystals drastically decrease the TMC bactericidal potential, whereas they are shielding the TMC N-quaternized groups, reducing the potential interaction of these sites with the bacteria cell membranes [10]. So, the dialysis may be used to remove the NaCl from TMC structure, purifying it (Figure 1). After dialysis, TMC chloride must be frozen and lyophilized to be obtained in an amorphous form [10]; feature of polysaccharide macromolecules.
TMC chlorides obtained by our research group was carried out according to the methodologies proposed by Sieval et al. [2] and Verheul et al. [3], using some modifications on experimental procedures [10]. TMC chloride O-methylated and TMC chloride free of O-methylation, containing the same DQ (≈15%) were evaluated, regarding their bactericidal potentials against Escherichia coli (E. coli) (ATCC 26922) [10]. In this case, TMC chlorides were produced at dialysis absence, i.e., TMCs with high crystallinity were performed due to ionic pair’s formation [10], as depicted in the reaction at the top on Figure 1. TMC salts were also successful synthesized and purified from dialysis method, i.e., amorphous TMCs free of ionic pairs were synthesized [10].
TMCs free of ionic pairs (purified by dialysis) showed stronger antimicrobial action against E. coli in only 6 h. Both O-methyl TMC and TMC free of O-methylation (both 15% DQ) have more than 90% inhibition. On the other hand, regarding the TMCs containing ionic pairs, their bactericidal performance does not exceed 40% inhibition [10]. These results showed that TMC should be purified by dialysis process to enable its bactericidal performance.
Another recent study depicted that TMC counterion species (counterions on -+N(CH3)3 sites) dramatically influences the antimicrobial effect [9]. Several TMC salts (TMC iodide, TMC bromide, TMC chloride, TMC acetate and TMC sulfate) with 60% quaternization degree were obtained and their microbial potentials investigated against E. coli (ATCC 25922) and Staphylococcus aureus (S. aureus) (ATCC 25923) [9]. The salts containing delocalized π counterions have better bactericidal activities toward both bacteria (E. coli and S. aureus). TMC iodide and TMC bromide showed weak potential to kill bacteria, due to the higher ionic volume (cm3 mol-1) and ionic radius (pm), as well as, the delocalized π absence for the Brand I- counterions [9]. In order, the bactericidal effect followed the sequence: TMC sulfate>TMC acetate>TMC chloride>TMC bromide ≈ TMC iodide [9]. So, TMC sulfate and TMC acetate presented the highest activities against both bacteria, whereas the number of − +N(CH3)3 groups “available” to interact with the bacteria cell membranes should be larger [9].
Most Recent TMC Applications
TMC-based materials have been much attention in these last years, mainly for the pharmaceutical and medical fields. The cationic property of TMC allows its association with polyanionic macromolecules, like heparin, sodium alginate, among others [20,21]. TMC/heparin multilayer thin films carried out from the layer-by-layer assembly [20,22]. Such assembled film prepared with O-methyl TMC, containing 80% DQ showed stronger bactericidal action (64.6% inhibition at 6 h) against E. coli (ATCC 26922) at physiologic pH condition (7.4) [22]. Sajomsang et al. [23] revealed that N,N,Ntrimethyl ammonium group showed high bactericidal activity toward E. coli (ATCC 25922) and S. aureus (ATCC 6538) [23].
Hydrogel beads based on TMC/alginate polyelectrolyte complexes showed bactericidal property against E. coli (ATCC 26922) cells, mainly when associated with silver nanoparticles [13]. In this case, these metallic nanomaterials enhanced TMC/alginate bactericidal performance to 91% inhibition at 24 h (pH 7.4) [13]. Quaternary ammonium TMC prepared from amino group moieties, exhibited improved water solubility and stronger bactericidal activity over an entire pH range. So, quaternized sites can increase the potential biomedical applications on anti-infection [19]. Electrospun membranes based on TMC/poly(vinyl pyrrolidone) had high antibacterial activity against S. aureus (749) and E. coli (3588) [15]. Another electrospun nanofibers, formulated from association of poly(e-caprolactone) coated by poly(acrylic acid)/TMC iodide polyelectrolyte complex, inhibited the growth of S. aureus and E. coli, as well as, suppressed the pathogenic bacteria adhesion of S. aureus [16]. Nanoparticles containing quaternized moieties on their surfaces also suppressed the E. coli growth [24]. Many TMC-formulations have been conducted and designed for technological applications, due to bactericidal action of these based-materials.
Future Trends
TMC is famous because it not only retains the chitosan properties, such as biocompatibility, biodegradability and non-toxicity, but also it can enhance the antimicrobial activity, water-solubility and mucoadhesion capacity [22]. Therefore, the TMC has attracted much attention and recent studies show its wide application range.
Zhou et al. [12] obtained TMC fibers (varying the TMC quaternization degree) with high water absorption performance and excellent bactericidal activity (than chitosan fibers) against E. coli (>63% inibição) and S. aureus (>99% inibição). TMC fibers regarding chitosan fibers could significantly enhance the contraction and wound re-epithelialization toward the mouse embryo fibroblast cells, leading to the wound dressing potential [12]. TMC-materials showed higher water absorbance capacity and stronger bactericidal effect, repressing excessive would maceration i.e., the eschar formation and chronic clinical events at only 12 days [12,14]. So, TMC fiber has potential to be used as components for wound healing performance.
Currently, TMC and TMC-based materials are being applied to obtain bactericidal materials. These are designed for orthopedic application [19], formulation of dental resin [11], repair engineering and wound healing/cell growth [12,14] and food industry [1,8], among others.
TMC has attracted attention on food industry due to its easy acquisition, preparation low cost and mainly due to its extraordinary bactericidal activity. Currently, there is a great race for the new technologies development, in order to obtain bactericidal films, aiming new food preservative strategies. In this way, TMC based film can be used to kill E. coli (O157:H7), one of the most infamous foodborne pathogen that has been detected in various foods, including cheese, neat milk, undercooked meat, and spinach [1].
Chitosan is the most bactericidal agent applied like food additive/ preservative [1]. However, the chitosan has lower bactericidal activity than TMC and chitosan based-materials in solid-state did not show considerable bactericidal action [6]. In this sense, the TMC synthesis may become a reality on industrial scale. The food industry has continually sought the development of bactericidal films that may increase the lifetime of food packaging. In this case, the TMC must be used for this purpose, in order to obtain bactericidal films with excellent performance for food preservation.
References
- Kong M, Chen XG, Xing K, Park HJ (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 144: 51-63.
- Sieval AB, Thanou M, Kotze´ AF, Verhoef JC, Brussee J, et al. (1998) Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. CarbohydrPolym 36: 157-165.
- Verheul RJ, Amidi M, van der Wal S, van Riet E, Jiskoot W, et al. (2008) Synthesis, characterization and in vitro biological properties of O-methyl free N,N,N-trimethylated chitosan. Biomaterials 29: 3642-3649.
- De Britto D, Assis OBG (2007) A novel method for obtaining a quaternary salt of chitosan. CarbohydrPolym 69: 305-310.
- Benediktsdóttir BE, Gaware VS, Rúnarsson ÖV, Jónsdóttir S, Jensen KJ, et al. (2011) Synthesis of N,N,N-trimethyl chitosan homopolymer and highly substituted N-alkyl-N,N-dimethyl chitosan derivatives with the aid of di-tert-butyldimethylsilyl chitosan. CarbohydrPolym 86: 1451-1460.
- Martins AF, Facchi SP, Follmann HD, Pereira AG, Rubira AF, et al. (2014) Antimicrobial Activity of Chitosan Derivatives Containing N-Quaternized Moieties in Its Backbone: A Review. Int J MolSci 15: 20800- 20832.
- Xu T, Xin M, Li M, Huang H, Zhou S, et al. (2010) Synthesis, characteristic and antibacterial activity of N,N,N-trimethyl chitosan and its carboxymethyl derivatives. CarbohydrPolym 81: 931-936.
- Hosseinnejad M, Jafari SM (2016) Evaluation of different factors affecting antimicrobial properties of chitosan. Int J BiolMacromol 85: 467-475.
- Follmann HD, Martins AF, Nobre TM, Bresolin JD, Cellet TS, et al. (2016) Extent of shielding by counterions determines the bactericidal activity of N,N,N-trimethyl chitosan salts. CarbohydrPolym 137: 418-425.
- Martins AF, Facchi SP, Follmann HD, Gerola AP, Rubira AF, et al. (2015) Shielding effect of ‘surface ion pairs’ on physicochemical and bactericidal properties of N,N,N-trimethyl chitosan salts. Carbohydr Res 402: 252-260.
- Song R, Zhong Z, Lin L (2016) Evaluation of chitosan quaternary ammonium salt-modified resin denture base material. Int J BiolMacromol 85: 102-110.
- Zhou Z, Yan D, Cheng X, Kong M, Liu Y, et al. (2016) Biomaterials based on N,N,N-trimethyl chitosan fibers in wound dressing applications. Int J BiolMacromol 89: 471-476.
- Martins AF, Monteiro JP, Bonafé EG, Gerola AP, Silva CTP, et al. (2015) Bactericidal activity of hydrogel beads based on N,N,N-trimethyl chitosan/alginate complexes loaded with silver nanoparticles. Chin ChemLett 26: 1129-1132.
- Fan L, Yang J, Wu H, Hu Z, Yi J, et al. (2015) Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int J BiolMacromol 79: 830-836.
- Ignatova M, Manolova N, Rashkov I (2007) Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) prepared by electrospinning. EurPolym J 43: 1112-1122.
- Kalinov K, Ignatova M, Maximova V, Rashkov I, Manolova N (2014) Modification of electrospun poly(ε-caprolactone) mats by formation of a polyelectrolyte complex between poly(acrylic acid) and quaternized chitosan for tuning of their antibacterial properties. EurPolym J 50: 18-29.
- Rúnarsson ÖV, Holappa J, Malainer C, Steinsson H, Hjálmarsdóttir M, et al. (2010) Antibacterial activity of N-quaternary chitosan derivatives: Synthesis, characterization and structure activity relationship (SAR) investigations. EurPolym J 46: 1251-1267.
- Belalia R, Grelier S, Benaissa M, Coma V (2008) New Bioactive Biomaterials Based on Quaternized Chitosan. J Agric Food Chem 56: 1582-1588.
- Tan H, Ma R, Lin C, Liu Z, Tang T (2013) Quaternized chitosan as an antimicrobial agent: antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int J MolSci 14: 1854-1869.
- Follmann HD, Naves AF, Martins AF, Félix O, Decher G, et al. (2016) Advanced fibroblast proliferation inhibition for biocompatible coating by electrostatic layer-by-layer assemblies of heparin and chitosan derivatives. J. Colloid Interface Sci 474: 9-17.
- Martins AF, Follmann HD, Monteiro JP, Bonafé EG, Nocchi S, et al. (2015) Polyelectrolyte complex containing silver nanoparticles with antitumor property on Caco-2 colon cancer cells. International Journal of Biological Macromolecules 79: 748-755.
- Follmann HD, Martins AF, Gerola AP, Burgo TA, Nakamura CV, et al. (2012) Antiadhesive and Antibacterial Multilayer Films via Layer-by-Layer Assembly of TMC/Heparin Complexes. Biomacromolecules 13: 3711-3722.
- Sajomsang W, Ruktanonchai UR, Gonil P, Warin C (2010) Quaternization of N-(3-pyridylmethyl) chitosan derivatives: Effects of the degree of quaternization, molecular weight and ratio of N-methylpyridinium and N,N,N-trimethyl ammonium moieties on bactericidal activity. CarbohydrPolym 82: 1143-1152.
- Wiarachai O, Thongchul N, Kiatkamjornwong S, Hoven VP (2012) Surface-quaternized chitosan particles as an alternative and effective organic antibacterial material. Colloids Surf, B 92: 121-129.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 13920
- [From(publication date):
August-2016 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 12993
- PDF downloads : 927