New Synergistic Potential of Silver Nanoparticles and it is Application in Pharmaceutical Production Areas
Received: 06-Dec-2018 / Accepted Date: 29-Dec-2018 / Published Date: 07-Jan-2019 DOI: 10.4172/2155-952X.1000288
Abstract
Aim of work: Silver nanoparticles play a vital role in the development of new antimicrobial substances against a number of pathogenic microorganisms. Assessment of the antimicrobial activity of biosynthesized silver nanoparticles (AgNPs) was achieved as an eco-friendly and cost-effective method alternative to complex chemical and physical techniques. Nanoparticles due to their smaller size could be very effective as they can improve the antibacterial activity through lysis of bacterial cell wall.
Methods: In the present work evaluation of extracellular biosynthesis of AgNPs by extracellular supernatant of Acinetobacter baumannii as a reducing agent was studied. Silver nanoparticles were characterized by means of UV-visible spectroscopy, Fourier Transform Infrared (FTIR), Scanning Electron Microscopy (SEM) and Energy dispersive X-ray microanalysis (EDX). The synthesized AgNPs were evaluated for their antibacterial activity against common pharmaceutical contaminants. The standard microorganisms designated for the study of antibacterial activity were Escherichia coli ATCC 8739, Salmonella typhimurium ATCC 6538, Klebsiella pneumonia ATCC 10031, Pseudomonas aeruginosa ATCC 15442, Bacillus spizizenii ATCC 6633, Staphylococcus aureus ATCC 6538, Candida albicans ATCC 10231 and Aspergillus niger ATCC 16404.
Results: FTIR analysis showed possible reducing the silver salt and stabilization of nanoparticles. The AgNPs surface morphology revealed from SEM images shows a formation of well-dispersed AgNPs of 45-55 nm. When AgNPs were mixed with hydrogen peroxide disinfectant they displayed strong synergistic antibacterial.
Improvement: Antimicrobial studies confirm the superior ability of biosynthesized silver nanoparticles to inhibit the growth of bacterial and fungal contaminants and their utilization in various applications particularly as antibacterial substance in disinfection and preservation to protect against various biomedical, pharmaceutical based activities.
Keywords: Silver nanoparticles, Acinetobacter baumannii, Surface plasmon resonance, Antimicrobial activity
Introduction
Nanoscience and nanotechnology are the study and application of extremely small things and can be used across all the other scientific fields, such as chemistry, biology, physics, materials science and engineering conducted at the nanoscale, which is about 1 to 100 nanometers [1]. Nanoparticle having one or more dimensions of the order of 100 nm or less have attracted considerable attraction due to their unusual and fascinating properties, with various applications, over their bulk counterparts. Currently, a large number of physical, chemical, biological, and hybrid methods are available to synthesize different types of nanoparticles [2].
Biological nanoparticles are finding important applications in the field of medicine. The antimicrobial potential of metal based nanoparticles has led to its incorporation in consume, health related and industrial products. Our area of interest lies in the biological synthesis of gold silver nanoparticles [3].
Biosynthesis methods are limited to synthesis of nanoparticles in small quantities and poor morphology [4].
Silver nanoparticles are nanoparticles of silver of between 1 nm and 100 nm in size. Silver nanoparticles have unique optical, electrical, and thermal properties and are being incorporated into products that range from photovoltaic to biological and chemical sensors [5]. Silver nanoparticles possess unique properties with numerous applications such as antimicrobial, anticancer, catalytic, and wound healing activities. Biogenic syntheses of silver nanoparticles and their pharmacological and other potential applications are gaining momentum owing to its assured rewards [6].
Fungi, bacteria, algae, yeasts, and plants have inherent capacities to reduce metal through their specific metabolic pathway. In fact, some biomimetic (peptides) procedures are also used. Silver nanoparticles has been increasing due to their high antimicrobial activity against bacteria, viruses and eukaryotic microorganisms. Ions are reduced by enzymes (e.g., reductase) or/and carbonyl groups leading to formation of metal nuclei, which further grow to frame crystals. Only in one example, nitrate reductase from the fungus F. oxysporum has been well documented to catalyze the reduction of silver nitrate to silver nanoparticles utilizing NADPH as reducing agent [7].
Silver nanoparticles have gained attention because of their antimicrobial activity which offers the possibility of their use for medical and hygiene purposes. Indeed, silver nanoparticles in different formulations and with different shapes and sizes exhibit variable antimicrobial activity. However, the mechanisms of antimicrobial activity of silver ions and silver nanoparticles, and their toxicity to human tissues are not fully characterized [8].
Silver exhibits low toxicity to mammalian cells and silver has a lower propensity to induce microbial resistance than many other antimicrobial materials. Silver nanoparticles have a high activity against a wide range of microbial pathogens (Gram-positive and Gram-negative bacteria, fungi, viruses, protozoa as well as multidrug resistance microorganisms) and, as opposed to ions, retain a longer a biological usage [9].
Silver nanoparticles that continuously release a low level of silver ions to provide protection against bacteria. The silver content has to be high enough to inhibit the growth of bacteria cells (minimal inhibitory concentration, MIC) or kill 99.9% of them (minimal bactericidal concentration, MBC). Silver nanoparticles have prolonged activity; thanks to this, they are much better for use as a medical factor than silver ions. However, the risk of such a mechanism decreases the sensitivity of the microorganism [10].
Multi resistance to antibiotics is a serious and disseminated clinical problem, common to several new compounds that block the resistance mechanism. The AgNPs could be evaluated as antimicrobial agents for potential use as a barrier control of hospital acquired infections.
Materials And Methods
Materials
Silver nitrate (AgNO3), anhydrous sodium di-hydrogen phosphate, di-sodium hydrogen phosphate. Tryptic Soya Broth (TSB), R2A Agar and Mueller-Hinton agar media were procured from Merck Pathogenic bacteria: Escherichia coli ATCC 8739, Salmonella typhimurium ATCC 6538, Klebsiella pneumonia ATCC 10031, Pseudomonas aerugenosa ATCC 15442, Bacillus spizizenii ATCC 6633, Staphylococcus aureus ATCC 6538, Candida albicans ATCC 10231 and Aspergillus niger ATCC 16404.
Sample Collection
Sample locations: Samples were taken from representative different classes and locations in pharmaceutical facility including:
1. Area Class A (the highest grade of cleanliness): Microbiology laboratory laminar air flow units.
2. Area Class C (intermediate measure of cleanliness): Syrup dosage form preparation area, soft gelatin area.
3. Area Class D (low measure of cleanliness): Solid dosage form preparation areas.
Sample collection: Air sampler was placed in the center of each room at a height of approximately 1 meter above the floor. One thousand liters of air sampling were obtained by Merck Mass 100 centrifugal sampler. The Tryptic soya agar plates (TSA) were incubated for 48 hours at 30ºC - 35ºC and then for 72 hours at 20ºC - 25ºC. After incubation, the colonies were counted and recorded in specific protocols [11].
Swab samples were collected by removing a sterile swab from a sterile tube, moistening it by inserting it into a second tube which contained a sponge soaked with sterile 1.5 ml of phosphate buffered saline (PBS) at pH 7.2. The selected surface was swabbed by moving the swab back and forth across the surface with several horizontal strokes, then several vertical strokes. The swab was rotated during sampling to ensure that the entire surface of the swab was used. After sampling, the swab was returned to its pre-labeled sampling tube containing appropriate amount of liquid media [10].
Water Samples were collected from regular sites across the Egyptian National Organization for Potable Water and Sanitary drainage (NOPWAS) in sterile containers. Under unidirectional laboratory safety cabinet a tenfold dilution was applied with normal saline solution (NaCl 0.9% w/v), one ml from each diluent was transferred into the surface of 9 ml sterile Petri dishes, about 70 – 80 ml of melted R2A Agar media was added at about 40-45°C, gently shacked with plate rotator. Petri dishes were labeled, inverted and incubated at 30-35°C for 48 hours. By using sterile transfer loop separate colonies were picked up and transferred into a surface of TSA media in a zigzag pattern, the petri dish labeled, inverted and incubated at 30-35°C for 48 hours. The bacterial cells were grown in streaks as single colonies. Separate grown colonies transferred into TSB media and incubated at 30-35°C for 48 hours. After growth occurred the cell suspensions centrifuged at 1000 rpm for 5 minutes and supernatants were separated in clean containers and stored in refrigerator at 2–8°C for further examinations [12].
Identification of isolates
Isolates were primarily differentiated by Gram staining then identified using the VITEK 2 system (Biomerieux) in Chemical Warfare Institute, the Defense Ministry of Egypt. A. baumannii isolate was used in the present study to test their ability to biosynthesize of AgNPs [13].
Synthesis of silver nanoparticles
A. baumannii isolate was freshly inoculated in sterile screw caped bottles containing 100 ml TSB media. The bottles were incubated on orbital shaker at 30-35˚C and agitated at 200 rpm for 24 hours. After incubation, the cell filtrates were obtained by centrifugation at 1000 rpm for 10 minutes and followed by decantation. From the final concentration of 50.0 mg/ml AgNO3 one ml was added in to 10 ml of cell filtrate in 20 ml screw capped test tube. The test tubes were incubated in a dark condition up to 24 hours. The control was maintained without addition of AgNO3 with the experimental bottle containing cell filtrate. The brown colored solution of was stored in refrigerator for further characterization and applications [14].
Characterization of silver nanoparticles
AgNPs detection was carried out primarily by visual observation of color change of the bacterial filtrate after treatment with silver nitrate. The appearance of dark brown color of bacterial cell filtrate indicates the formation of AgNPs. Further, one ml of the reaction solutions were removed after 24 hours and consequently subjected to UV-visible spectroscopy (Shimadzu UV- 1800) from 300 – 800 nm at regular intervals. Additional sample was examined by FTIR Spectrophotometer (Bruker Alpha II) the technique was used in measuring spectra of functional groups. Scanning Electron Microscopy (SEM) (Hitachi, model: S-3400N) was applied to observe entrapped AgNPs. Thin film of the sample were prepared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid, extra solution was removed using a blotting paper and then the film on the SEM grid were allowed to dry by putting it under a mercury lamp for 5 min. The combination of SEM with energy-dispersive X-ray spectroscopy (EDX) applied to examine silver powder morphology and conducting chemical composition analysis [15,16].
Antibacterial and antifungal activities
The antimicrobial activity of synthesized AgNPs were tested against” Escherichia coli ATCC 8739, Salmonella typhimurium ATCC 6538, Klebsiella pneumonia ATCC 10031, Pseudomonas aerugenosa ATCC 15442, Bacillus spizizenii ATCC 6633, Staphylococcus aureus ATCC 6538, Candida albicans ATCC 10231and Aspergillus niger ATCC 16404”. The test organisms were suspended in saline solution and adjusted to match 0.5 McFarland's Standard. Further, by using sterile swab the culture spread over MullerHinton Agar plates. The plates were allowed to dry and a sterile cork-borer with diameter of 6.0 mm was used to bore four wells in each agar plate. A 50 μL volume of Silver Nanoparticles was applied by micropipette in the wells. The bacterial filtrate without AgNO3 served as control. The plates were allowed to stand for 1h for complete diffusion and incubated at 30- 35ºC for 24 hours. Antimicrobial activity was determined by measuring the inhibition zone around each well for each extract in millimeter using zone reader; three replicate trials were conducted against each organism [17].
Statistical analysis
All experiments were carried out in triplicate, and the results were expressed as the mean. Means, standard deviations (SD) and P-value in this study was less than 0.05 were analyzed by using the SPSS 18 software.
Results And Discussion
Environmental monitoring samples taken from different locations in pharmaceutical facility
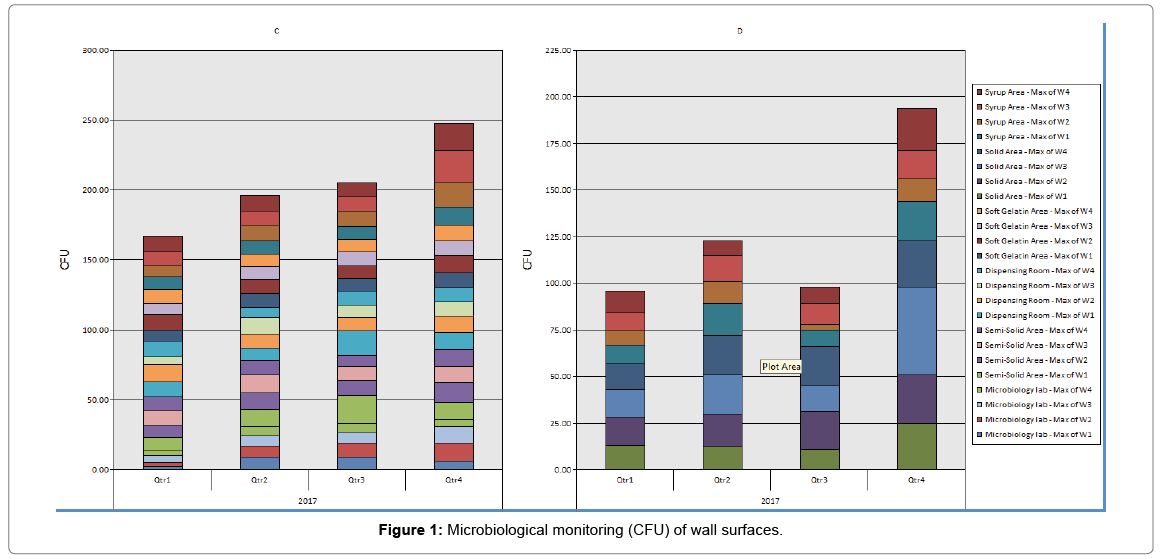
Surface monitoring: All samples were taken using contact plates from production area and microbiology laboratory in facility except sterility test isolator samples were taken by swab technique. Results were shown in Tables 1 and 2. All samples of class A showed no growth. All 517 of class C and D showed microbial count.
| Year 2017 |
Microbiology laboratory | Semi-Solid Area | Dispensing Room | Soft Gelatin Area | Solid Area | Syrup Area |
|---|---|---|---|---|---|---|
| Qrt.1 | 5 | 10 | 12 | 11 | 15 | 12 |
| Qrt.2 | 9 | 13 | 12 | 10 | 21 | 17 |
| Qrt.3 | 10 | 20 | 17 | 10 | 21 | 11 |
| Qrt.4 | 13 | 14 | 12 | 12 | 47 | 23 |
| Max. | 13 | 20 | 17 | 12 | 47 | 23 |
Table 1: Microbiological monitoring (CFU) of wall surfaces.
| Year 2017 |
Microbiology lab | Semi-Solid Area | Dispensing Room | Soft Gelatin Area | Solid Area | Syrup Area |
|---|---|---|---|---|---|---|
| Qrt.1 | 10 | 28 | 25 | 25 | 44 | 36 |
| Qrt.2 | 31 | 27 | 35 | 23 | 52 | 33 |
| Qrt.3 | 22 | 29 | 33 | 32 | 61 | 41 |
| Qrt.4 | 30 | 32 | 27 | 28 | 52 | 35 |
| Max. | 30 | 32 | 33 | 32 | 52 | 41 |
Table 2: Microbiological monitoring of active air.
Visual observation
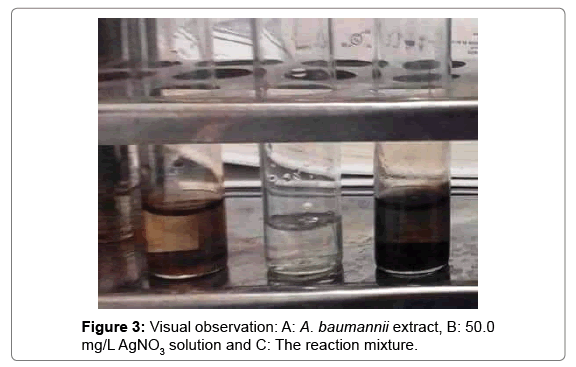
It is well known that silver nanoparticles exhibit dark brownish color in aqueous solution due to excitation of surface plasmon vibrations in silver nanoparticles [18]. As the A. baumannii extract which mixed with the aqueous solution of the silver nitrate, its color turned from watery to yellowish brown and finally to dark brown due to reduction of silver ion (Figures 1-3).
UV-Vis spectra analysis
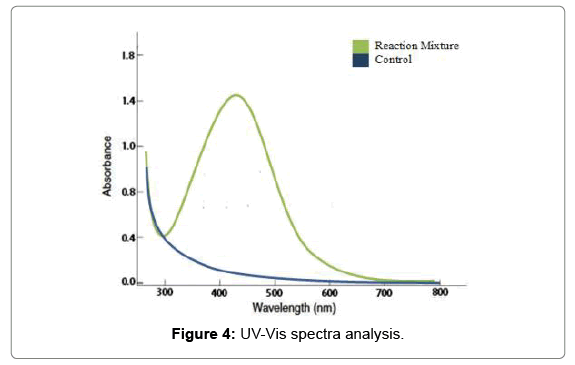
The confirmation of reduction AgNO3 to silver ions by A. baumannii extract was examined; the aqueous silver ions were reduced to silver nanoparticles when added to culture supernatant. It was observed that the color of the solution turned from yellow to bright yellow and then to dark brown after 5 hours of the reaction shown in the Figure 4, which indicated the formation of silver nanoparticles. Absorption spectra of silver nanoparticles formed in the reaction media has absorbance peak located between 300 to 600 nm and the broadening of peaks indicated that the particles are polydispersed. In the UV-Vis spectra; a strong and broad Surface Plasmon Resonance (SPR) peak was observed at a range of 420 nm that confirmed the synthesis of AgNPs and has been well documented for various metal nanoparticles with sizes ranging from 2 nm to 100 nm [19]. Recent studies suggested that a SPR peak located between 410 and 450 nm has been observed for AgNPs and might be attributed to spherical nanoparticles [20].
Fourier transform infrared spectroscopy (FTIR)
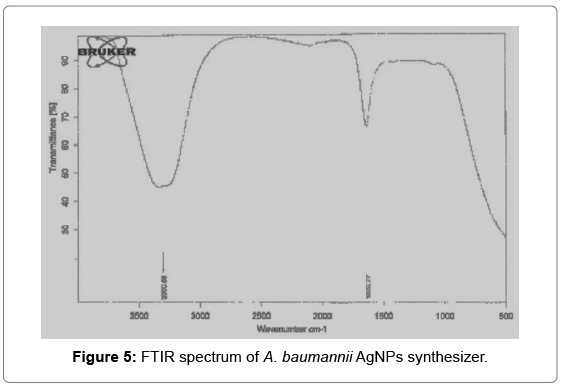
FT-IR measurements were carried out to hypothesize the possible interactions between silver salts and bioactive molecules, which could account for reduction of Ag+ ions and stabilization of AgNPs (20). The representative spectra in the region of 3000 to 500 cm-1 revealed the presence of different functional groups like 3290 secondary amide (N–H stretch, H-bonded) and 1635.0 cm-1 corresponds to carbonyl group [21]. The FTIR spectra recorded from AgNPs formed after 24 hours of incubation of the bacterial isolates. A possible interaction of biomolecules present in the culture filtrate and the vibrational stretches were predicted based on their earlier reports showed between 3280.0 to 3330.0 cm-1 which corresponds to primary amines [22]. The amide linkages between amino acid residues in proteins give rise to well-known signatures in the infrared region of the electromagnetic spectrum. The FTIR spectrum reveals band at 1625 - 1529 cm-1 that correspond to the bending vibrations of the amide bands of the proteins. FTIR analysis reveals the role of biomolecules which reduce the silver nitrate and bind onto the nanoparticles and stabilize them which prevents the aggregation (Figure 5).
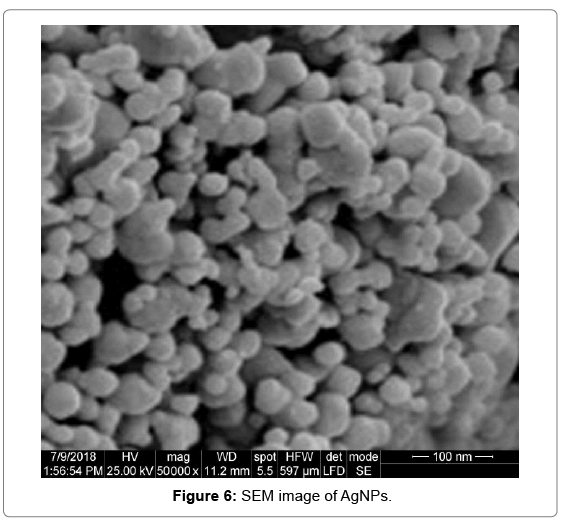
Scanning electron microscopy (SEM)
SEM was used to determine the size and shape of the biosynthesized nanoparticles. SEM images revealed the average size of particles as 45- 55 nm. SEM images show that they are relatively uniform in diameter and have a spherical shape (Figure 6).
Energy dispersive x-ray microanalysis
EDAX spectroscopy confirmed the presence of an elemental silver signal from the silver nanoparticles. The vertical axis shows the number of X-ray counts and the horizontal axis shows energy in keV. Identification lines for the major emission energies for silver are shown and these correspond with peaks in the spectra, indicating that silver was correctly identified. The EDAX profile for the silver nanoparticles showed strong silver signals from silver atoms (Table 3). The EDAX pattern shown clearly the silver nanoparticles were crystalline in nature due to the reduction of silver ions. The present study confirmed the presence of silver nanoparticles formed and showed strong signal energy peaks for silver atoms in the range 2–4 keV compared with another signals of sodium, oxygen, potassium, sulfur, carbon, oxygen and chloride [22].
| Element | Wt % | At % |
|---|---|---|
| C K | 48.30 | 65.26 |
| O K | 22.90 | 23.24 |
| NaK | 05.93 | 04.19 |
| P K | 01.46 | 00.76 |
| S K | 00.66 | 00.34 |
| ClK | 06.95 | 03.18 |
| AgL | 10.19 | 01.53 |
| K K | 03.61 | 01.50 |
Table 3: EDAX ZAF quantification standardless SEC.
Antimicrobial activity
The antibacterial activities of synthesized silver nanoparticles were tested against reference microorganisms as shown in Table 4 and Figure 7. The silver nanoparticles exhibited antibacterial activity against single cell and filamentous fungi, Gram positive and Gram-negative bacteria. The highest inhibition zone diameter was formed against E. coli and A. niger, however the lowest of 13.6 mm was produced against K. pneumonia. Additionally an in-vitro comparison between antibacterial activity of chemically and biosynthesized silver nanoparticles against standard organisms shown that no significant difference between inhibitory effect of biosynthesized silver nanoparticles compared with chemically synthesize. The highest efficacy against multidrug resistance bacteria [23]. Hydrogen peroxide (H2O2) and silver that their strong bactericidal activities have been studied on different bacteria [24].
| Standard Organisms | 1.1.0 µg 0 µg | 5.0 µg | 10.0 µg | 10.0 µg |
|---|---|---|---|---|
| Escherichia coli ATCC 8739 | 15.8 ± 0.1 | 19.4 ± 0.2 | 22.0 ± 0.3 | 23.5 ± 0.6 |
| Salmonella Typhimurium ATCC 6538 | 12.2 ± 0.5 | 14.8 ± 0.5 | 17.6 ± 0.2 | 19.2 ± 0.5 |
| Klebsiella pneumonia ATCC 10031 | 9.9 ± 0.2 | 12.7 ± 0.2 | 15.3 ± 0.1 | 17.6 ± 0.4 |
| Pseudomonas aerugenosa ATCC 15442 | 9.8 ± 0.1 | 13.1 ± 0.5 | 15.6 ± 0.3 | 16.8 ± 0.3 |
| Bacillus spizizenii ATCC 6633 | 14.1 ± 0.4 | 18.3 ± 0.5 | 20.2 ± 0.3 | 21.6 ± 0.3 |
| Staphylococcus aureus ATCC 6538 | 11.6 ± 0.6 | 15.3 ± 0.4 | 18.4 ± 0.4 | 19.1 ± 0.1 |
| Candida albicans ATCC 10231 | 13.8 ± 0.3 | 17.4 ± 0.3 | 19.8 ± 0.2 | 20.8 ± 0.3 |
| Aspergillus niger ATCC 16404 | 14.5 ± 0.6 | 18.8 ± 0.4 | 21.1 ± 0.5 | 21.6 ± 0.2 |
Table 4: Minimum inhibitory concentration (MIC) of AgNPs (μg /ml) against standard organisms (mean ± SD).
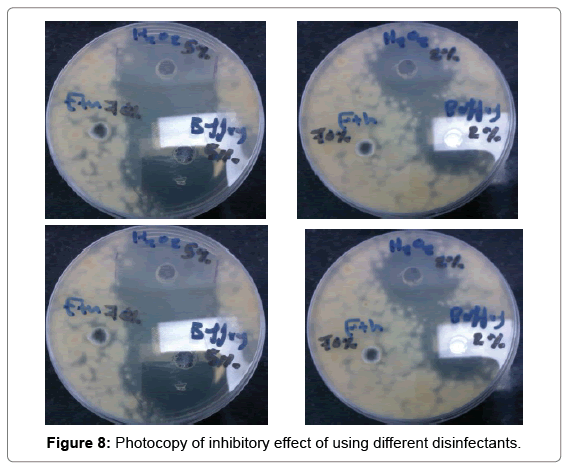
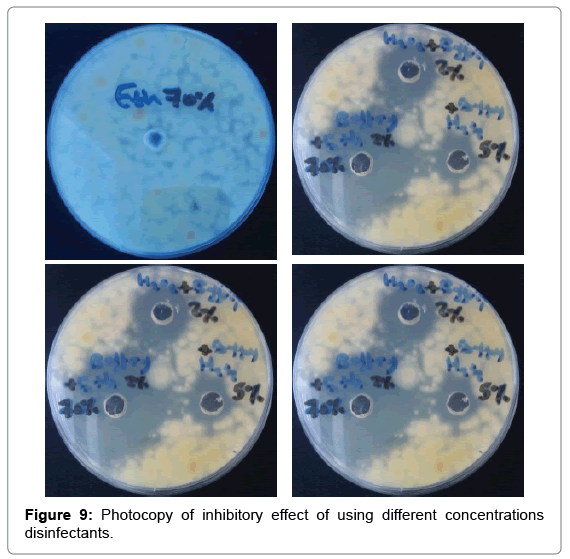
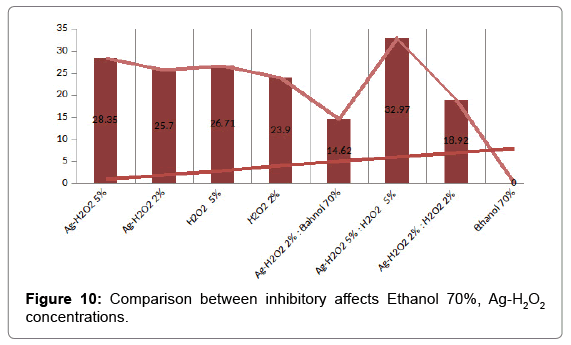
Strong disinfection effect of H2O2 + Ag+ against three human pathogens including Proteus mirabilis, Klebsiella pneumonia and Escherichia coli has been demonstrated (Table 5 and Figure 8). The efficacy of 30 ppb silver in 0.3% Hydrogen peroxide solution for disinfection of selected enterobacteria was assessed offers new possibilities (Figure 9). Silver nanoparticles account for greater than 23% of all nanoproducts and have been widely used for diagnostic and therapeutic applications (e.g. in wound healing, arthritic disease, etc.). These have been widely known for their antibacterial, antifungal, and antiviral effects (Table 6 and Figure 10).
| Disinfectant | Ag-H2O2 | Ag-H2O2 | H2O2 | H2O2 |
|---|---|---|---|---|
| Concentration | 5% | 2% | 5% | 2% |
| Zone (mm) | 28.35 | 25.70 | 26.71 | 23.90 |
Table 5: Comparison between inhibitory affects H2O2 Ag-H2O2 concentrations.
| Disinfectant | Ethanol | Ag-H2O2 & Ethanol | Ag-H2O2 | Ag-H2O2 |
|---|---|---|---|---|
| Concentration | 70% | 2% -70% | 5% | 2% |
| Zone (mm) | 0.0 | 14.62 | 32.97 | 18.92 |
Table 6: Comparison between inhibitory affects Ethanol 70%, Ag-H2O2 concentrations.
Conclusion
The more serious problem arising from microbial contamination of pharmaceutical environments. The study findings have shown that all tested samples were microbiologically contaminated. The majority of microbial contaminants in non-sterile pharmaceuticals. The antibacterial activities of synthesized silver nanoparticles were tested against reference microorganisms. Silver nanoparticles exhibited antibacterial activity against both single cell, filamentous fungi, Gram positive and Gram-negative bacteria.
After 3 days of incubation; the results shows the following:
1. Ethanol 70% has no effect on the microbial growth.
2. Ag-H2O2 solution (2% and 5%) Inhibit the microbial growth.
3. H2O2 solution (2% and 5%) Inhibit the microbial growth.
4. Ag-H2O2 solutions (2%, 5%) more effective than H2O2 (2%, 5%) solutions.
5. Mixing between Ag-H2O2 solution 2% with H2O2 2% solutions or Ethanol 70% decrease its efficacy (antagonistic effectiveness).
References
- Natarajan K, Selvaraj S, Murty VR (2010) Microbial production of silver nanoparticles. Dig J Nanomat Biostruct 5: 135-140.
- Gopinath SM, Saha NS, Jincy JV, Khanum NS, Ganesh S, et al. (2013) Biological Synthesis, Characterization and Application of Silver Nano Particles - A Review. Int J Pharm App 4: 19-28.
- Fulekar MH, Pathak B, Kale RK (2014) Nanotechnology: Perspective for environmental sustainability. In Envir Sustainable Devel Pp: 87-114.
- Antunes A, Fierro I, Guerrante R, Mendes F, Alencar MS (2013) Trends in nanopharmaceutical patents. Int J Mol Sci 14: 7016-7031.
- Sau TK, Rogach AL (2010) Nonspherical Noble Metal Nanoparticles: Colloid-Chemical Synthesis and Morphology Control. Adv Mat 22: 1781-1804.
- Firdhouse MJ, Lalitha P (2015) Biosynthesis of silver nanoparticles and its applications. J Nanotechn 1: 15-20.
- Irena Maliszewska (2011) Microbial Synthesis of Metal Nanoparticles. Metal Nanoparticle Microbio 153-175.
- Duran N, Marcato PD, Roseli DC, Alves OL, Costa FTM, et al. (2010) Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J Braz Chem Soc 21: 949-959.
- Kedziora A, Strek W, Kepinski L, Bugla PG, Doroszkiewicz W (2012) Synthesis and antibacterial activity of novel titanium dioxide doped with silver. J Sol Gel Sci Technol 62: 79-86.
- Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M (2009) Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedi Nanotechn Biol Med 5: 382-386.
- Ingle AGA, Pierrat S, Sonnichsen C, Rai M (2008) Mycosynthesis of Silver Nanoparticles Using the Fungus Fusarium acuminatum and its Activity Against Some Human Pathogenic Bacteria. Curr Nanosci 4: 141-144.
- Radzig MA, Nadtochenko VA, Koksharova OA, Kiwi J, Lipasova VA, et al. (2013) Antibacterial effects of silver nanoparticles on gram-negative bacteria: influence on the growth and biofilms formation, mechanisms of action. Colloids Surf B Biointerfaces 102: 300-306.
- Dar MA, Ingle A, Rai M (2013) Enhanced antimicrobial activity of silver nanoparticles synthesized by Cryphonectria sp evaluated singly and in combination with antibiotics. Nanomedi Nanotech Biol Med 9: 105-110.
- Aggarwal PJ, Vibhor A (2012) Synthesis, characterization and antimicrobial effects of silver nanoparticles from microorganisms - A Review. Int J Nano Mater Sci 1: 108-120.
- Ashrafpour S, Moghadam TT, Ranjbar B (2014) Fluorescence Spectroscopy of Lysozyme Silver Nanoparticles Complex. World A Sci Eng Tech Int J Chem Mol Eng 8: 985-988.
- Davoudi M, Vakili T, Absalan A, Ehrampoush MH, Ghaneian MT (2013) Antibacterial effects of Hydrogen peroxide and silver composition on selected pathogenic enterobacteria. Middle East J Sci Res 13: 710-715.
- Bata VI, Adanyi N, Beczner J, Farkas J, Szekacs A (2013) Nanotechnology and microbial food safety. Microb Pathog Strateg Combat Them Sci Technol Edu Formatex Zubaran Pp: 155-159.
- Antunes LCS, Visca P, Towner KJ (2014) Acinetobacter baumannii: Evolution of a global pathogens. Pathog Dis 71: 292-301.
- Buszewski B, Railean PV, Pomastowski P, Rafinska K, Szultka MM, et al. (2018) Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. J Microbiol Immunol Infect 51: 45-54.
- Faunce T, Watal A (2010) Nanosilver and global public health: International regulatory issues. Nanomed 5: 617-632.
- Jain N, Bhargava A, Majumdar S, Tarafdar JC, Panwar J (2011) Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: A mechanism perspective. Nanoscale 3: 635-641.
- Midha K, Singh G, Nagpal M, Arora S (2016) Potential Application of Silver Nanoparticles in Medicine. Nanosci Nanotech Asia 6: 82-91.
- Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin Microbio Rev 21: 538-582.
- Zapata GJ, Mena P, Galeano B, Escobar N, MejÃa MOIC, et al. (2017) Characterization of silver nanoparticles for potential use as antimicrobial agent. VII Latin A Congress Biomed Eng CLAIB 2016 Bucaramanga, Santander, Colombia, Pp: 245-247.
Citation: Mohammed ABA, Azzazy AM, Ibrahim AM (2019) New Synergistic Potential of Silver Nanoparticles and it is Application in Pharmaceutical Production Areas. J Biotechnol Biomater 8: 288. DOI: 10.4172/2155-952X.1000288
Copyright: © 2019 Mohammed ABA et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3252
- [From(publication date): 0-2018 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 2565
- PDF downloads: 687