Nilotinib Single-Dose Pharmacokinetics and Pharmacodynamics in Parkinson's Disease Patients
Received: 14-Nov-2023 / Manuscript No. JADP-23-120066 / Editor assigned: 16-Nov-2023 / PreQC No. JADP-23-120066 (PQ) / Reviewed: 30-Nov-2023 / QC No. JADP-23-120066 / Revised: 07-Dec-2023 / Manuscript No. JADP-23-120066 (R) / Published Date: 14-Dec-2023 DOI: 10.4172/2161-0460.1000586
Abstract
With the strongest affinity for inhibiting Abelson (c-Abl) and Discoidal Domain Receptors (DDR1/2), nilotinib is a broad-based tyrosine kinase inhibitor. According to preclinical data, nilotinib lowers brain alpha-synuclein levels and lessens inflammation in Parkinson's Disease (PD) animals. As we previously demonstrated, nilotinib crosses the Blood-Brain Barrier (BBB) and may help patients with Parkinson's disease (PD) and Dementia with Lewy Bodies (DLB) achieve better clinical outcomes. With a cohort of 75 PD subjects, we conducted a physiologically based Population Pharmacokinetic/Pharmacodynamic (popPK/PD) study to ascertain the effects of nilotinib. Five groups (n=15) were randomly assigned (1:1:1:1:1) and each group was given an open-label Random Single Dose (RSD) of 150:200:300:400 mg of nilotinib instead of a placebo. After taking nilotinib, samples of plasma and Cerebrospinal Fluid (CSF) were taken 1,2,3 and 4 hours later. The findings indicate that nilotinib penetrates the brain in a manner that is independent of dose and that nilotinib at a dose of 200 mg raises the levels of Homovanillic Acid (HVA) and 3,4-Dihydroxyphenylacetic Acid (DOPAC), which may indicate changes in dopamine metabolism. Both the CSF oligomeric: total alpha-synuclein ratio and plasma total alpha-synuclein are markedly decreased by nilotinib. Moreover, nilotinib dramatically raises the CSF concentration of TREM-2 activating receptors on myeloid cells, indicating an anti-inflammatory impact. When combined, 200 mg of nilotinib seems to be the ideal single dosage for reducing inflammation and activating alpha-synuclein and dopamine metabolism, two surrogate disease indicators.
Keywords: Alpha-synuclein; Dopamine; Nilotinib; Parkinson; TREM-2
Introduction
The Breakpoint Cluster-Abelson (BCR-Abl) tyrosine kinase inhibitor nilotinib (Tasigna®, AMN107, Novartis, Switzerland) is licensed by the FDA to treat adults with Chronic Myeloid Leukemia (CML) at oral doses of 600-800 mg per day [1-3]. Additionally, nilotinib has a strong inhibitory effect on Discoidin Domain Receptor (DDR) 1 and 2 [4,5]. In a number of animal models of neurodegenerative disorders, we have shown that a low dose of nilotinib (1-10 mg/kg daily) can cross the Blood-Brain Barrier (BBB), lower inflammation and break down misfolded alpha-synuclein [6-9]. Additionally, nilotinib raises Dopamine (DA) levels and enhances motor and cognitive functions in models of Alzheimer's Disease (AD) and Parkinson's disease (PD) [10-15]. In a tiny pilot research with 12 patients, nilotinib showed promise in treating both motor and nonmotor symptoms in Parkinson's disease patients and Dementia with Lewy bodies (DLB), as well as appearing to have an impact on alpha-synuclein and DA metabolism, two surrogate disease indicators. Sixteen the second most frequent neurodegenerative disease causing both motor and nonmotor symptoms is PD. The hallmarks of PD include the degeneration of dopaminergic neurons in the Substantia Nigra (SN) pars compacta and the development of intracellular inclusions called Lewy Bodies (LBs), which are largely composed of aggregated alpha-synuclein. Alpha-synuclein oligomer levels in Cerebrospinal Fluid (CSF) rise with time in Parkinson's Disease (PD) patients as compared to age-matched controls [16-19]. Furthermore, when compared to controls, the CSF of PD patients shows an elevated oligomeric to total alpha-synuclein ratio, which has been linked to motor deterioration [20,21]. Two useful primary metabolites of DA are 3,4-Dihydroxyphenylacetic Acid (DOPAC) and Hom Vanillic Acid (HVA) as a marker of DA metabolism in the CSF. It has been demonstrated that lower CSF levels of DOPAC are an early indicator of PD and that PD patients' CSFs had lower HVA levels than controls [22,23]. Alpha-synuclein levels, CSF HVA and DOPAC measurements may all have a significant impact on the pharmacodynamics of nilotinib therapy for parkinson's disease. Strong risk factors for Parkinson's Disease (PD) include the R47H and other variations of Triggering Receptors On Myeloid Cells-2 (TREM-2) that cause TREM-2 function to be lost [24-26]. The SN's activated microglia multiply and release pro-inflammatory cytokines and reactive oxygen species, which causes the DA neurons in Parkinson's disease to gradually deteriorate [26-28]. TREM-2 may control phagocytosis and the response of microglia. By suppressing TREM-2, inflammatory responses in microglia are inhibited as a marker of DA metabolism in the CSF. It has been demonstrated that lower CSF levels of DOPAC are an early indicator of Parkinson's Disease (PD) and that PD patients' CSFs had lower HVA levels than controls [22]. Nuclear Factor kappa B (NF-kB) pathways and innate immunity activation, whereas decreased microglial phagocytosis is the outcome of TREM-2 loss of activity [29-32]. Therefore, when PD patients receive nilotinib treatment, monitoring TREM-2 levels in the CSF may offer another significant pharmacodynamic effect suggesting neuroinflammation and the phagocytic activity of microglia to potentially reducen alpha-synuclein levels. We designed a physiologically based Population Pharmacokinetic/Pharmacodynamic (popPK/PD) study to ascertain the pharmacokinetics and pharmacodynamics of nilotinib in PD patients. We measured the levels of DOPAC, HVA, total and oligomeric nilotinib in plasma and CSF, as well as TREM-2. In an open-label, Random Single Dose (RSD) research, 75 patients were randomized into five groups (n=15) containing 150 mg, 200 mg, 300 mg and 400 mg of nilotinib or placebo. This study of RSD in a homogeneous cohort of PD participants offers important insight into the possible mechanisms of action of nilotinib, as well as its impact on neuroinflammation and possible disease biomarkers in the cerebrospinal fluid.
Literature Review
Study goals and design to ascertain the pharmacokinetics and pharmacodynamics of nilotinib, an RSD trial involving a total of 100 persons was screened and 75 participants were enrolled. Participants in this study had PD with Hoehn & Yahr stages ranging from 2.5 to 3. Before starting treatment, eligible individuals had to refrain from taking any Monoamine Oxidase (MAO)-B inhibitors, such as rasagine or selegiline, for at least six weeks. The maximum dosage for levodopa was ≤ 800 mg (Sinemet/carbidopa/levodopa/entacopone/stalevo) or IPX 066 (Rytary), which was later adjusted to 800 mg carbidopa/levodopa equivalent of 800 mg or less per day was permitted. According to the Novartis Investigator Brochure, nilotinib has substantial drug-drug interactions with CYP3A inhibitors, inducers and substrates, as well as multiple other Cytochrome (CYPs). Patients taking CYP3A inhibitors were not included in this trial. At screening, Mild Cognitive Impairment (MCI) was determined by a score of ≥ 22 on the Montreal Cognitive Assessment (MoCA). Prior to baseline assessments, all inclusion/exclusion criteria were satisfied, screening results were evaluated and baseline visits were planned two to four weeks after screening. Following enrollment, 75 patients were recruited for an open-label RSD research. They were randomly assigned to one of five groups (n=15, 1:1:1:1:1) and given a single daily dosage of either 150 mg, 200 mg, 300 mg or 400 mg of nilotinib as a placebo (Figure 1). Since we Nilotinib can be detected in PD and DLB patients up to 4 hours after the drug is administered, as previously shown. 16 blood collection and Lumbar Punctures (LPs) were spaced out between 1 and 4 hours, as depicted in Figure 1, with blood being taken 30 minutes before LPs. Approximately two hours passed after the last levodopa dosage for each LP. Additionally, nilotinib was taken at least two hours after a meal, on an empty stomach. All patients were randomized into three groups and given 150 mg, 300 mg or placebo for a duration of 12 months after a one-week wash-out phase (NCT02954978). At the conclusion of a year of treatment, LP and blood will be taken as part of this double-blind, placebo-controlled study to provide physiological population pharmacokinetics pharmacodynamics as well. This smooth, open-label RSD trial was conducted as a component of a longer, more comprehensive investigation that included two active arms (150 mg and 300 mg) and a placebo. Approvals, registrations and patient consent for standard protocols This research was authorized by the Institutional Review Board (IRB#) and carried out in compliance with the principles of good clinical practice. Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) scientific review board and Georgetown University Medical Center's (2016-0380). All research partners and participants gave their informed consent. The trial was filed on ClinicalTrials.gov (NCT02954978) and carried out under Food and Drug Administration Investigational New Drug (IND) # 123183. To oversee research safety and advancement, an external, independent Data Safety Review Board (DSMB) including a neurologist specializing in movement disorders, a biostatistician, a cardiologist and a clinical pharmacologist was appointed.
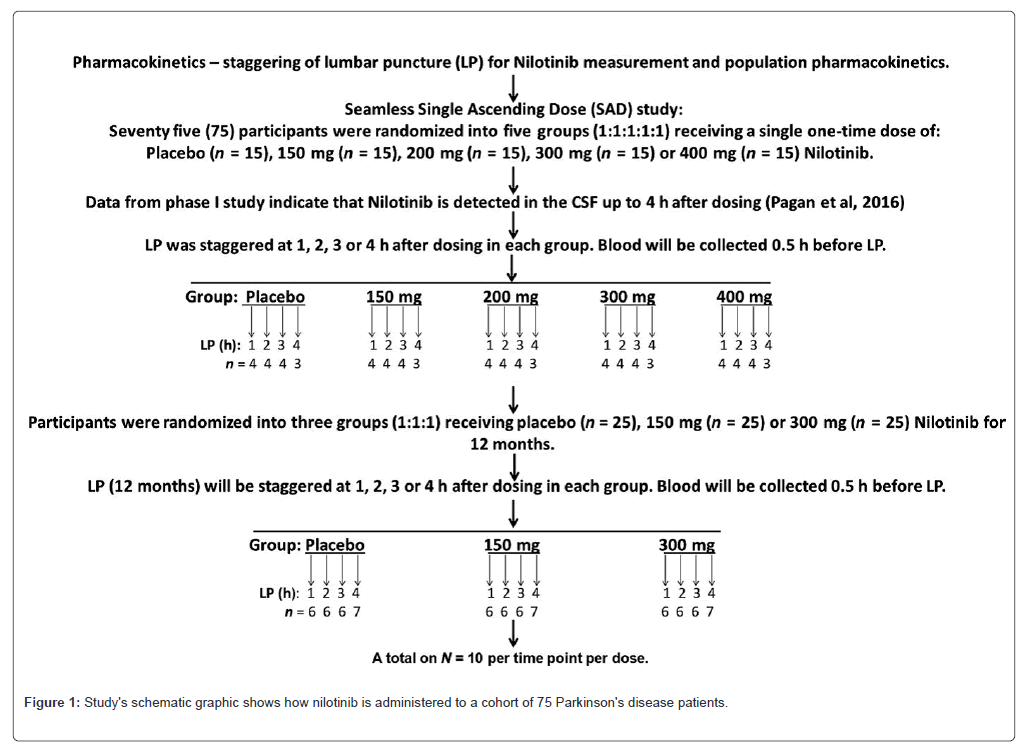
The phase II : This study's schematic graphic shows how nilotinib is administered to a cohort of 75 Parkinson's disease patients in order to ascertain the drug's pharmacokinetics and pharmacodynamics. The Random Single Dose (RSD) trial was conducted over the course of a year as a randomized, double-blind, placebo-controlled investigation. In the RSD investigation, a total of 15 people were enrolled in each group (placebo: 150 mg; 200 mg; 300 mg; 400 mg). At 1, 2, 3 and 4 hours, Lumbar Punctures (LPs) were randomly performed in each group. The concentration of nilotinib and illness biomarkers in each therapy group (dose study) and at each time point (exploratory time-dependent study) were measured using CSF and plasma analysis.
Collection of plasma and CSF
As we previously demonstrated, four hours after delivery, nilotinib was found in the human CSF. 16 All patients underwent blood draws (15 mL) and lymphograms (∼15 mL CSF) at 1, 2, 3 or 4 hours following oral nilotinib delivery and around two hours after their previous levodopa dosage. Following blood collection, plasma was separated, aliquoted and kept at -80°C. Aliquoted CSF was kept at -80°C. Cycles of freeze and thaw were avoided. The initial milliliter of CSF was discarded and all samples were centrifuged for 15 minutes at 1000 g to prevent blood from contaminating the CSF. Samples with hemoglobin concentrations more than 25 ng/mL were discarded and did not undergo biomarker testing. The amounts of hemoglobin in the human hemoglobin Enzyme-Linked Immunoassay (ELISA) quantitation set (Cat # E80-136) kit (Bethyl Lab Inc, Montgomery, TX) was used to quantify CSF samples in accordance with the manufacturer's instructions.
Assessing the pharmacokinetics of nilotinib using mass spectrometry
After being first thawed on ice at room temperature, 20 µL of plasma and CSF samples were transferred to eppendorf tubes holding 100 µL of water. The sample was mixed with 500 µL of acetonitrile/methanol (50:50) extraction solvent that included the internal standard (5 ng/mL of nilotinib_13C_2H3). To extract unbound or free Nilotinib, the mixture was vortexed and incubated for 20 minutes on ice to speed up protein precipitation and dialysis through 25 µm membranes. Following incubation, the samples were vortexed and centrifuged for 20 minutes at 4°C at 13,000 g. The unbound nilotinib-containing supernatant was rapidly vacuum-dried, then reconstituted in 200 µL of a 50:50 methanol:water mixture, the sample was subjected to Mass Spectrometry (MS) analysis. The samples were resolved online using a triple quadrupole mass spectrometer (Xevo-TQ- S, Waters Corporation) in the Multiple Reaction Monitoring (MRM) mode on an Acquity UPLC Ethylene Bridged Hybrid (BEH) C18 1.7 µm, 2.1 × 50 mm column. Using the "IntelliStart" function of the MassLynx software (Waters corporation), the instrument parameters were adjusted to achieve the highest possible specificity and sensitivity of ionization for the parent (m/z=530.27 nilotinib) and daughter ions (m/z=289.01 nilotinib).
Quantification of DA metabolites
DOPAC and HVA by Liquid Chromatography-Mass Spectroscopy/Mass Spectrometry (LC-MS/MSC) -standardized tissue samples normalized to the internal standard nilotinib_13C_2H3. This allowed for the calculation of the metabolite ratios. Using electrospray tandem mass spectrometry and Ultra-High Performance Liquid Chromatography (UHPLC-MS/MS), the concentrations of DOPAC and HVA in the CSF samples were determined. After the previously mentioned derivatization with benzoyl chloride. 34 A PAL autosampler (CTC Analytics, Switzerland) an Advance UHPLC pump and an EVOQ elite triple quadrupole mass spectrometer (Bruker Daltonics) with an Electrospray Ionization (EPI) source working in a positive mode at +4500 V comprised the UHPLC-MS/MS system, to put it briefly. Probe gas flow of 50, nebulizer gas flow of 60, probe temperature +300°C, cone gas flow of 30, cone temperature +200°C and Collision-Induced Dissociation (CID) gas Ar 1.5 mTorr were the specifications of the source. The benzoyl derivatives were separated using a Luna Omega C18 PS column, measuring 150 × 2.1 mm and 1.6 µm (Phenomenex). Water, 0.1% (v/v) formic acid and 6.7 mmol/L ammonium format made up mobile phase A. Acetonitrile and 0.1% formic acid made up mobile phase B. The gradient elution was 400 µL/min, with the following values (min-% A/%B): 0-98/2; 0.2-98/2; 6-30/70; 7.5-30/70; 7.6-98/2; 8.2-98/2. The internal standards were the stable isotope-labeled DOPAC-d5 and HVA-d3 (Toronto Research Chemicals, Canada). The process of derivatization was carried out at room temperature. The following components were pipetted and the mixture was vigorously shaken after each pipetting step, adding 10 µL of CSF sample or a calibration standard in CSF (148 mmol/L NaCl, 4 mmol/L KCl, 0.8 mmol/L MgCl2, 1.4 CaCl2, 1.2 mmol/L Na2HPO4, 0.3 mmol/L NaH2PO4, pH 7.2). 0.1 mol/L sodium tetraborate buffer, 6 µL of the internal standard mixture (1 µmol/L of each deuterated analyte standard in water) and 6 µL of the internal standard mixture µL freshly produced 1% v/v benzoyl chloride in acetonitrile every day. After five minutes of mixing the resultant solution, 26 µL of 1% formic acid in acetonitrile was added. To minimize the acetonitrile concentration, the samples were centrifuged for 8 minutes at 177.0912 g after being agitated for an additional 3 minutes. 40 µL of the supernatant was then aspirated and the resulting samples were placed in a vacuum centrifuge mini Vac Duo concentrator (Genevac) for 10 minutes. The column was injected with three microliters. The range in which the calibration curves were built was 0.25-16,384 nmol/L. As mentioned below, Pronexus Analytical AB, Bromma, Sweden used mass spectroscopy to assess the amounts of DOPAC and HVA in the CSF samples. ELISA was used to confirm the results.
ELISA-based quantification of DA metabolites human CSF
Samples were used for a solid-phase sandwich ELISA analysis of human HVA. Using solid-phase sandwich ELISA (MyBioSource, Cat# MBS064661), 100 µL of CSF or plasma sample is incubated for 1 hour at 37°C with 100 µL HRP-conjugate reagent. Following washing, the solution is mixed with 50 µL of chromogen solution A and 50 µL of chromogen solution B and it is incubated for 15 minutes at 37°C. At 450 nm, the optical density is measured and the process is terminated using 50 µL of stop solution. The amount of CSF and plasma HVA is correlated with the absorbance's magnitude. 2.7 | Total alpha-synuclein as determined by ELISA CSF was subjected to a solid phase alpha-synuclein sandwich ELISA (Cat#SIG38974, Biolegend) gaseous. Following LP and blood draws, 15 ml of CSF and 5 ml of plasma were aliquoted on ice into 0.5 ml tubes and maintained at -80°C to prevent freeze-thaw cycles. For the ELISA, fresh aliquots were utilized. The same reagents were used to analyze each sample side by side. After coating the microwells with a rabbit monoclonal antibody against total alpha-synuclein (amino acids 118-123), 200 µL of CSF or plasma was applied to the specified wells. Plasma samples were diluted 1:50 and CSF samples at 1:10, respectively. The coated antibody bound to alpha-synuclein after the sample was incubated at 4°C for the entire night. To identify the captured alpha-synuclein (amino acids 118-123), a biotinylated mouse monoclonal alpha-synuclein (amino acids 103-107) detection antibody was applied to each well after washing. The samples were cultured using 50 µL of the detecting antibody at room temperature for two hours. To identify the bound biotinylated detection antibody, 200 µL of streptavidin Horseradish Peroxidase (HRP) was added and incubated for an hour at room temperature after washing. Following a wash, 100 µL of chemiluminescent substrates were added to the samples and incubated. After shaking the plates for ten to fifteen seconds, a luminometer read them right away.
Oligomeric alpha-synuclein quantification via ELISA CSF
It was subjected to a solid phase human alpha-synuclein oligomer sandwich ELISA (Cat# MBS730762, Mybiosource). Following LP and blood draws, 15 ml of CSF and 10 mL of plasma were aliquoted on ice into 0.5 ml tubes and maintained at -80°C to prevent freeze-thaw cycles. For the ELISA, fresh aliquots were utilized. Every sample was examined side by side utilizing the same chemicals. To the corresponding wells, 50 microliters of standards or CSF samples were introduced. Samples only received five microliters of the balancing solution, which was thoroughly mixed. Each well received 100 microliters of conjugate added to it. After thoroughly mixing the sample solution, it was incubated at 37°C for one hour. Following washing, 50 µL of substrate A and 50 µL of substrate B were added to each well, along with a blank control well. 50 µL of stop solution was added to each well after the sample solution was incubated for ten to fifteen minutes at 37°C. a good blank control. Samples were immediately scanned at 450 nm using a microplate reader to determine Optical Density (OD).
Xmap multiplex magnetic microspheres with two fluorescent dyes internally coded are used in TREM2 ELISA Xmap technology. Multiple proteins inside a sample can be examined simultaneously by carefully combining these two colors. These spheres are all coated in a different capture antibody. The reaction on the bead's surface is finished when the capture antibody binds to the detection antibody and a reporter molecule. The same reagents were used to analyze each sample concurrently. A mixture bead solution comprising 25 µL of TREM2 (Millipore Cat# HNS2MAG-95K) was incubated overnight at 4°C with 25 microliters of human CSF or plasma. Samples were cleaned and then incubated using 25 µL of the detection antibody solution was left at room temperature for 1.5 hours. Each well containing 25 µL of the detection antibody solution received an addition of streptavidin-phycoerythrin (25 µL). Following a wash, the samples were suspended in 100 µL of sheath fluid. Next, samples were processed using Xponent software on MAGPIX. TREM-2 concentrations in samples were determined by analyzing the Median Fluorescence Intensity (MFI) data using a five-parameter logistic or spline curve-fitting approach.
Measuring Phosphorylated Neurofilament-H (pNF-H) CSF
Samples from the current popPK/PD RSD investigation and a prior phase I open-label study were used for solid phase pNF-H sandwich ELISA (CAT# NS170), Biolegend, on baseline and 6-month-old PD and DLB patient samples (see study description in reference 16). Preventing freeze-thaw cycles, 15 ml of CSF was aliquoted on ice into 0.5 ml tubes and frozen at -80°C right after LP and blood draw. For the ELISA, fresh aliquots were utilized. The same reagents were used to analyze each sample side by side. Before adding 50 µL of standards to the wells, Tris Buffered Saline (TBS) was added and then withdrawn. The plate was gently shaken and allowed to incubate for one hour at room temperature. Each well received 100 µL of the diluted anti-pNF-H antibody after washing. The plate was gently shaken and allowed to incubate for one hour at room temperature. Following the pNF-H antibody incubation period, the samples underwent a washing step and 100 µL of the diluted goat anti-rabbit polyclonal antibody conjugated with alkaline phosphatase was introduced into each well. The dish was gently shaken and incubated for 1 hour at room temperature. Rinse with 200 µL of 1 × TBS. After that, each well received 100 microliters of newly diluted 1 × p-Nitrophenyl Phosphate (pNPP) alkaline phosphatase substrate, which was left to incubate for 30 to 60 minutes at room temperature in the dark. At 405 nm, the plate was read right away and each well received 50 µL of 3N NaOH to halt the reaction.
Data analysis and experimental design: A single daily dosage of either 150 mg, 200 mg, 300 mg or 400 mg of placebo or five groups (n=15; 1:1:1:1:1) of nilotinib, was administered to patients (N=75) in an open-label RSD research (Figure 1). Given that nilotinib can be detected up to four hours after taking a medication administration in PD and DLB patients, 16 Lumbar Punctures (LP) and blood collection were spaced out over one to four hours (Figure 1), with blood being taken 30 minutes before LP. Important biomarkers were TREM-2, alpha-synuclein, HVA, DOPAC, CSF dopamine metabolites and various doses were contrasted with a placebo. To ascertain if a single dosage of nilotinib would alter CSF levels of biomarkers in a time-dependent way, additional exploratory research was conducted to examine changes in biomarkers at each time point when CSF was collected. When comparing different doses with a placebo, the data are reported as mean ± SEM (Standard Error of the Mean) for n=15 in each group. Version 5.01 of Graph Pad Prism Software was used to create all graphs and conduct statistical analysis (Graph CA-based Pad Prism Software, Inc). All estimations for means and Standard Deviation (SD) were based on the mean of relative changes rather than the mean value changes in order to account for group differences. The shapiro-wilk test was used to determine whether the biomarkers were normal, with 15 participants in each dosing group. To compare, we looked at the mean differences between each nilotinib therapy and the placebo using a one-tailed welch t-test. The sample allocation for pharmacokinetics was done at random for each group one, two, three or four hours after dosage. In order to gain a deeper understanding of the impact of nilotinib on biomarkers at every stage of CSF collection, we also conducted an exploratory study. However, because of the small sample size (n=3-4), we needed in order to increase power while maintaining statistical significance, a one-tailed welch t-test may be used. A one-sided P value of less than 0.05 was deemed statistically significant. Because of the very small sample size in relation to the number of biomarkers, multiple test correction was not taken into consideration. Each figure shows the sample size (n) and P-values for each test. In every HVA and DOPAC trial, n=4 was used for every 1-hour, n=4 for every 2-hour and n=4 for every 3-hour time point-with the exception of the 200 mg experiment, where n=3. For every 4-hour time point, N=3. For both oligomeric and total CSF alpha-synuclein time points, n=4 (with the exception of the 4-hour group, n=3) and the 1-hour 200 mg group, n=3. 400 mg at 2-hour n=4 and 300 mg at 3-hour n=3, with the exception of 400 mg. For our exploratory time-point study, we have a total of 17 time points with n=3 and 67 time points with n=4.
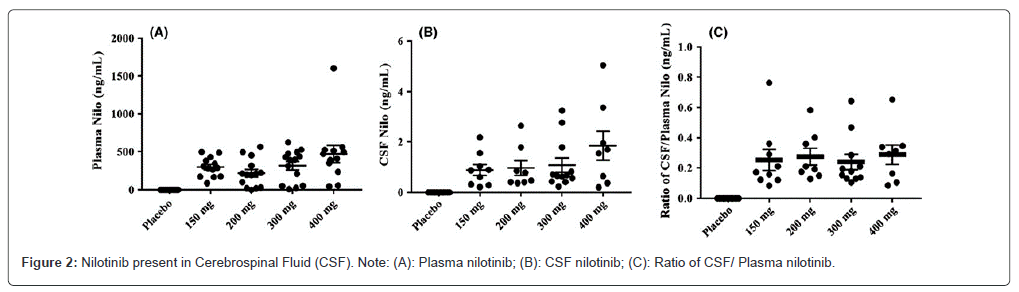
Nilotinib pharmacokinetics in plasma and CSF the concentration of nilotinib found in the plasma (Figure 2A) was consistent across all nilotinib dosage groups and nilotinib was not detectable in the plasma of the placebo group. There was one patient who was an outlier in the 400 mg nilotinib group. Comparing all groups to the placebo group, the level of nilotinib in the CSF (Figure 2B) was similar, with an increase in outliers observed with higher doses. According to these findings, nilotinib enters the brain without regard to dosage. The unbound CSF to plasma ratio nilotinib (Figure 2C) appears to be the same in all dose groups and an average of 0.5-1% nilotinib is detected in the CSF, suggesting that nilotinib crosses the BBB and is detected as free or unbound in the CSF at low concentrations.
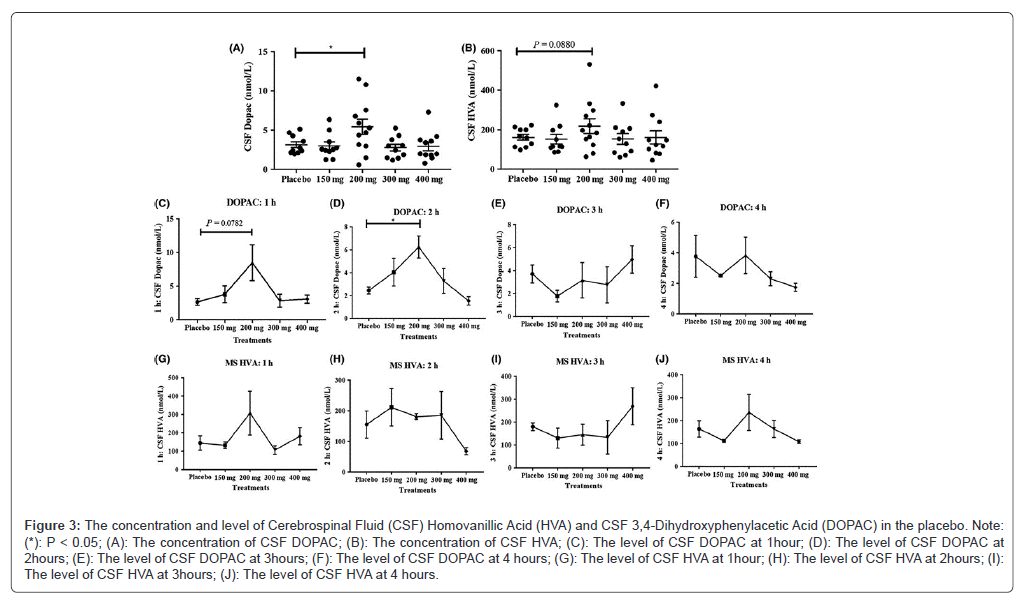
Nilotinib's effects on the metabolism of DA HVA and DOPAC concentrations of primary metabolites of DA were evaluated in the CSF in order to assess the impact of nilotinib on DA metabolism in the brain. All data were normalized to 200 mg levodopa, the lowest dose that PD participants were typically receiving, in order to normalize the impact of levodopa medication on the levels of DA metabolism. In comparison to the placebo and all other groups, the 200 mg nilotinib group (n=15) had a significantly higher concentration of CSF DOPAC obtained 1-4 hours after nilotinib dosage (Figure 3A). No significant differences were seen in the concentration of CSF HVA collected 1-4 hours after nilotinib dose across all treatment groups indicated a tendency toward growth in the 200 mg group (Figure 3B). In the CSF of participants receiving nilotinib, additional exploratory analysis of a time-dependent change in HVA and DOPAC shows that DOPAC rises at 1 hour (Figure 3C) and peaks to a significantly high level at 2 hours (Figure 3D) post-nilotinib administration in comparison with placebo. Following the delivery of nilotinib, DOPAC levels remained unaltered 3 hours (Figure 3E) and 4 hours (Figure 3F). Moreover, the HVA concentration remained constant throughout all time points (Figure 3G-J). The placebo group's CSF levels of HVA and DOPAC, however were constant over the course of the study, indicating that the removal of MOA-B inhibitors and the adjustment of levodopa levels to 200 mg in all treatment groups served as a control quantify the DA metabolites following nilotinib therapy. In every HVA and DOPAC trial, n=4 was used for every 1-hour, n=4 for every 2-hour and n=4 for every 3-hour time point with the exception of the 200 mg experiment, where n=3. For every 4-hour time point, N=3.
Figure 3: The concentration and level of Cerebrospinal Fluid (CSF) Homovanillic Acid (HVA) and CSF 3,4-Dihydroxyphenylacetic Acid (DOPAC) in the placebo. Note: (*): P < 0.05; (A): The concentration of CSF DOPAC; (B): The concentration of CSF HVA; (C): The level of CSF DOPAC at 1hour; (D): The level of CSF DOPAC at 2hours; (E): The level of CSF DOPAC at 3hours; (F): The level of CSF DOPAC at 4 hours; (G): The level of CSF HVA at 1hour; (H): The level of CSF HVA at 2hours; (I): The level of CSF HVA at 3hours; (J): The level of CSF HVA at 4 hours.
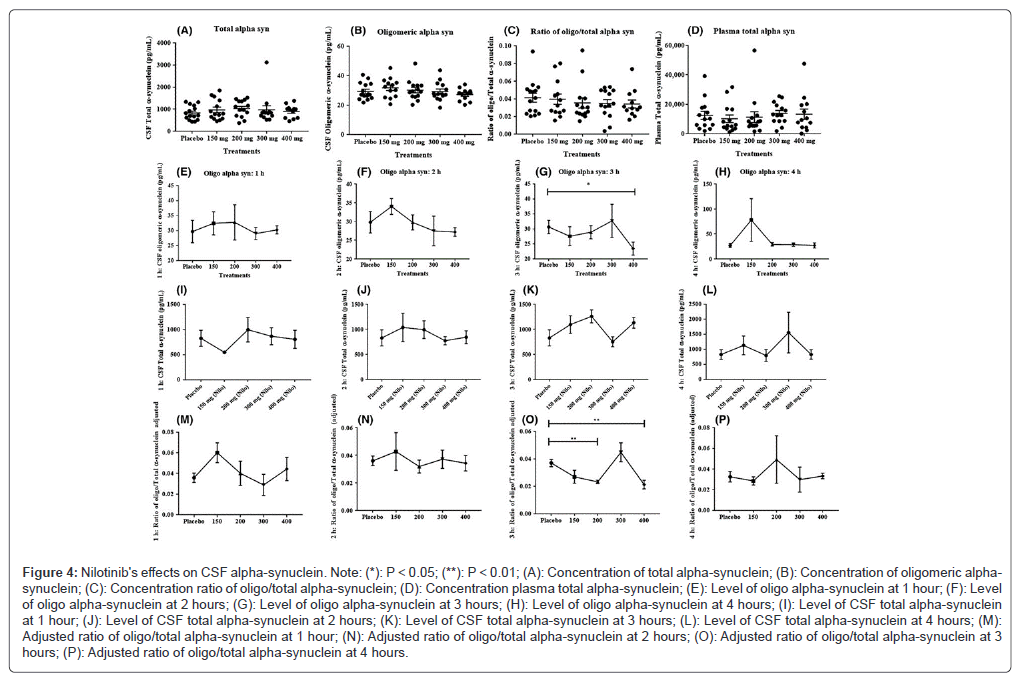
Nilotinib's effects on CSF alpha-synuclein: All nilotinib-treated groups (n=15) compared with placebo had the same concentration of total alpha-synuclein in the CSF (Figure 4A) and oligomeric alpha-synuclein in the same concentration in the CSF (n=15) compared with placebo (Figure 4B). The oligomeric: total alpha-synuclein ratio in the plasma (Figure 4C) and CSF (Figure 4D) did not differ from one another. Additional exploratory assessment of oligomeric alpha-synuclein levels in CSF throughout time demonstrates that oligomeric alpha-synuclein remains unchanged at 1 hour (Figure 4E), 2 hours (Figure 4F) and 4 hours (Figure 4G) following nilotinib administration, however after 3 hours of nilotinib dosage, a notable decrease in oligomeric alpha-synuclein was seen in the 400 mg group (Figure 4H). In the case of CSF total alpha-synuclein and oligomeric alpha-synuclein, time points n=4 (except from at 4 hours n=3), 200 mg group n=3 at hour 1, 400 mg group n=4 and 300 mg group n=3 at hour 2 and hour 3. When comparing the effects of nilotinib with a placebo, there were no changes in total CSF alpha-synuclein in any of the groups or time periods (Figure 4I-L). At one hour (Figure 4M) and two hours, the ratio of oligomeric: total CSF alpha-synuclein remained unchanged. (Figure 4N) and 4 hours (Figure 4O) following nilotinib dosage, nevertheless, in the 200 mg (Figure 4P) and 400 mg (Figure 4O) groups, there was a notable decrease in the oligomeric, total alpha-synuclein ratio in contrast to the placebo. Nilotinib's effects on plasma alpha-synuclein after one hour of nilotinib administration, the plasma concentration of total alpha-synuclein did not change in any of the nilotinib-treated groups (Figure 5A) when compared to the placebo. When compared to the placebo, the 150 mg group's plasma total alpha-synuclein dramatically decreased (Figure 5B) and trended downward in the 200 mg group. When compared to placebo, there was no discernible change in the overall levels of alpha-synuclein at 3 hours (Figure 5C) or 4 hours (Figure 5D). Every time point n=4 for plasma alpha-synuclein (except from 4 hours n=3). There was no oligomeric alpha-synuclein found in the plasma.
Figure 4: Nilotinib's efects on CSF alpha-synuclein. Note: (*): P < 0.05; (**): P < 0.01; (A): Concentration of total alpha-synuclein; (B): Concentration of oligomeric alphasynuclein; (C): Concentration ratio of oligo/total alpha-synuclein; (D): Concentration plasma total alpha-synuclein; (E): Level of oligo alpha-synuclein at 1 hour; (F): Level of oligo alpha-synuclein at 2 hours; (G): Level of oligo alpha-synuclein at 3 hours; (H): Level of oligo alpha-synuclein at 4 hours; (I): Level of CSF total alpha-synuclein at 1 hour; (J): Level of CSF total alpha-synuclein at 2 hours; (K): Level of CSF total alpha-synuclein at 3 hours; (L): Level of CSF total alpha-synuclein at 4 hours; (M): Adjusted ratio of oligo/total alpha-synuclein at 1 hour; (N): Adjusted ratio of oligo/total alpha-synuclein at 2 hours; (O): Adjusted ratio of oligo/total alpha-synuclein at 3 hours; (P): Adjusted ratio of oligo/total alpha-synuclein at 4 hours.
Figure 5: Nilotinib's effects on plasma alpha-synuclein. Note: (*): P < 0.05; (A): The plasma concentration of total alpha-synuclein at 1 hour; (B): The plasma concentration of total alpha-synuclein at 2 hours; (C): The plasma concentration of total alpha-synuclein at 3 hours; (D): The plasma concentration of total alpha-synuclein at 4 hours; (E): Graph shows Triggering Receptor Expressed on Myeloid cells (TREM-2) levels in the CSF; (F): Graph shows TREM-2 levels in the plasma; (G): Graph shows the Cerebrospinal Fluid levels of phosphorylated neurofilaments between baseline and after 6-months treatment with 150 mg Nilotinib; (H): Graph shows the CSF levels of phosphorylated neurofilaments between baseline and after 6-months treatment with 300 mg Nilotinib.
Nilotinib's effects on the CSF's soluble TREM-2 Figure 5E, n = 15, illustrates a marginal (10%) rise in the CSF level of soluble TREM-2 in the 150 mg nilotinib group when compared to the placebo and the TREM-2 levels are noticeably higher in the 200 mg group (n=15) than in the placebo group. When comparing the 300 and 400 mg nilotinib groups (n=15 per group) to the placebo group, nothing changed. The results of this investigation indicate that TREM-2 plasma levels were not affected by nilotinib at the dose employed, indicating that soluble CSF TREM-2 is affected by nilotinib regardless of its plasma levels (Figure 5F). As anticipated, all groups in the current investigation had the same amount of p-NF-H following a single dosage of nilotinib (data not shown). In order to illustrate how nilotinib affects cell death markers, we examined the levels of p-HF-H in CSF samples taken from a prior trial following 150 mg (n=5) of treatment. Additionally for six months, 300 mg (n=7) of nilotinib. Sixteen According to earlier findings, nilotinib may considerably lower CSF levels of Neuron-Specific Enolase (NSE) and S100B, which are indicators of glial and neuronal cell death, respectively. Sixteen examination of CSF samples indicates a significant decrease in the level of the cell death biomarker p-NF-H between baseline and 6-month treatment with 150 mg (Figure 5G) and 300 mg (Figure 5H) of nilotinib, indicating the possibility of neuroprotective benefits from nilotinib treatment.
Discussion
According to the data, nilotinib reaches the brain and modifies PD surrogate biomarkers in a way that is independent of dose. All dosing groups' CSFs showed the same level of unbound free nilotinib, indicating that the medication target (c-Abl, DDRs, etc.) might act as a sink for nilotinib in the brain. As we previously demonstrated, nilotinib was found in human CSF 4 hours after treatment, while CSF Abl inhibition persisted for up to 6 hours [16]. This suggests that the amount of nilotinib present in free CSF may not be the entire amount of medication that reaches the brain. Strong and irreversible binding to its target is exhibited by nilotinib and through its interaction with tyrosine kinase inhibitors, the amount of nilotinib in CSF may be indicative of the dynamic pharmacological characteristics of these agents. The p-glycoproteins (PgP), the Adenosine Triphosphate (ATP)-binding cassette efflux transporters and maybe aquaporin channels, contingent on a number of variables, including as individual genetic polymorphisms, gender, ethnicity and metabolic parameters [33-36]. The Novartis Investigator Brochure (IB) states that nilotinib is a PgP inhibitor with an IC50 of 1.7 µmol/L and considerable passive permeability, however, considering its low Cmax of 0.06 µmol/L, this is probably not significant at the BBB. Moreover, our experiments on mice demonstrated that the concentration of nilotinib in the brain rose in a way that was not proportionate to the dose. Plasma concentrations in humans were comparable across the board for dosages between 150 and 400 mg. The dose had no effect on CSF levels or CSF/plasma ratios. This makes sense given that plasma levels remain consistent between dosages, as the dispersion of nilotinib inside the brain amounts are dependent on plasma. The drug's effect at multiple targets, particularly at higher (400 mg) concentrations, may be the cause of the apparent dose-independent effect of nilotinib on potential disease biomarkers in the CSF. This suggests that multi-target engagement abrogates nilotinib action and may result in more side-and off-target effects in the Central Nervous System (CNS). Nilotinib may bind to c-Abl and/or DDR1/2 more specifically at lower concentrations, which may be the ideal way to strike a compromise between drug level in the CNS and target engagement. Similar to the effects of nilotinib in this investigation, we have demonstrated in multiple experimental models that knockdown of either Abl or DDRs alone leads to decrease of alpha-synuclein and protection of DA neurons. High binding is shown by nilotinib. It has a high affinity and can effectively inhibit DDRs (IC50 at 3-6 nmol/L) and Abl (IC50<30 nmol/L). Kinase receptor, which nilotinib potently targets could cause changes in TREM-2 signaling [4,5]. Postmortem Parkinson's disease brains have higher levels of DDRs and nilotinib improves the clearance of neurotoxic proteins, attenuates cell death and decreases TREM-2+microglia [37], indicating the modulation of myeloid-derived microglia and defense against neurotoxic proteins. Although oligomeric alpha-synuclein was not found in the plasma, we did find that lower single doses of nilotinib (150 and 200 mg) had a notable impact on overall alpha-synuclein levels. Patients with Parkinson's Disease (PD) have high blood levels of alpha-synuclein, which nilotinib considerably lowered [37-41]. These outcomes are in line with our earlier research, which showed that nilotinib decreased mice's blood levels of alpha-synuclein [7]. There is proof that PD patients' blood has dysregulated autophagy [42-45] and nilotinib's effects on plasma alpha-synuclein may be explained by its effects on autophagy. However, using the same technique and parameters as for the detection of plasma alpha-synuclein, a single dose of nilotinib showed no effect on the total CSF alpha-synuclein level. Three hours following the delivery of nilotinib, there was an apparent alteration in the levels of CSF oligomeric alpha-synuclein, especially at lower values. These findings are in line with elevated CSF concentrations of TREM-2, which improves microglia's capacity for phagocytosis and reduces inflammation [46] This suggests that elevated phagocytosis could be the cause of the decrease in oligomeric alpha-synuclein. In multiple models of dementia, we have shown that nilotinib improves motor and cognitive symptoms while promoting autophagic clearance of neurotoxic proteins without causing increased inflammation [6,7,9,11,13,47-49]. CSF alpha-synuclein measurement in conjunction with other PD-related biomarkers such as TREM2- and DA metabolism could confirm the usefulness of CSF and/or plasma alpha-synuclein as a biomarker for certain therapeutic effects such as nilotinib, rather than for the diagnosis or development of Parkinson's disease. Although oligomeric CSF alpha-synuclein varies somewhat, its relationship to TREM-2, DA metabolism and plasma alpha-synuclein implies that oligomeric alpha-synuclein, which longitudinally rises in the CSF of PD patients, may be a reliable indicator of how nilotinib affects PD-related biomarker changes over the course of prolonged drug exposure [17,19-21,50-52].
Conclusion
Nilotinib enters the brain in a dose-independent manner and 200 mg. Nilotinib increases the level of 3,4-Dihydroxyphenylacetic Acid (DOPAC) and Hom Vanillic Acid (HVA), suggesting alteration to dopamine metabolism. Nilotinib significantly reduces plasma total alpha-synuclein and appears to reduce CSF oligomeric: total alpha-synuclein ratio. Furthermore, nilotinib significantly increases the CSF level of Triggering Receptors on Myeloid cells (TREM-2), suggesting an anti-inflammatory effect. Taken together, 200 mg nilotinib appears to be an optimal single dose that concurrently reduces inflammation and engages surrogate disease biomarkers, including dopamine metabolism and alpha-synuclein. As a result, our study shows that nilotinib crosses the blood-brain barrier and is found in the CSF regardless of concentration. An ideal dosage of 200 mg nilotinib may concurrently alter DA metabolism, lower oligomeric alpha-synuclein and increase of TREM-2 concentrations in the CSF.
References
- Emelyanov A, Kulabukhova D, Garaeva L, Senkevich K, Verbitskaya E, et al. (2018) SNCA variants and alpha-synuclein level in CD45+ blood cells in Parkinson’s disease. J Neurol Sci 395:135-140.
[Crossref] [Google Scholar] [PubMed]
- Macchi B, Di Paola R, Marino-Merlo F, Rosa Felice M, Cuzzocrea S, et al. (2015) Inflammatory and cell death pathways in brain and peripheral blood in Parkinson’s disease. CNS Neurol Disord Drug Targets 14(3):313-324.
[Crossref] [Google Scholar] [PubMed]
- Miki Y, Shimoyama S, Kon T, Ueno T, Hayakari R, et al. (2018) Alteration of autophagy-related proteins in peripheral blood mononuclear cells of patients with Parkinson's disease. Neurobiol Aging 63:33-43.
[Crossref] [Google Scholar] [PubMed]
- Papagiannakis N, Xilouri M, Koros C, Stamelou M, Antonelou R, et al. (2015) Lysosomal alterations in peripheral blood mononuclear cells of Parkinson's disease patients. Mov Disord 30(13):1830-1834.
[Crossref] [Google Scholar] [PubMed]
- Schwienbacher C, Foco L, Picard A, Corradi E, Serafin A, et al. (2017) Plasma and white blood cells show different miRNA expression profiles in Parkinson’s disease. J Mol Neurosci 62(2):244-254.
[Crossref] [Google Scholar] [PubMed]
- Wu G, Wang X, Feng X, Zhang A, Li J, et al. (2011) Altered expression of autophagic genes in the peripheral leukocytes of patients with sporadic Parkinson's disease. Brain Res 1394:105-111.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Feng S, Nie K, Li Y, Gao Y, et al. (2018) TREM2 modulates microglia phenotypes in the neuroinflammation of Parkinson's disease. Biochem Biophys Res Commun 499(4):797-802.
[Crossref] [Google Scholar] [PubMed]
- Hebron ML, Lonskaya I, Olopade P, Selby ST, Pagan F, et al. (2014) Tyrosine kinase inhibition regulates early systemic immune changes and modulates the neuroimmune response in α-synucleinopathy. J Clin Cell Immuno 5:259.
[Crossref] [Google Scholar] [PubMed]
- Lonskaya I, Hebron ML, Selby ST, Turner RS, Moussa CH (2015) Nilotinib and bosutinib modulate pre-plaque alterations of blood immune markers and neuro-inflammation in Alzheimer’s disease models. Neuroscience 304:316-327.
[Crossref] [Google Scholar] [PubMed]
- Moussa CE (2015) Parkin is dispensable for mitochondrial function, but its ubiquitin ligase activity is critical for macroautophagy and neurotransmitters: therapeutic potential beyond Parkinson's disease. Neurodegener Dis 15(5):259-270.
[Crossref] [Google Scholar] [PubMed]
- Eusebi P, Giannandrea D, Biscetti L, Abraha I, Chiasserini D, et al. (2017) Diagnostic utility of cerebrospinal fluid α‐synuclein in Parkinson's disease: a systematic review and meta‐analysis. Mov Disord 32(10):1389-1400.
[Crossref] [Google Scholar] [PubMed]
- Shahnawaz M, Tokuda T, Waragai M, Mendez N, Ishii R, et al. (2017) Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 74(2):163-172.
[Crossref] [Google Scholar] [PubMed]
- Karuppagounder SS, Brahmachari S, Lee Y, Dawson VL, Dawson TM, et al. (2014) The c-Abl inhibitor, nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson's disease. Sci Rep 4(1):4874.
[Crossref] [Google Scholar] [PubMed]
- Lonskaya I, Desforges NM, Hebron ML, Moussa CE (2013) Ubiquitination increases parkin activity to promote autophagic α-synuclein clearance. PLoS One 8(12):e83914.
[Crossref] [Google Scholar] [PubMed]
- Lonskaya I, Hebron M, Chen W, Schachter J, Moussa (2014) Tau deletion impairs intracellular β-amyloid-42 clearance and leads to more extracellular plaque deposition in gene transfer models. Mol Neurodegener 9:46.
[Crossref] [Google Scholar] [PubMed]
- Lonskaya I, Hebron ML, Desforges NM, Franjie A, Moussa CE (2013) Tyrosine kinase inhibition increases functional parkin‐B eclin‐1 interaction and enhances amyloid clearance and cognitive performance. EMBO Mol Med 5(8):1247-1262.
[Crossref] [Google Scholar] [PubMed]
- Mahul-Mellier AL, Fauvet B, Gysbers A, Dikiy I, Oueslati A, et al. (2014) c-Abl phosphorylates α-synuclein and regulates its degradation: implication for α-synuclein clearance and contribution to the pathogenesis of Parkinson's disease. Hum Mol Genet 23(11):2858-2879.
[Crossref] [Google Scholar] [PubMed]
- Salomoni P, Calabretta B (2009) Targeted therapies and autophagy: new insights from chronic myeloid leukemia. Autophagy 5(7):1050-1051.
[Crossref] [Google Scholar] [PubMed]
- Pagan F, Hebron M, Valadez EH, Torres-Yaghi Y, Huang X, et al. (2016) Nilotinib effects in Parkinson’s disease and dementia with Lewy bodies. J Parkinsons Dis 6(3):503-517.
[Crossref] [Google Scholar] [PubMed]
- El‐Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, et al. (2006) Detection of oligomeric forms of α‐synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J 20(3):419-425.
[Crossref] [Google Scholar] [PubMed]
- Mollenhauer B, El-Agnaf OM, Marcus K, Trenkwalder C, Schlossmacher MG (2010) Quantification of α-synuclein in cerebrospinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomark Med 4(5):683-699.
[Crossref] [Google Scholar] [PubMed]
- Park MJ, Cheon SM, Bae HR, Kim SH, Kim JW (2011) Elevated levels of α-synuclein oligomer in the cerebrospinal fluid of drug-naïve patients with Parkinson's disease. J Clin Neurol 7(4):215-222.
[Crossref] [Google Scholar] [PubMed]
- Majbour NK, Vaikath NN, Eusebi P, Chiasserini D, Ardah M, et al. (2016) Longitudinal changes in CSF alpha‐synuclein species reflect Parkinson's disease progression. Mov Disord 31(10):1535-1542.
[Crossref] [Google Scholar] [PubMed]
- Majbour NK, Vaikath NN, Van Dijk KD, Ardah MT, Varghese S, et al. (2016) Oligomeric and phosphorylated alpha-synuclein as potential CSF biomarkers for Parkinson’s disease. Mol Neurodegener 11:1-5.
[Crossref] [Google Scholar] [PubMed]
- Goldstein DS, Holmes C, Sullivan P, Jinsmaa Y, Kopin IJ, et al. (2016) Elevated cerebrospinal fluid ratios of cysteinyl-dopamine/3, 4-dihydroxyphenylacetic acid in parkinsonian synucleinopathies. Parkinsonism Relat Disord 31:79-86.
[Crossref] [Google Scholar] [PubMed]
- Herbert MK, Kuiperij HB, Bloem BR, Verbeek MM (2013) Levels of HVA, 5-HIAA and MHPG in the CSF of vascular parkinsonism compared to Parkinson’s disease and controls. J Neurol 260:3129-3133.
[Crossref] [Google Scholar] [PubMed]
- Benitez BA, Cruchaga C (2013) TREM2 and neurodegenerative disease. N Engl J Med 369(16):1567-1568.
[Crossref] [Google Scholar] [PubMed]
- Jonsson T, Stefansson K (2013) TREM2 and neurodegenerative disease. N Engl J Med 369(16):1568-1569.
[Crossref] [Google Scholar] [PubMed]
- Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, et al. (2013) TREM2 in neurodegeneration: evidence for association of the p. R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener 8(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Zhang D, Pang H, Caudle WM, Li Y, et al. (2008) Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J Immunol 181(10):7194-7204.
[Crossref] [Google Scholar] [PubMed]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, et al. (2003) NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A 100(10):6145-6150.
[Crossref] [Google Scholar] [PubMed]
- Sims R, Van Der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, et al. (2017) Rare coding variants in PLCG2, ABI3 and TREM2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat Genet 49(9):1373-1384.
[Crossref] [Google Scholar] [PubMed]
- Garcia-Reitboeck P, Phillips A, Piers TM, Villegas-Llerena C, Butler M, et al. (2018) Human induced pluripotent stem cell-derived microglia-like cells harboring TREM2 missense mutations show specific deficits in phagocytosis. Cell Rep 24(9):2300-2311.
[Crossref] [Google Scholar] [PubMed]
- Hong S, Stevens B (2017) TREM2: keeping microglia fit during good times and bad. Cell Metab 26(4):590-591.
[Crossref] [Google Scholar] [PubMed]
- Sasaki A (2017) Microglia and brain macrophages: An update. Neuropathology 37(5):452-464.
[Crossref] [Google Scholar] [PubMed]
- Song P, Mabrouk OS, Hershey ND, Kennedy RT (2012) In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography–mass spectrometry. Anal Chem 84(1):412-419.
[Crossref] [Google Scholar] [PubMed]
- Bi C, Tham DK, Perronnet C, Joshi B, Nabi IR, et al. (2017) The oxidative stress-induced increase in the membrane expression of the water-permeable channel aquaporin-4 in astrocytes is regulated by caveolin-1 phosphorylation. Front Cell Neurosci 11:412.
[Crossref] [Google Scholar] [PubMed]
- Hegedűs C, Özvegy‐Laczka C, Apati A, Magocsi M, Nemet K, et al. (2009) Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti‐cancer effects and pharmacological properties. Br J Pharmacol 158(4):1153-1164.
[Crossref] [Google Scholar] [PubMed]
- Hira D, Terada T (2018) BCRP/ABCG2 and high-alert medications: Biochemical, pharmacokinetic, pharmacogenetic and clinical implications. Biochem Pharmacol 147:201-210.
[Crossref] [Google Scholar] [PubMed]
- Parkinson Study Group (1995) Cerebrospinal fluid homovanillic acid in the DATATOP study on Parkinson’s disease. Arch Neurol 52(3):237-45.
[Crossref] [Google Scholar] [PubMed]
- Stefani A, Pierantozzi M, Olivola E, Galati S, Cerroni R, et al. (2017) Homovanillic acid in CSF of mild stage Parkinson's disease patients correlates with motor impairment. Neurochem Int 105:58-63.
[Crossref] [Google Scholar] [PubMed]
- Ulland TK, Colonna M (2018) TREM-a key player in microglial biology and Alzheimer disease. Nat Rev Neurol 14(11):667-675.
[Crossref] [Google Scholar] [PubMed]
- Jay TR, von Saucken VE, Landreth GE (2017) TREM2 in neurodegenerative diseases. Mol Neurodegener 12(1):1-33.
[Crossref] [Google Scholar] [PubMed]
- Konishi H, Kiyama H (2018) Microglial TREM2/DAP12 signaling: a double-edged sword in neural diseases. Front Cell Neurosci 12:206.
[Crossref] [Google Scholar] [PubMed]
- Lill CM, Rengmark A, Pihlstrøm L, Fogh I, Shatunov A, et al. (2015) The role of TREM2 R47H as a risk factor for Alzheimer's disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis and Parkinson's disease. Alzheimers Dement 11(12):1407-1416.
[Crossref] [Google Scholar] [PubMed]
- Sayed FA, Telpoukhovskaia M, Kodama L, Li Y, Zhou Y, et al. (2018) Differential effects of partial and complete loss of TREM2 on microglial injury response and tauopathy. Proc Natl Acad Sci U S A 115(40):10172-10177.
[Crossref] [Google Scholar] [PubMed]
- Belloli S, Pannese M, Buonsanti C, Maiorino C, Di Grigoli G, et al. (2017) Early upregulation of 18-kDa translocator protein in response to acute neurodegenerative damage in TREM2-deficient mice. Neurobiol Aging 53:159-168.
[Crossref] [Google Scholar] [PubMed]
- Ren M, Guo Y, Wei X, Yan S, Qin Y, et al. (2018) TREM2 overexpression attenuates neuroinflammation and protects dopaminergic neurons in experimental models of Parkinson's disease. Exp Neurol 302:205-213.
[Crossref] [Google Scholar] [PubMed]
- Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, et al. (2010) TREM2-and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal 3(122):ra38.
[Crossref] [Google Scholar] [PubMed]
- Hebron M, Peyton M, Liu X, Gao X, Wang R, et al. (2017) Discoidin domain receptor inhibition reduces neuropathology and attenuates inflammation in neurodegeneration models. J Neuroimmunol 311:1-9.
[Crossref] [Google Scholar] [PubMed]
- Caranci G, Piscopo P, Rivabene R, Traficante A, Riozzi B, et al. (2013) Gender differences in Parkinson’s disease: focus on plasma alpha-synuclein. J Neural Transm (Vienna) 120(8):1209-1215
[Crossref] [Google Scholar] [PubMed]
- Emamzadeh FN, Surguchov A (2018) Parkinson’s disease: biomarkers, treatment and risk factors. Front Neurosci 12:612.
[Crossref] [Google Scholar] [PubMed]
Citation: Sonwani HP, Sahu P, Bandey R (2023) Nilotinib Single-Dose Pharmacokinetics and Pharmacodynamics in Parkinson's Disease Patients. J Alzheimers Dis Parkinsonism 13:586. DOI: 10.4172/2161-0460.1000586
Copyright: © 2023 Sonwani HP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1624
- [From(publication date): 0-2024 - Dec 04, 2025]
- Breakdown by view type
- HTML page views: 1301
- PDF downloads: 323