Review Article Open Access
New Insight into Alzheimer's Disease via Caspase 3-cleaved Tau: Pathogenic Role in Tau Oligomer Formation and Memory Deficits
Young Doo Kima, Jisu Parka and Yong Keun Jung*Global Research Laboratory, School of Biological Sciences, Seoul National University, Seoul 151-747, Korea
- Corresponding Author:
- Youn Keun Jung
School of Biological Sciences
Seoul National University, 1 Gwanak-ro
Gwanak-gu, Seoul 151-747, Korea
Tel: 8228804401
E-mail: ykjung@snu.ac.kr
Received date: June 21, 2017; Accepted date: June 28, 2017; Published date: July 05, 2017
Citation: Kima YD, Parka J, Jung YK (2017) New Insight into Alzheimer’s Disease via Caspase 3-cleaved Tau: Pathogenic Role in Tau Oligomer Formation and Memory Deficits. J Alzheimers Dis Parkinsonism 7:344. doi:10.4172/2161-0460.1000344
Copyright: © 2017 Kima YD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer’s disease (AD) is the most common and leading cause of dementia. AD has two different pathological hallmarks, extracellular amyloid beta plaques and intracellular neurofibrillary tangles (NFT). NFTs consist of abnormally modified tau protein that forms protein aggregates. Tau protein aggregates are prevalently observed as disease progresses and are believed to cause neuronal dysfunction. The caspase-3 cleaved form of tau, TauC3, is generated in cultured neurons under stress and is found in the brains of patients with AD in the early stages of disease when patients are asymptomatic. TauC3 accelerates tau oligomerization in vitro and in vivo, and induces neuronal degeneration. Moreover, the neuronal expression of TauC3 in transgenic mice causes memory deficits at a young age, which is concomitant with the appearance of tau oligomers. The removal of TauC3-containing oligomers and aggregates using drug treatment improves both memory and synaptic function. These findings demonstrate that TauC3 is critical for the formation of tau oligomers and small aggregates and may ultimately play a role in the rapid memory impairments observed in AD. Overall, TauC3 may represent a new therapeutic target for the prevention of AD.
Keywords
Alzheimer’s disease; Memory deficit; Neurofibrillary tangle; Tau aggregate; Tau oligomer; TauC3
Introduction
Alzheimer’s disease (AD) accounts for 70% of all dementias and the number of patients with AD is increasing every year [1]. Some medications are available to improve the symptoms of AD; however, disease-modifying medicine is not adequately developed and studies on the pathogenesis of AD are urgently required. AD has two major pathological hallmarks: One is the extracellular amyloid beta (Aβ) plaque [2], and the other is the intracellular neurofibrillary tangles (NFT) that consists of tau protein [3]. NFTs are primarily found in the entorhinal cortex and hippocampus, the primary brain regions that show functional deficits and degeneration in patients with AD in early stages of the disease, and spread to the cortex and other parts of the brain as disease progresses [4]. Nonetheless, it is under discussion whether NFTs themselves are toxic [5,6].
While turning off tau protein expression mitigates neuronal cell death and results in the recovery of memory [7], overexpression of normal full-length tau does not induce either memory deficits or tau oligomers in most cases [8]. In general, it is accepted that conformers between monomer and large tangle level aggregates, such as intermediate tau oligomers/small aggregates, may be the cause of the disease. Compared to normal tau, genetic mutation in or post-translational modification of tau facilitates the formation of tau aggregates. Similar intracellular protein oligomer and aggregate conformers found in other neurodegenerative diseases, such as mutant Huntingtin in Huntington’s disease [9,10], Spinocerebellar ataxia type 1 (SCA) in spinocerebellar ataxia [11], alpha-synuclein in Parkinson’s disease [12], and TAR DNAbinding protein-43 (TDP-43) in amyotrophic lateral sclerosis [13] are also thought to induce the respective diseases [14]. Therefore, it is important to study the tau species between oligomers and aggregates found in Alzheimer’s disease, with particular attention to their role in memory impairment and the loss of synaptic function.
Tau Oligomers and Aggregates in Tau Pathology
How can tau proteins form oligomers or small aggregates? There are two species of tau protein that can readily assemble into oligomers; abnormally phosphorylated tau [15] and the caspase-cleaved form of tau (TauC3) [16]. Although no genetic tau mutation has been uncovered in AD, other neurodegenerative diseases, such as FTD with Parkinsonism linked to chromosome 17 (FTDP-17), have several tau mutants (TauP301S, TauP301L, or TauR406W) that are likely to become highly phosphorylated [17]. Overexpression of these mutants in neuronal cells or mouse brain results in hyperphosphorylation of tau protein, and the formation of tau oligomers and aggregates in the late stages of disease in mice.
However, the tau pathologies that result from these genetic mutations do not lead to memory deficits in many in vivo mouse models. The induction of TauR406W expression in the mouse brain fails to induce memory deficits until the end of the life span of the mouse [18]. Moreover, overexpression of TauP301S in mice does not induce significant memory deficits, although the brains display massive neuronal loss [19]. Other mice expressing human TauV337M, THYTau22 or hTau40/ΔK280 show memory deficits. However, the memory loss observed in these mouse models appears in the late stages of disease (>9 months), after significant neuronal loss, which differs from the events occurring in the brains of patients with AD where neuronal loss occurs after memory loss [20-22]. On the other hand, an AD mouse model expressing mutant TauP301L at an extremely high level (>13 times the normal tau level in mice) exhibits tau aggregation and tangles, and early memory deficits (<3 months) that occur before neuronal loss (>6 months) [7]. Interestingly, this transgenic tau model shows caspase activation and rapid aggregation of tau together with TauC3 at the early stages of memory deficits, and only the cells in which TauC3 is present show electron microscopy-detectible or Thioflavin S-positive tau aggregates, indicating a potential link between tau pathology and TauC3 generation [23,24].
A Role of TauC3 in Tau Oligomer Formation and Aggregate-based Memory Impairments
It had been assumed that caspase activation is limited to cell death only. However, today, many proteins essential for cell function are known to be activated by caspase cleavage [25]. For example, low-level activation of caspase can regulate the α-amino-3-hydroxy-5-methyl-4- isooxazolepropionic acid (AMPA) receptor in the synapses of neurons and mediate synaptic transmission [26]. Aβ and multiple stressors appear to induce aberrant activation of caspases in neurons [27]. Tau is also known to be a substrate for multiple caspases: at least three caspases have been shown to participate in tau cleavage and the major form of caspase-cleaved tau found in AD brains is the caspase-3-cleaved tau, TauC3 [16]. TauC3 is also found in the brains of patients with AD with mild cognitive impairment [28], indicating that caspase-cleavage of tau is an early event in AD tangle pathology.
Tau contains a caspase-3 cleavage motif (DMVD) located in the 20th amino acid (421th Asp) from the C-terminus [16,28], and cleavage at this site can generate TauC3. TauC3 tends to form TauC3 self-oligomers or acts as a seed to produce oligomers with full-length tau in vitro [29]. Moreover, neurite regression and neuronal loss were observed with TauC3/tau aggregates in cultured primary neurons [16,30] and in vivo in a TauC3 drosophila model [31], respectively. Interestingly, expression of TauC3 in a transgenic mouse (similar level as endogenous mouse tau expression) results in learning and memory deficits, with synaptic dysfunction occurring as early as two months after birth [32]. Notably, tau oligomers appear in the brains of TauC3 mice showing memory deficits. Similar to TauC3 mice, a Tau35 transgenic mouse model expressing another truncated form of tau (Tau187-441) at a relatively low level (<10% of endogenous mouse tau expression) also shows memory deficits [33]. It will be interesting to determine whether tau oligomers or aggregates also appear together with tau pathology in this Tau35 mouse model.
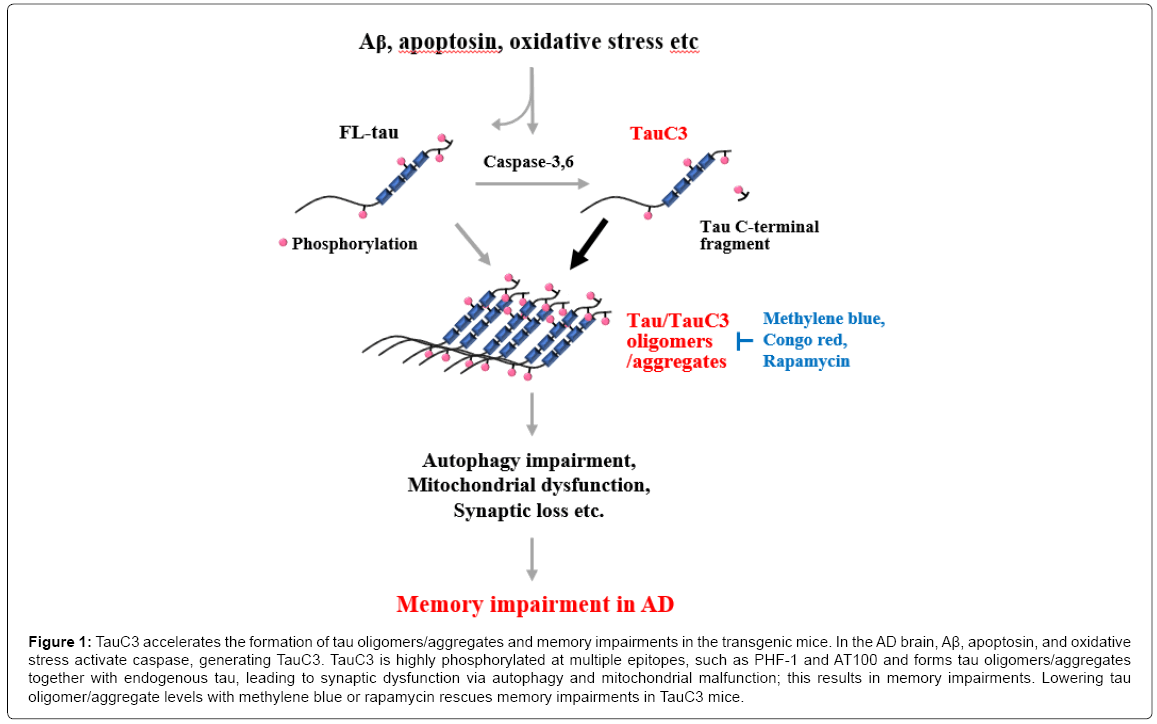
Then, an important question remains; how does TauC3 cause rapid memory deficits in the mouse model? As seen in an in vitro aggregation assay and in a fly model [31], TauC3 seems to be critical for the formation of both low-n tau oligomers and detergent-insoluble tau aggregates, and thereby for memory impairments in the mouse brain. TauC3 is found only in tau oligomers in the brains of mice showing memory deficits. In addition, soluble oligomers and detergent-insoluble small aggregates of tau can be detected in TauC3 mouse brains at a similar time point to that of the start of the memory deficits [32]. Moreover, when tau oligomers and aggregates are removed by treatment with aggregation blockers, such as methylene blue and Congo-red [32] or an autophagy inducer, such as rapamycin and pimozide memory [34] and synaptic function can be rescued (Figure 1). Similar data are also observed in an apoptosin-induced caspase-cleaved tau transgenic mouse model. Caspase-cleaved tau boosts the rapid formation of tau aggregates and synaptic protein loss [35]. These observations suggest a critical role of TauC3 in the formation of tau oligomers and aggregates, which leads to tau pathology, synaptic dysfunction and memory deficits.
Figure 1: TauC3 accelerates the formation of tau oligomers/aggregates and memory impairments in the transgenic mice. In the AD brain, Aβ, apoptosin, and oxidative stress activate caspase, generating TauC3. TauC3 is highly phosphorylated at multiple epitopes, such as PHF-1 and AT100 and forms tau oligomers/aggregates together with endogenous tau, leading to synaptic dysfunction via autophagy and mitochondrial malfunction; this results in memory impairments. Lowering tau oligomer/aggregate levels with methylene blue or rapamycin rescues memory impairments in TauC3 mice.
Of course, other roles of TauC3 in neuronal degeneration are possible. FKBP52 (52-kDa FK506 binding protein) and mitochondria are essential factors for axonal growth and synaptic function, respectively [36,37]. Recently it was shown that TauC3 and its oligomers might deplete FKBP52, an autophagy regulator, leading to lysosomal malfunction [38]. Given that mitochondria are critical for high energy demand fast protein turn-over in synapses [39], TauC3 might impair mitochondrial dynamics in neuronal cells [40]. Even in these situations, it might be the TauC3-harboring oligomers and small aggregates that affect axonal growth and mitochondrial dynamics, although this requires further clarification. Finally, propagation of disease-inducing protein oligomers has emerged as one of the causes of neurodegenerative diseases [41]. Propagation of pathogenic tau protein is a key issue for AD and other tauopathy progression, and the major form of tau conformers responsible for disease is the soluble low-n tau oligomers [42]. A recent report also suggested that transmitting tau oligomers also build up with TauC3: Treatment with a TauC3 monoclonal antibody effectively blocked tau transmission in vitro [43], highlighting the crucial role of TauC3 in disease propagation.
In summary, the TauC3 mouse might be a disease-relevant model of AD and TauC3 is an important therapeutic target for the prevention or treatment of AD. In the preclinical study by a biopharmaceutical company, TauC3 monoclonal antibody, which binds uniquely to Asp421 at the C-terminus of TauC3, reduces certain phosphorylated forms of Tau [44]. This kind of therapeutic approach targeting TauC3 might be useful to treat Alzheimer’s disease and other tauopathies in the near future.
Conclusion
Disease-related protein aggregation is indicated as the critical source of neurotoxicity in many neurodegenerative diseases. Many forms of wild-type and mutant tau proteins, including TauC3, are prone to form tau oligomers and aggregates both in vitro and in vivo. Of these, TauC3 is known to induce the formation of tau oligomers and aggregates in a transgenic mouse model, and this event appears to play a role in early and rapid memory impairments. Thus, TauC3 mice may be useful for investigating the role of tau in the pathogenesis of AD, and for testing drug candidates that interfere or block with the formation of tau oligomers and aggregates. To conclude, it is now more important than before to better understand tau-based memory loss in AD, in particular that related to TauC3.
Acknowledgement
This work was supported by a CRI grant (NRF-2016R1A2A1A05005304) and Global Research Laboratory program (NRF-2010-00341) funded by the Ministry of Education, Science and Technology in Korea.
References
- Williams SC (2013) Alzheimer's disease: Mapping the brain's decline. Nature 502: S84-85.
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297: 353-356.
- Hanger DP, Brion JP, Gallo JM, Cairns NJ, Luthert PJ, et al. (1991) Tau in Alzheimer's disease and Down's syndrome is insoluble and abnormally phosphorylated. Biochem J 275: 99-104.
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: 239-259.
- Shafiei SS, Guerrero-Munoz MJ, Castillo-Carranza DL (2017) Tau oligomers: Cytotoxicity, propagation and mitochondrial damage. Front Aging Neurosci 9: 83.
- Gerson JE, Castillo-Carranza DL, Kayed R (2014) Advances in therapeutics for neurodegenerative tauopathies: Moving toward the specific targeting of the most toxic tau species. ACS Chem Neurosci 5: 752-769.
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, et al. (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309: 476-481.
- Götz J, Probst A, Spillantini MG, Schäfer T, Jakes R, et al. (1995) Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J 14: 1304-1313.
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, et al. (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36: 585-595.
- Fan HC, Ho LI, Chi CS, Chen SJ, Peng GS, et al. (2014) Polyglutamine (PolyQ) diseases: Genetics to treatments. Cell Transplant 23: 441-458.
- Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, et al. (2012) Brain pathology of spinocerebellar ataxias. Acta Neuropathol 124: 1-21.
- Visanji NP, Brotchie JM, Kalia LV, Koprich JB, Tandon A, et al. (2016) α-Synuclein-based animal models of Parkinson's disease: Challenges and opportunities in a new era. Trends Neurosci 39: 750-762.
- Mann DM, Snowden JS (2017) Frontotemporal lobar degeneration: Pathogenesis, pathology and pathways to phenotype. Brain Pathol.
- Giacomelli C, Daniele S, Martini C (2017) Potential biomarkers and novel pharmacological targets in protein aggregation-related neurodegenerative diseases. Biochem Pharmacol 131: 1-15.
- Tepper K, Biernat J, Kumar S, Wegmann S, Timm T, et al. (2014) Oligomer formation of tau protein hyperphosphorylated in cells. J Biol Chem 289: 34389-34407.
- Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, et al. (2001) Pro-apoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis 8: 162-172.
- Baizabal-Carvallo JF, Jankovic J (2016) Parkinsonism, movement disorders and genetics in frontotemporal dementia. Nat Rev Neurol 12: 175-185.
- Ikeda M, Shoji M, Kawarai T, Kawarabayashi T, Matsubara E, et al. (2005) Accumulation of filamentous tau in the cerebral cortex of human tau R406W transgenic mice. Am J Pathol 166: 521-531.
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, et al. (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53: 337-351.
- Tanemura K, Murayama M, Akagi T, Hashikawa T, Tominaga T, et al. (2002) Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci 22: 133-141.
- Schindowski K, Bretteville A, Leroy K, Bégard S, Brion JP, et al. (2006) Alzheimer's disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol 169: 599-616.
- Van der Jeugd A, Hochgräfe K, Ahmed T, Decker JM, Sydow A, et al. (2012) Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau. Acta Neuropathol 123: 787-805.
- de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, et al. (2010) Caspase activation precedes and leads to tangles. Nature 464: 1201-1204.
- Lin WL, Dickson DW, Sahara N (2011) Immunoelectron microscopic and biochemical studies of caspase-cleaved tau in a mouse model of tauopathy. J Neuropathol Exp Neurol 70: 779-787.
- Snigdha S, Smith ED, Prieto GA, Cotman CW (2012) Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci Bull 28: 14-24.
- Li Z, Sheng M (2012) Caspases in synaptic plasticity. Mol Brain 5: 15.
- Tu S, Okamoto S, Lipton SA, Xu H (2014) Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease. Mol Neurodegener 9: 48.
- Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, et al. (2014) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Investig 114: 121-130.
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, et al. (2003) Caspase cleavage of tau: Linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A 100: 10032-10037.
- Chung CW, Hong YM, Song S, Woo HN, Choi YH, et al. (2003) Atypical role of proximal caspase-8 in truncated Tau-induced neurite regression and neuronal cell death. Neurobiol Dis 14: 557-566.
- Khurana V, Elson-Schwab I, Fulga TA, Sharp KA, Loewen CA, et al. (2010) Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS Genet 6: e1001026.
- Kim Y, Choi H, Lee W, Park H, Kam TI, et al. (2016) Caspase-cleaved tau exhibits rapid memory impairment associated with tau oligomers in a transgenic mouse model. Neurobiol Dis 87: 19-28.
- Bondulich MK, Guo T, Meehan C, Manion J, Rodriguez Martin T, et al. (2016) Tauopathy induced by low level expression of a human brain-derived tau fragment in mice is rescued by phenylbutyrate. Brain 139: 2290-2306.
- Kim Y, Jeong EI, Nah J, Yoo SM, Lee W, et al. (2017) Pimozide reduces toxic forms of tau in TauC3 mice via AMPK-mediated autophagy. J Neurochem.
- Zhao Y, Tseng IC, Heyser CJ, Rockenstein E, Mante M, et al. (2015) Appoptosin-mediated caspase cleavage of tau contributes to progressive supranuclear palsy pathogenesis. Neuron 87: 963-975.
- Shim S, Yuan JP, Kim JY, Zeng W, Huang G, et al. (2009) Peptidyl-prolyl isomerase FKBP52 controls chemotropic guidance of neuronal growth cones via regulation of TRPC1 channel opening. Neuron 64: 471-483.
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G (2013) Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep 5: 1564-1575.
- Meduri G, Guillemeau K, Dounane O, Sazdovitch V, Duyckaerts C, et al. (2016) Caspase-cleaved Tau-D(421) is colocalized with the immunophilin FKBP52 in the autophagy-endolysosomal system of Alzheimer's disease neurons. Neurobiol Aging 46: 124-137.
- Son JH, Shim JH, Kim KH, Ha JY, Han JY (2012) Neuronal autophagy and neurodegenerative diseases. Exp Mol Med 44: 89-98.
- Pérez MJ, Vergara-Pulgar K, Jara C, Cabezas-Opazo F, Quintanilla RA (2017) Caspase-cleaved tau impairs mitochondrial dynamics in Alzheimer's Disease. Mol Neurobiol.
- Jucker M, Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501: 45-51.
- Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, et al. (2016) Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 19: 1085-1092.
- Nicholls SB, DeVos SL, Commins C, Nobuhara C, Bennett RE, et al. (2017) Characterization of TauC3 antibody and demonstration of its potential to block tau propagation. PLoS ONE 12: e0177914.
- Rohn TT (2015) Caspase cleaved tau in Alzheimer's disease: A therapeutic target realized. Int J Neurol Neurother 2: 014.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 3713
- [From(publication date):
August-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 2861
- PDF downloads : 852