Review Article Open Access
New Insights about Congenital Infection by Human Cytomegalovirus: Unveiling the Role of PPARγ
Stephane Chavanas1,2,3*1Centre de Physiopathologie Toulouse Purpan, Inserm Umr 1043, Toulouse, France
2CNRS UMR 5282 Toulouse, France
3Université Paul Sabatier, Toulouse, France
- *Corresponding Author:
- Stephane Chavanas
Centre de Physiopathologie Toulouse Purpan
INSERM UMR 1043, Toulouse, France
Tel: 33-0562744539
E-mail: stephane.chavanas@inserm.fr
Received date: June 22, 2016; Accepted date: July 08, 2016; Published date: July 13, 2016
Citation: Chavanas S (2016) New Insights about Congenital Infection by Human Cytomegalovirus: Unveiling the Role of PPARγ. J Infect Dis Ther 4:288. doi:10.4172/2332-0877.1000288
Copyright: © 2016 Chavanas S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Congenital infection by human cytomegalovirus (HCMV) might result in permanent neurological sequelae, including sensorineural deafness, cerebral palsies or devastating neurodevelopmental abnormalities. Neural progenitors have been suspected to be key targets of infection, hence a number of studies have shown that HCMV is able to infect neural cells and alter their differentiation. However, little was known about the molecular and genetic bases underlying homeostatic changes in the infected progenitor. We recently disclosed that Peroxisome Proliferator-Activated Receptor gamma (PPARγ), a transcription factor of the nuclear receptor superfamily, is a key determinant of HCMV pathogenesis in the developing brain. Using neural stem cells from human embryonic stem cells, we showed that HCMV infection strongly increases levels and activity of PPARγ in NSCs. Further in vitro experiments showed that PPARγ activity inhibits the neuronogenic differentiation of NSCs into neurons. Consistently, increased PPARγ expression was found in brain section of fetuses infected by HCMV, but not in uninfected controls, what strongly supported the in vitro data. Here we review and discuss past and recent findings on the neuropathogenesis of HCMV congenital infection.
Keywords
Congenital infection; Human cytomegalovirus; HCMV; Neurotropic viruses
Introduction
Infections by neurotropic viruses in pregnancy may lead to neurodevelopmental abnormalities in the fetus. As infections by rubella, varicella zoster, or HIV to name a few, congenital infection by human cytomegalovirus (HCMV) may cause spontaneous abortion, fetal death, or neurodevelopmental abnormalities. Indeed, HCMV is the most frequent infectious cause of permanent neurological sequelae. About 1% of newborns are congenitally infected with HCMV each year [1]. Five to 10% among them show severe neurological or neurodevelopmental defects at birth, presenting with deafness, blindness, mental retardation, cerebral palsy, microcephaly, hydrocephalus... whereas 10 to 15% additional infected infants are asymptomatic at birth but subsequently develop sensorineural hearing loss, seizure or epilepsy [1]. Spastic cerebral palsies cause severe orthopaedic issues. Overall, patients with permanent sequelae represent 0.1%-0.2% of all live births, a frequency comparable to that of Down syndrome or fetal alcohol syndrome [1]. Not only HCMV congenital infection is a public health issue, it is also a key social and economic burden due to the amount of time and money needed for care and education of patients: the direct annual care costs for patients are estimated at $1-$2 billion in the USA [2]. No vaccine is available. Early neonatal antiviral treatment might provide benefit for affected newborns despite limitations due to toxicity [3]. Here we review past and recent findings on the neuropathogenesis of HCMV congenital infection.
HCMV Congenital Infection
HCMV is a BetaHerpes virus with a worldwide distribution. It is highly prevalent in the general population: seroprevalence ranges between 40% and 90%, with the greatest values among racial/ethnic minorities and persons of lower socio-economic background status [1]. HCMV is transmitted through close non-sexual or sexual contact, breastfeeding, blood transfusions, and organ transplantation [2]. For the pregnant woman, the most likely source of infection is the contact with the urine or saliva of young children, including her own children [2]. Lifelong latency is established after a primary infection. Though infection of immunocompetent adults is almost always benign, HCMV is responsible for serious illness and death in immunocompromised hosts, and is a major hazard for the fetus after infection during pregnancy. HCMV infection in utero is believed to occur through transplacental hematogenous spread [4]. Congenital HCMV disease is associated with a wide range of neurodevelopmental disabilities, including hearing and vision loss and mental retardation, as well as structural brain abnormalities including intracranial calcifications, microcephaly, hydrocephalus, ventriculomegaly, ventriculomegaly, polymicrogyria, porencephaly, and schizencephaly [5]. Neurological outcomes are more severe when infection occurs during the first trimester [5].
HCMV Tropism in the Developing Brain
HCMV is known to infect a wide spectrum of target cell types, either in parenchyma or connective tissue. Epithelial cells, endothelial cells, fibroblasts, smooth muscle cells, hematopoietic cells are the predominant targets of HCMV [6]. Characterizing HCMV tropism in the brain is critical to understand its neuropathy. Studies in the mouse revealed that the HCMV murine counterpart, namely murine cytomegalovirus (MCMV), infected the developing brain, and more precisely, the cerebral ventricular walls, a region known to contain neural progenitors [7]. Mouse neurons were also found to be sensitive to infection by MCMV [7].
Because such studies in human were not possible, studies were performed using primary or secondary cultures of human brain cells prepared from deceased, uninfected fetus, which were eventually infected by HCMV in vitro. These studies reported that brain microvascular endothelial cells, astrocytes, neuronal cells, oligodendroglial cells, microglia/macrophages, and neural progenitor/ stem cells were sensitive to HCMV infection ex vivo [8-12]. Besides, immunohistopathological and in situ hybridization analyses of brain samples from AIDS patients infected by HCMV detected HCMV in neurons and astrocytes, oligodendrocytes, ependyma, choroid plexus, endothelia, in periand endoneurium and in leptomeninges [13]. However, no histological study identifying the different cell types actually infected in utero during congenital HCMV infection were available, except one which reported HCMV inclusion bodies in the brain of premature infants with lethal congenital cytomegalovirus infection [14]. In our study, we explored the expression of the immediate early HCMV antigen (IE), a factor encoded by the HCMV genome and critical for virus replication, in histopathological slides from infected or non-infected fetus. We observed cells clearly immunoreactive to IE in the ependymal and germinative zones of the brain of infected cases [15]. Together these studies indicate that a variety of brain cell types are sensitive to HCMV infection, including neural stem/progenitor cells.
Noteworthy, it has been reported that the susceptibility of primary human NPCs to HCMV is retained concomitantly with differentiation into glial cells but is actively repressed following differentiation into neurons [8].
HCMV Replication in Neural Cells
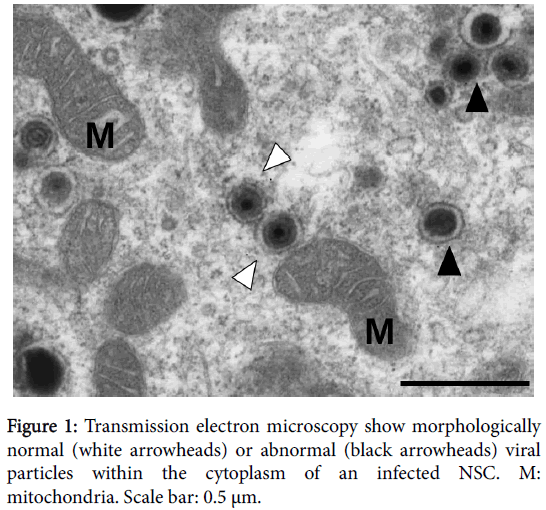
Since IE expression is required, but not always sufficient to virus replication, our results did not formally show that progenitors are permissive to HCMV infection in vivo, and did not rule out the possibility of abortive replication. However, it was not possible to show actual production of infectious particles in utero from fixed samples. Therefore we investigated the permissivity of neural progenitors to HCMV in vitro, using a new disease model: human neural stem cells (NSCs) generated from embryonic stem (ES) cells. NSCs were generated through early neuroepithelial differentiation of human ES cells in a monolayer system using SMAD inhibitors and the defined medium N2B27 [16]. This method allowed for efficient neural commitment, generation of highly neuronogenic NSCs, and, as compared to progenitors prepared from deceased fetus, avoided factors as donor variability (including gestation age), and allowed for deep in vitro investigations given their high proliferative capacity and stability in culture. NSCs displayed a cortical phenotype with positive immunostaining and/or high levels of expression of polarized neural stem cells and radial glia markers, in the absence of immunoreactivity to non-cortical markers [17]. This phenotype was particularly relevant with respect to the fact that congenital HCMV infection targets cortical areas of the developing brain as well as radial glia cells [11]. We showed that NSCs supported viral replication, exhibited strong expression of early and late antigens, showed assembled viral particles (Figure 1), and allowed for efficient spreading and allowed efficient production of infectious particles in vitro [15]. This result was in agreement with other studies using progenitors from human fetal brain [8,10,12] or from iPS cells [18,19]. Importantly, infected NSCs or progenitors remained immunoreactive to the stemness markers nestin, SOX2 [15] and A2B25 [8], suggesting that infection did not cause detectable changes in their stem cell status. It is worth noting however that permissivity seems to be dependent upon the differentiation status of the neural progenitor, as shown by a recent study [20]. More experiments are needed to investigate the molecular networks underlying the relation between permissivity and differentiation status of the host NSC. Such a connection has been already reported in ES cells [21,22] or the hematopoietic lineage [23].
Apart from progenitors, permissivity of neurons and astrocytes to HCMV has been investigated in vitro. Convergent studies showed that primary human astrocytes as well as astrocytes generated in vitro from human neural progenitors support highly productive, cytopathic replication of CMV [9,24,25]. In contrast, studies on neuron permissivity lead to conflicting results. Primary mature human neurons showed no viral antigen expression or cytopathic effect following infection in vitro [9], whereas neurons derived from human neural progenitors prepared from fetus were reported to be permissive [10,25] or not [8] to HCMV infection.
PPARγ: A Key Transcription Factor in Pathogenesis of HCMV Congenital Infection
HCMV infection alters the levels and/or activity of various host transcription factors of the host cell [26]. Notably, some host factors such as NF-κB [27] are used by HCMV for its own replication, through their recruitment on cognate DNA segments within HCMV Major Immediate Early Promoter (MIEP). Likewise, we showed that Peroxisome Proliferator-Activated Receptor gamma (PPARγ) is activated during HCMV infection of placenta cells, and is required for efficient virus replication [28]. PPARγ is a ligand-dependent transcription factor, member of the nuclear receptor superfamily, which plays key roles in regulating cellular function and tissue homeostasis [29]. Notably, PPARγ was thought to play a role in brain development or neurogenesis since brain development abnormalities were reported in PPARγ−/− and PPARγ−/+mice embryos [30] and various, though sometimes discordant, effects of PPARγ agonists on neural cells models were reported (reviewed [31]). Therefore we hypothesized that PPARγ could be a pathogenic effector of HCMV congenital infection. As expected, we observed that HCMV infection dramatically impaired the rate of neuronogenesis from NSCs. We showed that HCMV infection strongly increased PPARγ levels and activity in NSCs [15]. Further, we showed that PPARγ was sufficient and necessary to hamper neuronogenic differentiation from NSCs by using pharmacological activation or inhibition of endogenous or ectopically expressed PPARγ. The role of PPARγ in the disease phenotype was strongly supported by the immunodetection of nuclear PPARγ in brain germinative zones of congenitally infected fetuses (N=20), but not in control samples [15]. These findings revealed that PPARγ activation is a key molecular determinant of the pathology induced by HCMV infection in neural precursors, in vitro and presumably in vivo.
Lipid Metabolism and HCMV Infection
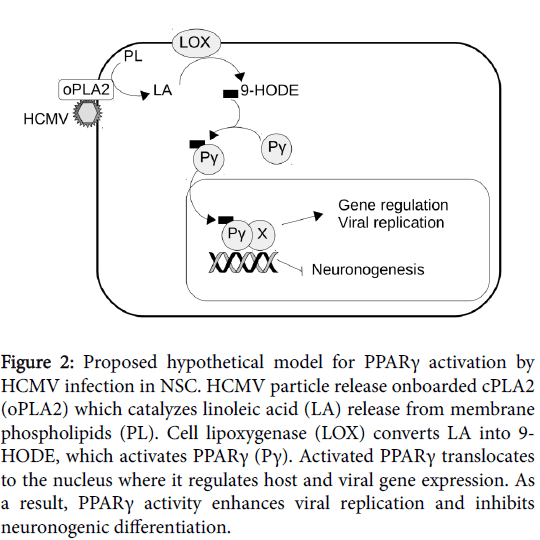
We investigated the mechanisms of PPARγ activation in NSCs. We found that levels of 9-hydroxyoctadecadienoic acid (9-HODE) were significantly increased in infected NSCs. 9-HODE is a polyunsaturated fatty acid (PUFA) known to be an activating ligand of PPARγ. Notably, other PUFAs, namely 13-HODE and 15-hydroxyeicosatetraenoic acid (15-HETE) activate PPARγ in human cytotrophoblast cells and placenta histocultures infected by HCMV [32]. Also, it has been shown that HCMV infection of fibroblasts caused increased biosynthesis of prostaglandin E2 (PGE2), and that PGE2 was required for efficient viral replication [33]. Noteworthy, PGE2 is also a PPARγ activating ligand. Together these studies underscored the importance of lipid metabolism in PPARγ activation in cells infected by HCMV, and, notably, the role of HCMV “onboarded” cPLA2 (oPLA2). oPLA2 is a cell-derived cPLA2, packaged in the tegument of the viral particle during release of HCMV from the infected cell [34]. oPLA2 is required for infectivity in human fibroblasts [34], placenta trophoblast cells [32] and NSCs [15]. Indeed, oPLA2 catalyzes the release of linoleic acid (LA) or arachidonic acid (AA) from host membrane phospholipids (PL) upon infection. LA oxidization driven by 15-lipoxygenase (15- LOX) generates 9-HODE and 13-HODE, whereas AA oxidization driven by 15-LOX or COX-2 generates 15-HETE or PGE2, respectively. In short, HCMV particles carry in their tegument oPLA2 as an “ignition factor” for synthesis of PPARγ activating ligands, which, in turn, will favor viral replication (Figure 2).
Figure 2: Proposed hypothetical model for PPARγ activation by HCMV infection in NSC. HCMV particle release onboarded cPLA2 (oPLA2) which catalyzes linoleic acid (LA) release from membrane phospholipids (PL). Cell lipoxygenase (LOX) converts LA into 9- HODE, which activates PPARγ (Pγ). Activated PPARγ translocates to the nucleus where it regulates host and viral gene expression. As a result, PPARγ activity enhances viral replication and inhibits neuronogenic differentiation.
Conclusion
Activation of PPARγ by HCMV infection illustrates that hijacking of host nuclear factors by the virus may have devastating outcomes on the host development. Our findings disclosed that PPARγ play a critical role in the neurodevelopmental sequelae of HCMV congenital infection. NSCs turned out to be a relevant tool for modeling functional correlates of HCMV infection. Such a cell platform will allow characterization of PPARγ gene targets in the infected cell, and thereby identify new pathogenic effectors of HCMV infection in the developing brain.
Primary HCMV infection is followed by a lifelong persistence of the virus in a latent state, namely latency, and reactivation may occur later in life. NSCs may help investigating if HCMV reactivation from latently infected brain cells might occur and be deleterious. Noteworthy, it has been reported that murine cytomegalovirus is able to establish latency and reactivate in mouse brain slice histocultures [7]. Last, NSCs may probably be extended to other viral pathologies of the central nervous system such as Zika virus (ZIKV) congenital infection.
References
- Cannon MJ (2009) Congenital cytomegalovirus (CMV) epidemiology and awareness. J ClinVirol 4: S6-10.
- Cannon MJ, Davis KF (2005) Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 5: 70.
- Oliver SE, Cloud GA, Sánchez PJ, Demmler GJ, Dankner W, et al (2009) Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J ClinVirol 4: S22–S26.
- Fisher S, Genbacev O, Maidji E, Pereira L (2000) Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol 74: 6808-6820.
- Cheeran MC, Lokensgard JR, Schleiss MR (2009) Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. ClinMicrobiol Rev 22: 99-126.
- Sinzger C, Digel M, Jahn G(2008) Cytomegalovirus cell tropism. Curr Top MicrobiolImmunol 325: 63-83.
- Tsutsui Y, Kosugi I, Kawasaki H, Arai Y, Han GP, et al. (2008) Roles of neural stem progenitor cells in cytomegalovirus infection of the brain in mouse models. PatholInt 58: 257-267.
- Cheeran MC, Hu S, Ni HT, Sheng W, Palmquist JM, et al. (2005) Neural precursor cell susceptibility to human cytomegalovirus diverges along glial or neuronal differentiation pathways. J Neurosci Res 82: 839-850.
- Lokensgard JR, Cheeran MC, Gekker G, Hu S, Chao CC, et al. (1999) Human cytomegalovirus replication and modulation of apoptosis in astrocytes. J Hum Virol 2: 91-101.
- Luo MH, Schwartz PH, Fortunato EA (2008) Neonatal Neural Progenitor Cells and Their Neuronal and Glial Cell Derivatives Are Fully Permissive for Human Cytomegalovirus Infection. J Virol82: 9994-10007.
- van Den Pol AN, Mocarski E, Saederup N, Vieira J, Meier TJ (1999) Cytomegalovirus Cell Tropism, Replication, and Gene Transfer in Brain. J Neurosci 19: 10948-10965.
- Odeberg J, Wolmer N, Falci S, Westgren M, Seiger A, et al. (2006) Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J Virol 80: 8929-8939.
- Schmidbauer M, Budka H, Ulrich W, Ambros P (1989) Cytomegalovirus (CMV) disease of the brain in AIDS and connatal infection: a comparative study by histology, immunocytochemistry and in situ DNA hybridization. ActaNeuropathol 79: 286-293.
- Perlman JM, Argyle C (1992) Lethal cytomegalovirus infection in preterm infants: clinical, radiological, and neuropathological findings. Ann Neurol 31: 64-68.
- Rolland M, Li X, Sellier Y, Martin H, Perez-Berezo T, et al. (2016)PPARγ Is Activated during Congenital Cytomegalovirus Infection and Inhibits Neuronogenesis from Human Neural Stem Cells. PLoSPathog 12: e1005547.
- Boissart C, Nissan X, Giraud-Triboult K, Peschanski M, Benchoua A (2012) miR-125 potentiates early neural specification of human embryonic stem cells. Development 139: 1247-1257.
- Boissart C, Poulet A, Georges P, Darville H, JulitaE et al. (2013) Differentiation from human pluripotent stem cells of cortical neurons of the superficial layers amenable to psychiatric disease modeling and high-throughput drug screening. Transl Psychiatry 3: e294.
- Nakamura H, Liao H, Minami K, Toyoda M, Akutsu H, et al. (2013) Human cytomegalovirus induces apoptosis in neural stem/progenitor cells derived from induced pluripotent stem cells by generating mitochondrial dysfunction and endoplasmic reticulum stress. Herpesviridae 4: 2.
- D'Aiuto L, Di Maio R, Heath B, Raimondi G, Milosevic J, et al. (2012) Human Induced Pluripotent Stem Cell-Derived Models to Investigate Human Cytomegalovirus Infection in Neural Cells. PLoS One 7: e49700.
- Belzile JP, Stark TJ, Yeo GW, Spector DH (2014) Human cytomegalovirus infection of human embryonic stem cell-derived primitive neural stem cells is restricted at several steps but leads to the persistence of viral DNA. J Virol 88: 4021-4039
- Matsukage S, Kosugi I, Kawasaski H, Miura K, Kitani H, et al. (2006) Mouse embryonic stem cells are not susceptible to cytomegalovirus but acquire susceptibility during differentiation. Birth Defects Res A ClinMolTeratol 76: 115-125.
- Kawasaki, H. Pluripotent stem cells are protected from cytomegalovirus infection at multiple points: Implications of a new pathogenesis for congenital anomaly caused by cytomegalovirus. CongenitAnom (Kyoto) 52: 147-154.
- Sinclair J, Reeves M (2014) The intimate relationship between human cytomegalovirus and the dendritic cell lineage. Front Microbiol 5: 389.
- Ho WZ, Song L, Douglas SD (1991) Human cytomegalovirus infection and trans-activation of HIV-1 LTR in human brain-derived cells. J Acquir Immune DeficSyndr 4: 1098-1106.
- McCarthy M, Auger D, Whittemore SR (2000) Human cytomegalovirus causes productive infection and neuronal injury in differentiating fetal human central nervous system neuroepithelial precursor cells. J Hum Virol 3: 215-228.
- Fortunato EA, McElroy AK, Sanchez I, Spector DH (2000) Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol 8: 111-119.
- Speir E, Shibutani T, Yu ZX, Ferrans V, Epstein SE (1996) Role of Reactive Oxygen Intermediates in Cytomegalovirus Gene Expression and in the Response of Human Smooth Muscle Cells to Viral Infection. Circ Res 79:1143-1152.
- Rauwel B, Mariamé B, Martin H, Nielsen R, Allart S, et al. (2010) vActivation of peroxisome proliferator-activated receptor gamma by human cytomegalovirus for de novo replication impairs migration and invasiveness of cytotrophoblasts from early placentas. J Virol 84: 2946-2954.
- Heikkinen S, Auwerx J, Argmann CA (2007)PPARgamma in human and mouse physiology. BiochimBiophysActa 1771: 999-1013.
- Wada K, Nakajima A, Katayama K, Kudo C, Shibuya A, et al. (2006) Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J BiolChem 281: 12673-12681.
- Stergiopoulos A, Politis PK (2013) The role of nuclear receptors in controlling the fine balance between proliferation and differentiation of neural stem cells. ArchBiochemBiophys 534; 27-37.
- Leghmar K, Cenac N, Rolland M, Martin H, Rauwel B, et al. (2015) Cytomegalovirus Infection Triggers the Secretion of the PPARgamma Agonists 15-Hydroxyeicosatetraenoic Acid (15-HETE) and 13-Hydroxyoctadecadienoic Acid (13-HODE) in Human Cytotrophoblasts and Placental Cultures. PLoS One 10: e0132627.
- Zhu H, Cong JP, Yu D, Bresnahan WA, Shenk TE (2002) Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. ProcNatlAcadSci99: 3932-3937.
- Allal C, Buisson-Brenac C, Marion V, Claudel-Renard C, Faraut T, et al. (2004)Human cytomegalovirus carries a cell-derived phospholipase A2 required for infectivity. J Virol 78: 7717-7726.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 11523
- [From(publication date):
August-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10810
- PDF downloads : 713