Nirmatrelvir Treatment Duration and Frequency of COVID-19 Rebound
Received: 15-Jul-2024 / Manuscript No. JIDT-24-142255 / Editor assigned: 17-Jul-2024 / PreQC No. JIDT-24-142255 (PQ) / Reviewed: 31-Jul-2024 / QC No. JIDT-24-142255 (QC) / Revised: 07-Aug-2024 / Manuscript No. JIDT-24-142255 (R) / Published Date: 14-Aug-2024 DOI: 10.4172/2332-0877.1000606
Abstract
Background: Nirmatrelvir has been shown to reduce morbidity and mortality associated with COVID-19. However, it is underutilized due to concerns regarding COVID-19 symptom rebound following nirmatrelvir’s standard 5-days to 10-days course. This study aims to identify and evaluate a nirmatrelvir dosage regimen that lowers symptom rebound.
Methods: Based on nirmatrelvir pharmacokinetics, we propose a novel 8-days regimen, 2 doses twice-daily followed by 6 doses once-daily to reduce rebound frequency. We then carried out a retrospective case series study of clinical outcomes among our patients to investigate their frequency of COVID-19 symptom rebound following nirmatrelvir usage.
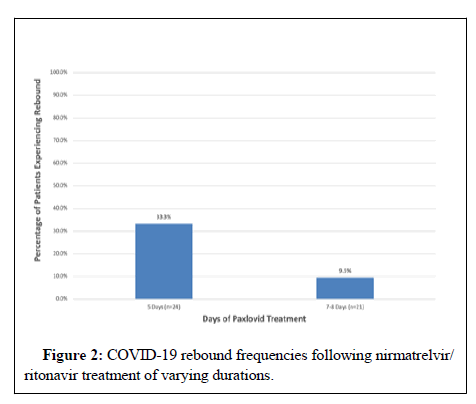
Results: Among the 58 prescribed case patients, 49 filled and initiated the prescription. Of those 49 patients, 4 took the medication for fewer than 5 days, 24 for 5 days and 21 for 7 days or 8 days. Among 5-day treatment cases (n=24), 8(33.3%) experienced clinical rebound, while among the 7-day or 8-day treatment cases (n=21), 2(9.5%) experienced rebound.
Conclusion: These findings suggest that a longer nirmatrelvir/ritonavir course might reduce rebound symptoms compared to the standard 5-days regimen.
Keywords: Paxlovid; COVID-19; Rebound; Nirmatrelvir
Introduction
Current COVID-19 treatment is a 5-days course of twice-daily nirmatrelvir/ritonavir [1]. Despite its effectiveness, nirmatrelvir/ritonavir remains underutilized due to concerns of symptom recurrence following treatment [2]. While US authorities concluded no association between nirmatrelvir/ritonavir treatment and a rebound in COVID-19 symptoms, many may avoid treatment due to concerns regarding overall symptom prolongation [2,3].
To reduce the frequency of symptom recurrence, we prescribed a novel extended regimen based on the pharmacokinetics of nirmatrelvir/ ritonavir: Twice-daily dosing the first 2 days, followed by daily dosing for the next 6 days, totaling 8 days of treatment. This dosing regimen provides a 2-days loading dose and the longest duration of additional dosing days available with a standard 10-dose nirmatrelvir/ritonavir pack.
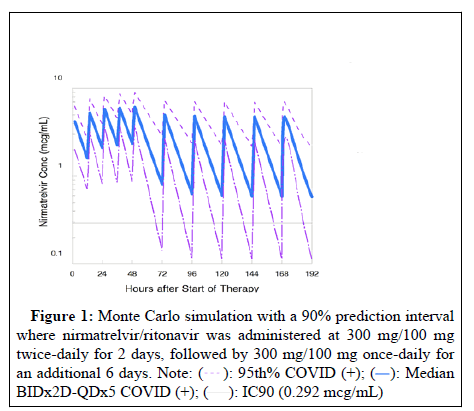
A Monte Carlo simulation was conducted simulating nirmatrelvir/ ritonavir administration at a 300 mg/100 mg twice-daily dosing for 2 days, followed by 300 mg/100 mg daily dosing for 6 days using a pharmacokinetic model of 5,149 measurements in 1,237 participants with COVID-19 [4]. The model predicted the median nirmatrelvir concentrations with a 90% prediction interval (5th percentile-95th percentile). We compared these values to the nirmatrelvir concentration required to inhibit 90% of in vitro replication (IC90) of SARS-CoV-2 virus (0.292 mcg/mL) [5]. The median trough on the 8-day nirmatrelvir/ritonavir regimen was 0.58 mcg/mL, with 79% of troughs above the IC90 value (Figure 1). These pharmacokinetic data suggest that the 8- days regimen may inhibit SARS-CoV-2 viral growth throughout treatment.
To understand the clinical validity of this model, we examined differences between the 5-days regimen and our longer-duration regimen among our patients.
We asked patients who were prescribed and completed nirmatrelvir/ritonavir in our telehealth practice to report any worsening clinical symptoms following a period of clinical improvement after completion of treatment.
Of 49 patients, 4 took the medication for fewer than 5 days, 24 for 5 days and 21 for 7 days or 8 days. Among the 5-days treatment cases 8(33.3%) of 24 reported clinical rebound, while among the 7-day or 8- day treatment cases only 2(9.5%) of 21 reported rebound (Figure 2). None experienced severe disease, hospitalization or death.
Conclusion
Our theoretical model and clinical reports suggest that a longer nirmatrelvir/ritonavir course might reduce clinical rebound frequency compared to the 5-days course. Additional studies are needed to determine the optimal treatment duration that maximizes the clinical benefits of while lowering frequency of rebound.
Acknowledgment
We would like to acknowledge Dr. Edmund V. Capparelli for performing a Monte Carlo simulation for this report and producing Figure.
Author Contributions
Jeffrey D. Klausner conceptualized and designed the report. Nathan Sudeep collected and analyzed clinical data. Noah Kojima analyzed pharmacokinetic data. Nathan Sudeep wrote the first draft of the manuscript with input from Jeffrey D. Klausner and Noah Kojima. All authors reviewed and approved the final version of the manuscript.
References
- COVID-19 Treatment Guidelines (2024) Ritonavir-boosted nirmatrelvir (Paxlovid).
- Jewett C (2024) Paxlovid cuts covid death risk. But those who need it are not taking it. Health, The New York Times.
- Smith DJ, Lambrou A, Patel P (2023) SARS-CoV-2 rebound with and without use of COVID-19 oral antivirals. Morb Mortal Wkly Rep 72:1357-1364.
[Crossref] [Google Scholar] [PubMed]
- Chan PLS, Singh RSP, Cox DS, Shi H, Damle B, et al. (2023) Dosing recommendation of nirmatrelvir/ritonavir using an integrated population pharmacokinetic analysis. CPT Pharmacometrics Syst Pharmacol 12:1897-1910.
[Crossref] [Google Scholar] [PubMed]
- Center for Drug Evaluation and Research Review (2024) Emergency use authorization for paxlovid. Pfizer Inc.,
Citation: Sudeep N, Kojima N, Klausner JD (2024) Nirmatrelvir Treatment Duration and Frequency of COVID-19 Rebound. J Infect Dis Ther 12:606. DOI: 10.4172/2332-0877.1000606
Copyright: © 2024 Sudeep N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 591
- [From(publication date): 0-0 - Apr 27, 2025]
- Breakdown by view type
- HTML page views: 393
- PDF downloads: 198