NMR Chemical Shifts of Carbon Atoms in the Light and Heavy Oils
Received: 03-Nov-2022 / Manuscript No. ogr-22-80408 / Editor assigned: 07-Nov-2022 / PreQC No. ogr-22-80408(PQ) / Reviewed: 21-Nov-2022 / QC No. ogr-22-80408 / Revised: 25-Nov-2022 / Manuscript No. ogr-22-80408(R) / Published Date: 29-Nov-2022 DOI: 10.4172/2472-0518.1000270
Abstract
With the help of high-resolution 13 C nuclear magnetic resonance (NMR) spectroscopy, the structural-group features of light and heavy oils were established. The molar fractions of primary, secondary, quaternary, tertiary, and aromatic groups, the aromatic factor, and the average length of the hydrocarbon chain of aliphatic hydrocarbons in these oil samples were determined. The 13 C attached proton test (APT) NMR experiments made it possible to carry out a detailed assignment of the 13 C NMR signals in light and heavy oil samples to compare them. Structural differences between light and heavy oil samples were fixed by means of a detailed study of their 13 C NMR spectra.
Keywords
13C NMR spectroscopy; Light oil; heavy oil; Functional group; Qualitative analysis; Quantitative composition
Introduction
Knowledge of the characteristics of oils from different fields and their petroleum products is of great importance for science and industry. The use of spectroscopic methods can provide important unique information for these purposes. 13C NMR spectroscopy has powerful tools with great potential for the development and application of this method to the study of oil [1, 2, 3, 4, 5, 6,7]. With the help of NMR spectroscopy, it is possible to expand research in the field of geological and chemical problems [8, 9]. There are some important advantages and disadvantages regarding this analytical technique [10, 11].
The method of NMR spectroscopy allows us to carry out a study very quickly and without destroying the sample. As a result of the NMR study, it is possible to obtain information on the quantitative and qualitative content of individual hydrocarbon groups in oil samples. Among the disadvantages of NMR spectroscopy the following can be noted: the high cost, the risk of magnetic disturbances, requiring magnetic shielding, and the overlap of frequency ranges, complex information, and requirement of statistical approach to correlate the spectral data with the characterization of crude oil. 1H NMR spectroscopy makes it possible to identify fractions, evaluate the content and distribution of hydrogen atoms in the functional groups of an oil sample. It is also possible to estimate the molecular weight through knowledge of the structural characteristics. 13C NMR spectroscopy provides information about the carbon skeleton of molecules in an oil sample. To elucidate the structure and chemical composition of liquid samples, the NMR method has no analogues. NMR has been used in the analysis of heavy petroleum fractions in many researches [12, 13, 14, 15, 16]. The NMR method, through knowledge of the structural group composition, can really answer the question of how crude oil can be processed more efficiently and economically.
NMR spectroscopy has a large arsenal of professional techniques for improving the quality of spectra and obtaining qualitative quantitative information about the object under study. The aim of various “spectral editing” techniques in NMR spectroscopy is to participate to the separation of primary (CH3), secondary (CH2), tertiary (CH), and quaternary carbons and to a sensitivity improvement. One of the “spectral editing” techniques used in this work is Attached Proton Test (APT) NMR experiments. This method is also known as polarization transfer method, transmitting the large excess polarization of the 1H to the insensitive 13C nuclei before its perturbation. The Attached Proton Test (APT) experiment is a common way to assign C-H multiplicities in 13C NMR spectra. It provides the information on all sorts of carbons within one experiment. The essence of the APT NMR method is the dependence of the spin vector after the initial pulse on the number of hydrogen atoms associated with the carbon atom (C, CH, CH2, CH3). If the delay in pulse sequence is set to the inversed value of the C–H coupling constant (1/JCH), CH and CH3 vectors will have opposite phases compared to C and CH2. Therefore, the phase of CH and CH3 peaks differs from that of C and CH2 peaks by 180°.
The objective of this work is to carried out the comparison between light and heavy oils: obtaining their structural-group characteristics and the complete 13C NMR chemical shift assignments.
Materials and Methods
NMR experiments on studied oil samples were performed on a Bruker Avance III HD 700 NMR spectrometer. The studied oil sample was diluted with deuterated chloroform. Field lock and shimming were achieved using the deuterium signal from CDCl3 solvent. 13C NMR spectra were recorded using 90° pulses with broadband proton decoupling (pulse program zgig); relaxation delay between consecutive accumulations were 3.3 s (and acquisition time was 2.3 s); spectrum width was set to 220.0 ppm; number of scans was 1300. Exponential digital filter with the LB parameter of 10 Hz was applied prior to Fourier transformation. Measurements were made at the temperature of 25°C. All the NMR spectra were integrated after baseline correction, and a mean of minimum three integration values has been taken for each calculation. The relative standard deviation of the results of manual integration did not exceed 3%.
Results and discussion
Structural and group characterization of light and heavy oils by high-resolution 13С NMR spectroscopy
The method of structural group analysis makes it possible to obtain the percentage of the main types of molecules in an oil sample. Oil fractions can be described as an average ratio of structural groups [17].
Information obtained by integration of aromatic signals in individual spectral ranges is represented by the fraction of the corresponding carbon atoms relative to their total number. Fraction of aromatic carbons Car can be straightforwardly found from NMR spectra:

where Car is the fraction of aromatic carbons, Iar is the total integral intensity of aromatic carbons, and Ij is the integral intensity of all functional groups in the 13C NMR spectrum of the sample. It is impossible to obtain unambiguous information on the content of hydrocarbons (alkanes, cyclanes) from 13C NMR spectra, although this information is contained in the fragmentary composition, which can be determined with high accuracy. If integral intensities of individual group signals in the 13C NMR spectrum are known, then corresponding molar fractions of tertiary, primary, secondary and quaternary carbons can be calculated by the following formulas:
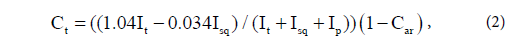
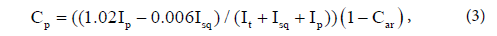
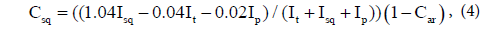
where Ct is the fraction of tertiary carbons; Cp, fraction of primary carbons; Csq, fraction of secondary and quaternary carbons (due to the complexity of separation of methylene and methine signals, their summary contents is estimated); It is the total integral intensity of tertiary (CH) groups; Isq, total integral intensity of secondary (CH2) and quaternary groups; Ip, total integral Intensity of primary (CH3) groups in 13C NMR spectrum of the oil sample. Mean chain length (MCL) was calculated as:

The aromaticity factor (FCA) can be calculated from the equation:

where Cal = Cp + Csq + Ct is the total aliphatic carbon content.
Estimation of molar fractions Cp, Csq, Ct, Car and the aromaticity factor (FCA), and mean chain length (MCL) of light and heavy oils by integration of the corresponding areas of 13C NMR spectra was carried out in a way similar to our previous works [18, 19, 20, 21, 22]. [Table 1]
| Group type | Relative molar content, % | |
|---|---|---|
| light oil | heavy oil | |
| Cp | 25.8 | 12.0 |
| Csq | 53.0 | 46.2 |
| Ct | 10.0 | 12.6 |
| Car | 12.5 | 29.2 |
| FCA | 0.123 | 0.292 |
| MCL | 6.9 | 11.8 |
Table 1: Molar fractions (%) of primary (Cp), secondary and quaternary (Csq), tertiary (Ct), aromatic (Car) groups, aromaticity factor (FCA) and mean chain length (MCL) of light and heavy oil samples based on 13C NMR spectra
13С NMR spectra of light and heavy oils according to NMR spectroscopy data
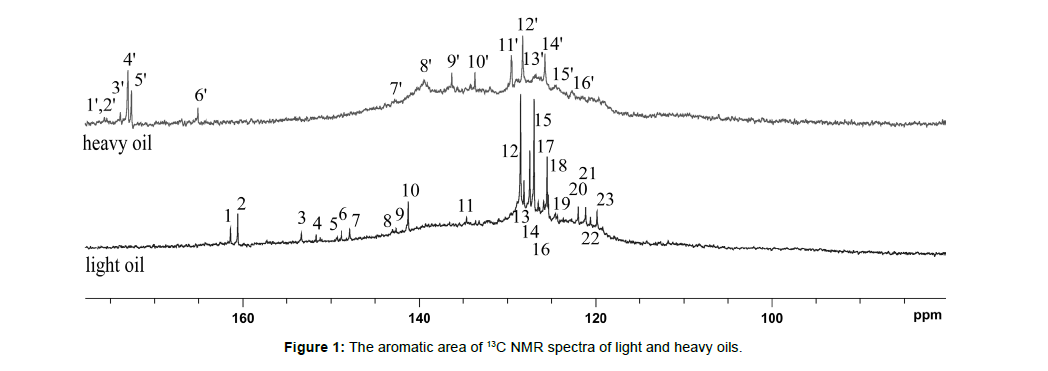
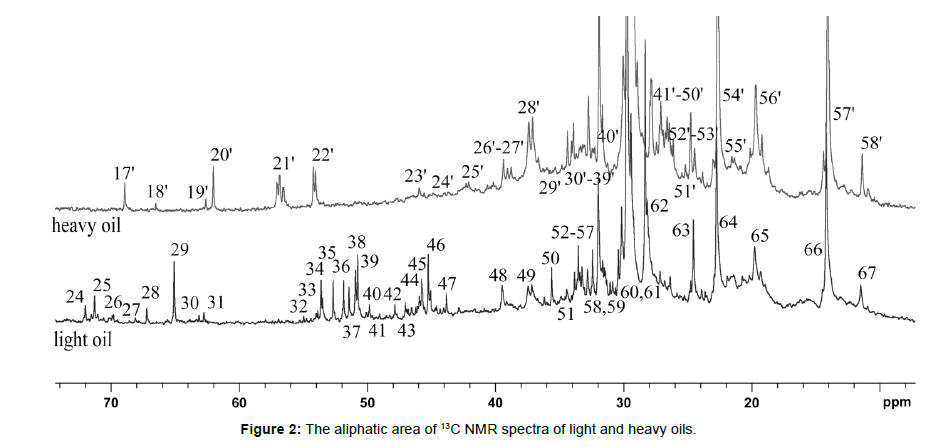
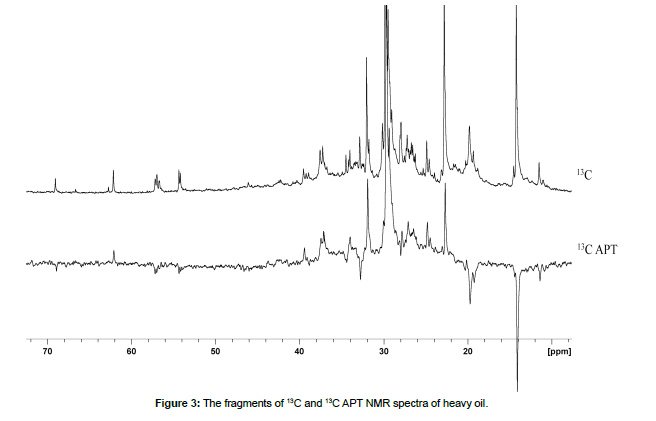
The purpose of the article is to demonstrate the possibilities of 13C NMR spectroscopy in performing a detailed assignment of their NMR signals in light and heavy oil samples, as well as to compare their structural-group characteristics (Tables 2,3). 13C NMR spectra of light and heavy oils were analyzed using a simultaneous consideration of 13C and 13C Attached Proton Test (APT) NMR experiments. 13C NMR spectra of studied oil samples were detailed analyzed and the assignment of all signals were carried out (Figs. 1,2, Tables 2,3). Different zoomed parts (aromatic, aliphatic) of the spectra with enumerated peaks are presented in Figs. 1,2. To distinguish between secondary (CH2) and tertiary (CH) hydrocarbon groups in heavy oil 13C NMR Attached Proton Test (APT) experiment was applied, similar to our previous work (Fig. 3). [18, 19, 20, 21, 22] [Figure 1, Table 2]
| light oil | heavy oil | ||||
|---|---|---|---|---|---|
| № | Group | Chemical shift, ppm | № | Group | Chemical shift, ppm |
| 1 | C | 161.40 | 1’ | C | 175.68 |
| 2 | C | 160.61 | 2’ | C | 175.34 |
| 3 | C | 153.33 | 3’ | C | 173.86 |
| 4 | C | 151.68 | 4’ | C | 173.07 |
| 5 | C | 149.34 | 5’ | C | 172.61 |
| 6 | C | 148.83 | 6’ | C | 165.10 |
| 7 | C | 147.86 | 7’ | C | 142.74 |
| 8 | C | 143.03 | 8’ | C | 139.44 |
| 9 | C | 142.63 | 9’ | C | 136.31 |
| 10 | C | 141.26 | 10’ | C | 133.64 |
| 11 | CH | 134.61 | 11’ | CH | 129.54 |
| 12 | CH | 128.46 | 12’ | CH | 128.18 |
| 13 | CH | 128.06 | 13’ | CH | 126.87 |
| 14 | CH | 127.44 | 14’ | CH | 125.73 |
| 15 | CH | 126.98 | 15’ | CH | 124.54 |
| 16 | CH | 126.42 | 16’ | CH | 122.66 |
| 17 | CH | 125.85 | |||
| 18 | CH | 125.45 | |||
| 19 | CH | 124.54 | |||
| 20 | CH | 121.98 | |||
| 21 | CH | 121.12 | |||
| 22 | CH | 120.61 | |||
| 23 | CH | 119.87 | |||
Table 2: The detailed characterization of 13C NMR spectra of light and heavy oils (aromatic area)
The spectrum of heavy oil is characterized by a wide broadening of signals in the aromatic region of chemical shifts. For the heavy oil sample, distinct signals were seen in the highly aromatic region (1’-5’) related to the carboxyl groups (COOH). Also in the heavy oil sample, signals from carbon atoms with a double bond in the presence of heteroatoms (6’-8’) are observed. [Figure 2, Table 3]
light oil |
heavy oil | ||||
|---|---|---|---|---|---|
| № | Group | Chemical shift, ppm | № | Group | Chemical shift, ppm |
| 24 | CH2 | 72.03 | 17’ | CH | 68.95 |
| 25 | CH2 | 71.34 | 18’ | CH2 | 66.51 |
| 26 | CH | 70.03 | 19’ | CH | 62.58 |
| 27 | CH | 68.10 | 20’ | CH2 | 62.01 |
| 28 | CH | 67.19 | 21’ | CH | 56.78 |
| 29 | CH2 | 65.08 | 22’ | CH | 54.10 |
| 30 | CH | 63.15 | 23’ | CH | 45.83 |
| 31 | CH | 62.75 | 24’ | CH2 | 42.09 |
| 32 | CH2 | 54.96 | 25’ | CH | 40.19 |
| 33 | CH2 | 53.93 | 26’ | CH2 | 39.27 |
| 34 | CH2 | 53.59 | 27’ | CH | 38.92 |
| 35 | CH2 | 52.68 | 28’ | CH | 38.63 |
| 36 | CH2 | 51.83 | 29’ | CH2 | 37.22 |
| 37 | CH2 | 51.43 | 30’ | CH2 | 36.94 |
| 38 | CH2 | 50.80 | 31’ | CH2 | 35.81 |
| 39 | CH2 | 50.12 | 32’ | CH2 | 35.25 |
| 40 | CH2 | 49.84 | 33’ | CH | 34.62 |
| 41 | CH2 | 49.10 | 34’ | CH2 | 34.26 |
| 42 | CH2 | 47.90 | 35’ | CH2 | 33.84 |
| 43 | CH2 | 46.99 | 36’ | CH | 32.92 |
| 44 | CH2 | 46.54 | 37’ | CH | 32.57 |
| 45 | CH2 | 45.80 | 38’ | CH2 | 32.22 |
| 46 | CH2 | 45.23 | 39’ | CH2 | 31.73 |
| 47 | CH2 | 43.81 | 40’ | CH | 31.44 |
| 48 | CH2 | 39.43 | 41’ | CH2 | 29.89 |
| 49 | CH2 | 37.26 | 42’ | CH2 | 29.54 |
| 50 | CH2 | 35.61 | 43’ | CH2 | 29.19 |
| 51 | CH2 | 34.59 | 44’ | CH2 | 28.83 |
| 52 | CH2 | 33.79 | 45’ | CH | 28.13 |
| 53 | CH | 33.51 | 46’ | CH2 | 27.99 |
| 54 | CH | 33.23 | 47’ | CH2 | 27.21 |
| 55 | CH | 32.83 | 48’ | CH2 | 26.72 |
| 56 | CH2 | 32.37 | 49’ | CH2 | 26.30 |
| 57 | CH | 31.92 | 50’ | CH2 | 25.45 |
| 58 | CH | 31.01 | 51’ | CH2 | 24.89 |
| 59 | CH | 30.84 | 52’ | CH2 | 24.60 |
| 60 | CH2 | 30.10 | 53’ | CH2 | 23.97 |
| 61 | CH2 | 29.53 | 54’ | CH2 | 22.70 |
| 62 | CH3 | 28.26 | 55’ | CH | 21.57 |
| 63 | CH3 | 24.56 | 56’ | CH3 | 19.81 |
| 64 | CH2 | 22.77 | 57’ | CH3 | 14.17 |
| 65 | CH3 | 19.78 | 58’ | CH3 | 11.49 |
| 66 | CH3 | 14.19 | |||
| 67 | CH3 | 11.49 | |||
Table 3: The detailed characterization of 13C NMR spectra of light and heavy oils (aliphatic area)
In Fig. 2 and Table 3 presented the signals of 13C nuclei and their chemical shifts for light and heavy oil samples. In a heavy oil sample, fewer signals from 13C nuclei are observed compared to light oil by reducing the number of signals from primary carbons. [Figure 3]
13C APT NMR spectrum is shown in Fig. 3. This experiment makes it possible to distinguish primary and tertiary carbon, on the one hand, from secondary and quaternary, on the other hand. In this case, we observe a signal with a positive phase if the 13C atom is bound to an odd number of neighboring protons. In another case, a signal with a negative phase is observed in the 13C APT NMR spectrum. Thus, even overlapping resonance signals from the CH and CH2 groups can be easily distinguished.
Conclusions
The structural-group characteristics of samples of light and heavy oils are compared. It is noted that for the studied samples of light and heavy oils, the proportions of primary and aromatic hydrocarbon groups have a mutually inverse relationship. Differences in 13С NMR signals for samples of light and heavy oils were recorded after detailed assignment of chemical shifts of their signals in 13С NMR spectra.
Acknowledgement
This work was supported by the Ministry of Science and Higher Education of the Russian Federation under agreement No. 075-15- 2022-299 within the framework of the development program for a world-class Research Center “Efficient development of the global liquid hydrocarbon reserves.”
References
- Speight JG (2014) The Chemistry and Technology of Petroleum, fifth ed., CRC Press.
- Shukla AK (2018) Analytical Characterization Methods for Crude Oil and Related Products. JohnWiley & Sons Ltd.
- Mamin GV, Gafurov MR, Yusupov RV, Gracheva IN, Ganeeva YuM, et al. (2016) Toward the asphaltene structure by electron paramagnetic resonance relaxation studies at high fields (3.4 T). Energy Fuels 30: 6942-6946.
- Gafurov M, Mamin G, Gracheva I, Murzakhanov F, Ganeeva Y, et al. (2019) High-field (3.4 T) ENDOR investigation of asphaltenes in native oil and vanadyl complexes by asphaltene adsorption on alumina surface. Geofluids 3812875: 1-9.
- Kvalheim OM, Aksnes DW, Brekke T, Eide MO, Sletten E (1985) Crude oil characterization and correlation by principal component analysis of 13C nuclear magnetic resonance spectra. Anal Chem 57: 2858-2864.
- Gao G, Cao J, Xu T, Zhang H, Zhang Y, et al. (2020) Nuclear magnetic resonance spectroscopy of crude oil as proxies for oil source and thermal maturity based on 1H and 13C spectra. Fuel 271: 117622.
- Netzel DA, Thompson LF (1986) Aromatic tertiary carbons as a check on the validity of NMR data on fossil fuels. Fuel 65: 597-598.
- Edwards JC (2011) A review of applications of NMR spectroscopy in the petroleum industry, in: Nadkarni, R.A. (Eds.), Spectroscopic analysis of petroleum products and lubricants; ASTM International, West Conshohocken. 423-473.
- Günther H (2013) NMR Spectroscopy: Basic Principles, Concepts, and Applications in Chemistry, third ed., Wiley.
- Rudzinski WE, Aminabhavi TM (2000) A review on extraction and identification of crude oil and related products using supercritical fluid technology. Energy Fuels 14: 464-475.
- Silva SL, Silva AMS, Ribeiro JC (2011) Chromatographic and spectroscopic analysis of heavy crude oil mixtures with emphasis in nuclear magnetic resonance spectroscopy: A review. Analytica Chimica Acta 707: 18-37.
- Friedel RA, (1959) Absorption spectra and magnetic resonance spectra of asphaltene. J Chem Phys 31: 280-281.
- Cookson DJ, Smith BE (1987) One- and two-dimensional NMR methods for elucidating structural characteristics of aromatic fractions from petroleum and synthetic fuels. Energy Fuel 1: 111-120.
- Bansal V, Kapur GS, Sarpal AS (1998) Estimation of total aromatics and their distribution as mono and global di-plus aromatics in diesel-range products by NMR spectroscopy. Energy Fuels 12: 1223-1227.
- Lee SW, Glavincevski BB (1999) NMR method for determination of aromatics in middle distillate oils. Fuel Process. Technol 60: 81-86.
- Woods J, Kung J, Kingston D (2008) Canadian crudes: A comparative study of SARA fractions from a modified HPLC separation technique. Oil Gas Sci Technol 63: 151-163.
- Kalabin GA, Kanitskaya LV, Kushnarev DF (2000) Quantitative NMR Spectroscopy of Natural Organic Raw Materials and its Processing Products, Moscow, Chemistry.
- Rakhmatullin IZ, Efimov SV, Margulis BYa, Klochkov VV (2017) Qualitative and quantitative analysis of oil samples extracted from some Bashkortostan and Tatarstan oilfields based on NMR spectroscopy data. J Petrol Sci Eng 156: 12-18.
- Rakhmatullin IZ, Efimov SV, Tyurin VA, Al-Muntaser AA, Klimovitskii AE, et al. (2018) Application of high resolution NMR (1H and 13C) and FTIR spectroscopy for characterization of light and heavy crude oils. J Petrol Sci Eng 168: 256-262.
- Rakhmatullin IZ, Efimov SV, Tyurin VA, Gafurov MR, Al-Muntaser AA, et al. (2020) Qualitative and quantitative analysis of heavy crude oil samples and their SARA fractions with 13C nuclear magnetic resonance. Processes 8: 995.
- Rakhmatullin IZ, Efimov SV, Klochkov AV, Gnezdilov OI, Varfolomeev MA, et al. (2022) NMR chemical shifts of carbon atoms and characteristic shift ranges in the oil sample. Petroleum Research 7: 269-274.
- Rakhmatullin IZ, Efimov SV, Klochkov AV, Varfolomeev MA, Klochkov VV (2022) NMR structural-group characteristics and detailed shift ranges in light, heavy and catalyzed oils. Znanstvena misel journal 69: 7-11.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Varfolomeev MA (2022) NMR Chemical Shifts of Carbon Atoms in the Light and Heavy Oils. Oil Gas Res 8: 270. DOI: 10.4172/2472-0518.1000270
Copyright: © 2022 Varfolomeev MA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2280
- [From(publication date): 0-2022 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 1888
- PDF downloads: 392