Nivolumab Bullous Pemphigoid: Case Description and Literature Review
Received: 22-Feb-2019 / Accepted Date: 01-Mar-2019 / Published Date: 08-Mar-2019 DOI: 10.4172/2161-0681.1000364
Abstract
Nivolumab is a monoclonal antibody, belonging to the checkpoint inhibitors, recently approved for treatment of recurrent or metastatic non-small cell lung cancer, melanoma, and renal cell carcinoma. Although very efficacious, oncologic immunotherapy is associated with a new spectrum of dermatological adverse events, and spontaneous reporting is advisable to define presentation, prognosis and real-life management. In particular, development of bullous pemphigoid (BP), characterized by pathologic autoantibody formation and complement deposition in the skin. is a rare, but severe event, potentially life-threatening, which requires high doses corticosteroids, or even more aggressive immunosuppression. We describe a 73-year-old Caucasian man, under nivolumab therapy for metastatic renal carcinoma, with 2-month-history of a bullous itching eruption on the trunk and arms, rapidly responding to standard corticosteroids treatment and nivolumab dismission, supporting the decision to taper and completely interrupt steroids. Unfortunately, 3 weeks after complete steroidal washout, the itching bullous eruption relapsed, confirming that the autoimmune disease, once aroused by the drug-induced immune unbalance, has a clinical course superimposable to the idiopathic disease. Prompt referral to the dermatologist is advisable in patients under oncologic immunotherapy, developing pruritus and skin eruptions to address the correct diagnostic workup and management.
Keywords: IgG4 antibody; Cancer; Nivolumab
Introduction
Nivolumab is an IgG4 antibody classified among the novel anticancer immune checkpoint inhibitors, recently approved for the treatment of advanced or recurrent non-small cell lung cancer, melanoma, and renal cell carcinoma [1]. The human monoclonal antibody is designed to interact with the programmed death cell receptor-1 (PD-1), preventing the inhibitory signaling on cytotoxic T cells that several types of cancer cells are able to produce by the release of the natural ligands (PD-L1, PD-L2). Besides the revolutionary significant benefits of this immune activation strategy, Nivolumab and in general checkpoint inhibitors are associated with a new spectrum of side effects, termed immune-related adverse events (irAEs), that include dermatologic, gastrointestinal, hepatic, endocrine and other less common inflammatory events [2,3]. Reactions are usually mild and self-limiting, but the occurrence of bullous pemphigoid (BP), is a rare, potentially life-threatening event, characterized by pathologic autoantibody formation and complement deposition in the skin. Treatment with high doses of corticosteroids, or even more aggressive immunosuppressant, such as rituximab is reported [4-6]. The rarity and novelty of this iatrogenous entity questions the definition of prognostic factors and differences from the idiopathic autoimmune disease, which has a chronic relapsing course, requires long-term treatment and potential complications management, with a 3.6 -fold increased mortality in respect to the general population [7,8].
We describe a mild case, rapidly responding to standard corticosteroids treatment and nivolumab dismission, thus supporting the hypothesis of a self-limiting reaction and the decision to taper and completely interrupt steroids. Unfortunately, 3 weeks after complete steroidal washout, without nivolumab re-exposure, the itching bullous eruption relapsed. Our experience provides an extra proof that the drug-induced autoimmune disease follows a natural course superimposable to the idiopathic disease.
Case Report
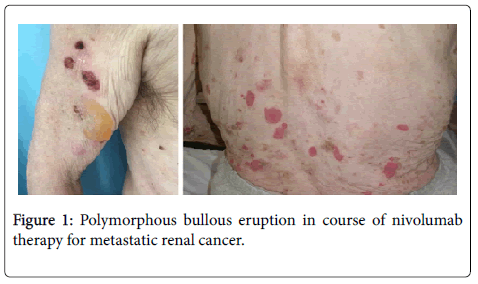
A 73-year-old man presented with 2 months history of a bullous itching eruption. Physical examination demonstrated large erythematous-edematous bullous lesions, some with a clear serous content, some covered with serum-hematic crusts, on the trunk and arms (Figure 1); mucous membranes were unaffected.
His medical history was marked by an aggressive renal cancer, with pulmonary metastasis under nivolumab treatment from 1 year. Other co-morbidity included hypertension, and prostatic hypertrophy, treated with Fosinopril, Hydrochlorothiazide, Silodosin.
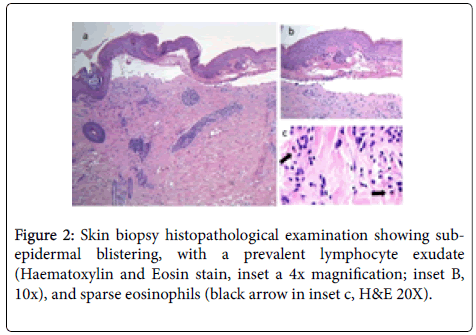
Blood tests were performed with normal range results, except for the elevated serum BP180 antibody levels (171 U/mL; normal value<20). The following diagnostic work-up included a skin biopsy for routine histology and direct immunofluorescence, which confirmed the sub-epidermal blistering, with a prevalent lymphocyte exudate, and sparse eosinophils (Figure 2). Direct immunofluorescence showed linear deposition of IgG and C3c along the dermo-epidermal junction.
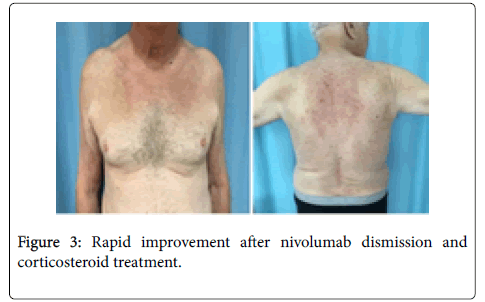
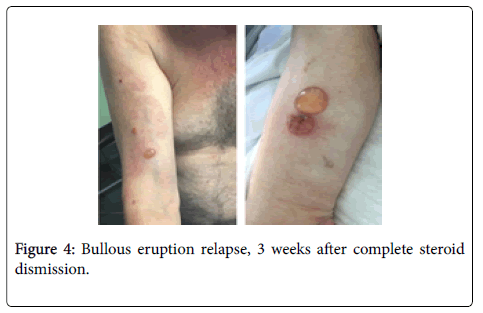
Clinical, immunological and histopathological findings were diagnostic for bullous pemphigoid, and the patient started treatment with intravenous methylprednisolone 0.5 mg/Kg. in accordance with the oncologists, the decision to suspend nivolumab treatment was taken, and the eruption immediately improved (Figure 3). After 10 days, the skin lesions were completely healed, treatment passed to oral steroids and progressively tapered to 20 mg/daily. The oncologists decided to perform pulmonary metastasis ablation, and find unnecessary to reintroduce nivolumab. After 2 month of complete BP remission, the corticosteroid treatment was further tapered until complete dismission. Unfortunately, 3 weeks from complete corticosteroids dismission, severe itching announced the bullous eruption recurrence, requiring corticosteroid re-introduction (Figure 4).
Discussion and Conclusion
Dermatologic toxicity is the most common immune-related adverse event associated with checkpoint inhibitors, with approximately 30 to 40 percent of patients treated with Nivolumab affected [2-4]. For many patients, dermatologic toxicity is the earliest irAE experienced, about 3-6 weeks after treatment initiation, but delayed onset is reported, even after therapy discontinuation [2]. A revision of previous nivolumab BP cases (Table 1) suggests an interval variable from 6 weeks to 20 months (3-14). The occurrence of BP is considered a class-effect, being reported during other anti–PD-1 or programmed death ligand 1 (PDL1) therapy [3]. The type of malignancy seems not relevant, being reported in association with renal, lung, and melanoma treatment [4-15].
| Age | Nivolumab indication | Prior treatment | Latency | Treatment | Reference |
|---|---|---|---|---|---|
| 73 | Renal cancer | Sutinib | 12 months | methylprednisolone | Current observation |
| 80 | Lung cancer | none | 12 months | Methylprednisolone | [6] |
| 77 | Lung cancer | none | 6 weeks | Prednisone | [8] |
| 68 | Melanoma | none | 20 months | Clobetasol | [11] |
| 80 | Melanoma | Ipilimumab | 24 weeks | Topical tacrolimus, oral nicotinamide | [12] |
| 85 | Lung cancer | Carboplatino+ Gemcitabine |
6.1 weeks | Prednisone, topical corticosteroids | [12] |
| 48 | Melanoma | none | Not declared | Corticosteroids | [9] |
| 60 | Renal cancer | Chemotherapy | 3 months | Triamcinolone, Prednisone |
[4] |
| 60 | Lung cancer | none | 12 months | Prednisone | [14] |
Table 1: Nivolumab associated BP cases from the literature retrieval.
The therapeutic effects of PD-1 inhibition is mediated by a specific alteration of the T cell functions that results from the block of the PD-1 interaction with its ligands (PD-L1 and PD-L2), reducing cell-death signaling and promoting tumor cell recognition and destruction [1]. However, B cells also express PD-1, and the signaling inhibition can directly activate B cells in a T-cell–independent fashion [1-3]. In the case of nivolumab-associated bullous pemphigoid, altered B-cells probably start secreting the pathogenic antibodies, reproducing the same conditions as in the spontaneous idiopathic BP [2-4].
Our observation was peculiar for the rapid control of the eruption, causing the illusion that drug withdrawal and a short course of steroids could be followed by BP effective remission, as suggested by other Authors [14]. However, recurrence without nivolumab or other anti- PD-1 exposure confirmed that once the autoimmune disease is triggered advocates same advertisement and guidelines than the idiopathic form [7]. In previous observations, development of BP required discontinuation of immunotherapy in 76% of cases [3]. Prompt dermatological consulting is mandatory to avoid diagnostic delay, establish the appropriate treatment and support the oncologist decision whether to continue the oncologic therapy or not [14]. In our patient the oncologists decided to definitely interrupt the immunotherapy, not for the skin conditions which were rapidly healed or eventually controlled with low corticosteroids doses, but for the surgical opportunity to remove the pulmonary metastasis. Expanding the case collections, including mild forms can reassure the medical community on the possibility to continue efficacious immunotherapy, even when dermatological toxicity occurs.
Financial Disclosure
None.
References
- Rajan A, Kim C, Heery CR, Guha U, Gulley JL (2016) Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: Role in advanced cancers. Hum VaccinImmunother 12: 2219-2231.
- Sibaud V, Boulinguez S, Pagès C, Riffaud L, Lamant L, et al. (2018) Dermatologic toxicities of immune checkpoint inhibitors. Ann DermatolVenereol 145: 313-330.
- Lopez AT, Khanna T, Antonov N, Audrey-Bayan C, Geskin L (2018) A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol 57:664-669.
- Kwon CW, Land AS, Smoller BR, Scott G, Beck LA, et al. (2017) Bullous pemphigoid associated with nivolumab, a programmed cell death 1 protein inhibitor. J EurAcadDermatolVenereol 31: e349-e350.
- Ridpath AV, Rzepka PV, Shearer SM, Scrape SR, Olencki TE, et al. (2018) Novel use of combination therapeutic plasma exchange and rituximab in the treatment of nivolumab-induced bullous pemphigoid. Int J Dermatol 57: 1372-1374.
- Sowerby L, Dewan AK, Granter S, Gandhi L, LeBoeuf NR (2017) Rituximab Treatment of Nivolumab-Induced Bullous Pemphigoid. JAMA Dermatol 153: 603-605.
- Cozzani E, Marzano AV, Caproni M, Feliciani C, Calzavara-Pinton P, et al. (2018) Bullous pemphigoid: Italian guidelines adapted from the EDF/EADV guidelines. G ItalDermatolVenereol 153: 305-315.
- Kidrin K, Schwarts N, Cohen AD, Zelber-Sagi S (2018) Mortality in bullous pemphigoid: A systematic review and meta-analysis of standardized mortality ratios.JDermatol 45: 1094-1100.
- Wang LL, Patel G, Chiesa-Fuxench ZC, McGettigan S, Schuchter L, et al. (2018) Timing of Onset of Adverse Cutaneous Reactions Associated with Programmed Cell Death Protein 1 Inhibitor Therapy. JAMA Dermatol 154: 1057-1061.
- Damsky W, Kole L, Tomayko MM (2016) Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep 2: 442-444.
- Anastasopoulou A, Papaxoinis G, Diamantopoulos P, Christofidou E, Benopoulou O, et al. (2018) Bullous Pemphigoid-like Skin Lesions and Overt Eosinophilia in a Patient With Melanoma Treated With Nivolumab: Case Report and Review of the Literature. J Immunother 41:164-167.
- Hwang SJ, Carlos G, Chou S, Wakade D, Carlino MS, et al. (2016) Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Res 26: 413-416.
- Naidoo J, Schindler K, Querfeld C, et al. (2016) Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol Res 4: 383-389.
- Jour G, Glitza IC, Ellis RM, Torres-Cabala CA, Tetzlaff MT, et al. (2016) Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J CutanPathol 43:688-696.
- Panariello L, Fattore D, Annunziata MC, Piantedosi F, Gilli M, et al. (2018) Bullous pemphigoid and nivolumab: Dermatologic management to support and continue oncologic therapy. Eur J Cancer 103: 284-286.
Citation: Anedda J, Atzori L, Rongioletti F, Pilloni L (2019) Nivolumab Bullous Pemphigoid: Case Description and Literature Review. J Clin Exp Pathol 9:364. DOI: 10.4172/2161-0681.1000364
Copyright: © 2019 Anedda J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2760
- [From(publication date): 0-2019 - Dec 19, 2024]
- Breakdown by view type
- HTML page views: 2141
- PDF downloads: 619