Research Article Open Access
New Approach to Use Phage Therapy against Aeromonas hydrophila Induced Motile Aeromonas Septicemia in Nile Tilapia
El-Araby DA1*, Gamal El-Didamony2 and Marihan Megahed TH2
1Department of Fish Diseases and Management, Central Laboratory of Aquaculture Research Center Ponds of Abbassa, Abou-Hammad, Sharkia, Egypt
2Department of Botany, Zagzig University, Egypt
- Corresponding Author:
- El-Araby DA
Department of Fish Diseases and Management
Central Laboratory of Aquaculture Research Center Ponds of Abbassa
Abou-Hammad, Sharkia, Egypt
Tel: 2629-2261
E-mail: dr.doaae@gmail.com
Received Date: May 07, 2016; Accepted Date: May 28, 2016; Published Date: May 31, 2016
Citation: El-Araby DA, El-Didamony G, Megahed MTH (2016) New Approach to Use Phage Therapy against Aeromonas hydrophila Induced Motile Aeromonas Septicemia in Nile Tilapia. J Marine Sci Res Dev 6:194. doi:10.4172/2155-9910.1000194
Copyright: © 2016 El-Araby DA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Infections with Aeromonas hydrophila is a progressive problem in aquaculture. The use of antibiotic such as Ciprofloxacine has contributed to the rapid and effective treatment of disease cause by this organism. However the Fast-paced increase of resistance to the Said antibiotics has posed problems and there is now a new approach to look for alternative method to control this bacterial pathogen. Phage therapy comes in as a new method to respond to these growing problems. This study demonstrated the promising action of isolated bacteriophage ΦZH1 and ΦZH2 for therapy against Motile Aeromonas Septicemia in Nile tilapia caused by Aeromonas hydrophila.
Keywords
Phage therapy; Aeromonas hydrophila; Motile Aeromonas Septicemia; Fish; Nile tilapia
Introduction
Egyptian aquaculture has developed rapidly in recent years, where there are many problems facing fish, one of them is bacterial infection for fish, which constitutes a huge menace for aquaculture farming, leading to disastrous economic loss and health risks for the consumer [1].
Aeromonas hydrophila a gram negative, rod shaped enterobacterium and distributed widely in aquatic environments [2]. It is one of the most important agents of the outbreaks in fresh water fish. The main problem involving the use of antibiotics against Aeromonas infections is the development of resistance by these bacteria [3].
A bacteriophage is a virus that infects bacteria and can either instantly kill a bacterial cell or integrate its DNA into the host bacterial chromosome [4]. If the phage DNA is integrated into the host, the phage can then stay within the bacteria causing no harm. This pathway is called the lysogenic cycle. On the other hand, the phage can also cause eventual lysis and death of the host after it reproduces inside the host and escapes with numerous progeny through the lytic cycle [5]. Phages are effective against multidrug resistant pathogenic bacteria because the mechanisms by which they induce bacteriolysis differ completely from those antibiotics. Moreover, phages have self-limitation, meaning that the number of phages remain in very low level after killing the target bacteria [6].
The role of bacteriophages in the environment has been the subject of intense investigation over the past several years [7]. The development of techniques to study natural viral populations in situ has progressed tremendously. Various aspects of bacteriophage ecology in natureincluding abundance, role in microbial mortality and water column trophodynamics, viral decay rates, repair mechanisms and lysogeny are gradually being understood [8]. Much of research has focused on using phages to control diseases caused by a variety of human pathogenic bacteria including Salmonella [9], Listeria [10] and Campylobacter [11] species. In addition, the current attempts to apply phages in the control of human pathogens, aquatic animal pathogens have also been investigated as a target for phage therapy.
A number of phages have been isolated for potential use in phage therapy against important aquatic animal pathogens such as Aeromonas salmonicida in brook trout (Oncorhynchus fontinalis) [12], Vibrio harveyi in shrimp (Penaeus monodon) [13], Pseudomonas plecoglossicida in ayu (Plecoglossus altivelis) [14] and Lactococcus garvieae in yellowtail (Seriolaquin queradiata) [14].
The efficiency of an Aeromonas hydrophila bacteriophage isolated from ponds of Abbassa was compared to that of the antibiotic Ciproflixacine for the treatment of “Motile Aeromonas Septicemia” (MAS) in Oreochromis niloticus [15]. Hence, this study aims to isolate and identify of A. hydrophila of lytic phages and efficiency of phages to control A. hydrophila in aquria.
Material and Methods
Isolation of Aeromonas hydrophila bacteriophages
Bacteriophages were isolated from sewage samples by the specific enrichment method of Adams. The supernatant were filtered through a 0.45 μm pore size syringe filter and assayed for phage activity by double layer agar technique. The presence of phage in filtrate was detected by spot test and plaque assay methods as described by Eisenstark [16]. Phages were propagated and purified from single-plaque isolates according to Adams [17]. Plaques were distinguished by differences in plaque morphology, size and turbidity and were purified by successive single plaque isolation using the propagating host strain. Afterward, Phage suspensions of high titer lysates were prepared in two ways first Confluent plate lysates were prepared according to the method of Eisenstark [16].
Physical characterization of isolated bacteriophages
Effect of temperature on isolated phage: The effect of temperature on the viability of phage was studied by the method described by Clokie [18]. Phage suspension was incubated at 30, 40, 50, 60, 70, 80 and 90ºC in water bath for 10 min. Phages survival was determined.
Effect of irradiation by ultra violet light on the isolated bacteriophages: The effect of UV light on the viability of phages was studied by method described by Clokie [18]. UV sensitivity was determined by exposing 5 ml of phage lysate (4.8×1011 pfu/ml and 5.0×1011 pfu/ml) for phage ΦZH1 and ΦZH2 respectively (diluted 0.1 in saline solution) in an uncovered small Petri dish to UV-light at distance 20 cm from Cosmolux UVA, A1-11-40 W, PREHEAT- BIPIN, Mode In W-Germany, lamp was used as a UV source for the following times: 20, 40, 60, 80,100 and 120 minutes. Phages survival was determined by plaque assay technique [17].
Biological Characteristics
Host range
The host ranges of the phages were determined by spot tests [16]. The adsorption experiments and single step growth curve of phages were carried out as described by Adams [17].
Effect of different MOI on bacterial growth
Multiplicity of infection (MOI) was defined as the ratio of virus particles to potential host cells and prepared according to Birge [19].
Morphological characteristics (Electron microscopy)
High stock titer of phages (4.8×1011 pfu/ml, 5.0×1011 pfu/ml ) were negatively stained with 2% (w/v) aqueous uranyl acetate (pH 4.0) on a carbon-coated grid and examined by transmission electron microscopy JEOL( JEM-1400cx) at an accelerating voltage of 80 KV.
Effect of bacteriophages on mortality of Nile tilapia by Aeromonas hydrophila infections
Nile tilapia (O. niloticus) (weight range: 25-40 g) were obtained from ponds of Fish Research Center of Abbassa, Abo- Hammad, Sharkia. “Where these fishes were transferred alive to Microbiological laboratory, Faculty of Veterinary Medicine, Zagazig University. All fishes were kept in tanks (40 cm×70 cm×60 cm) with approximately 45 l de-chlorinated tap water. Acclimatized for 1 week prior to the experiment and fed with organic feeds. Four aquarium were used for the experiment and each aquarium contained 5 fishes. The aquaria were maintained at 28 ± 1°C with a pH of 7. Bacterial inoculum of A.hydrophila was prepared using a 24 hrs old culture of A. hydrophila inoculated in TSB. The inoculum was subjected to to 10-7, 10-8 and 10-9 dilutions. These were transferred to falcon tubes and were centrifuged for 30 min at 3,000×g. After centrifugation, the supernatant was removed and 5 mL of normal saline solution (0.9%) was added. The lethal dose (LD) of A. hydrophila was determined by intraperitoneally injecting the fishes with 0.5 mL of doses [20]. The dose sufficient to cause death among the fishes within 72-96 h was taken as the optimum LD100 (lethal dose causing 100% mortality). Clinical signs of MAS such as skin lesions, hyperemia, rotting of caudal and dorsal fins, and hyperemia in fin bases were observed prior to the experimental treatment [21].
The mortality in the tanks was monitored after 15 days for each challenge. The concentration of bacteria and phage in the water tanks was monitored by inoculating the corresponding dilution in TSA plates to detect the bacteria, and using the double-layer agar plaque assay to determine the phage concentration [17].
Two groups only were injected with A. hydrophila. Group 1, which contain water only. Group 2, which contain water and fishes served as the negative control. Group 3, which was used as positive control contain water and fishes were injected with A. hydrophila but was not treated with bacteriophages. Group 4, was also injected with A. hydrophila and was treated with bacteriophage. Administration of bacteriophage (Group 4) was done 24 hrs after injection of A.hydrophila.
Results
Morphology of plaques and phage(s)
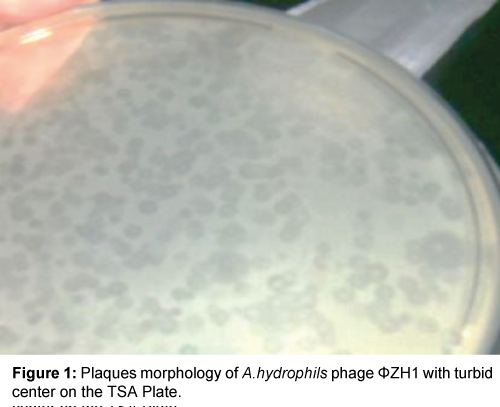
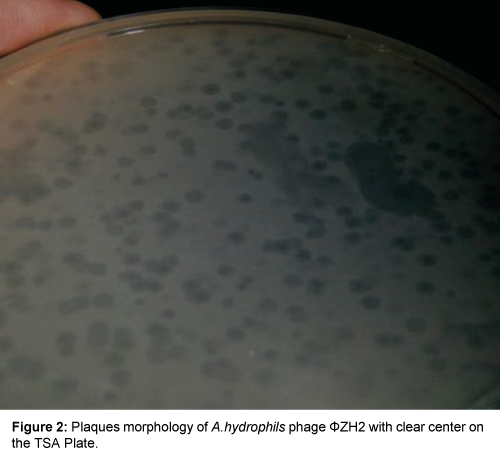
Result in Table 1 and Figures 1 and 2 showed that two phenotypic plaques were appeared. One of them remarked as ΦZH1 which measured 3.0 mm in diameter with turbid center (LTC), while other remarked as ΦZH2 which measured 4.0 mm in diameter with clear center (LCC).
| Phage No. | Appearance of plaques | Diameter of isolated phages (nm) | |
|---|---|---|---|
| Head | Tail | ||
| φZH1 | LT | 100 | 30 |
| 100 | 30 | ||
| φZH2 | LC | 50 | 7.0 |
Table 1: Morphology of plaques and isolated phages under electronmicroscope after negatively staining.
Morphology of isolated plaques under electron microscope
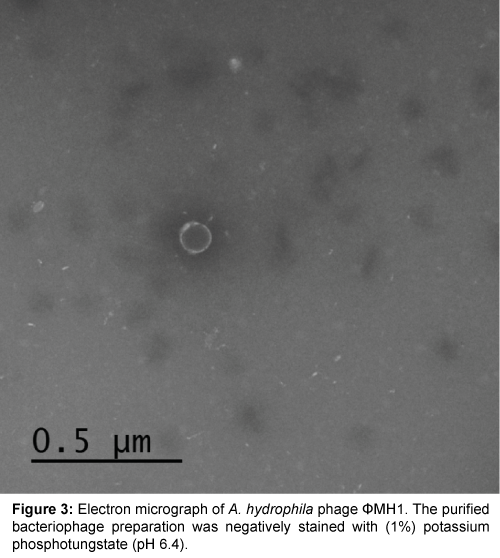
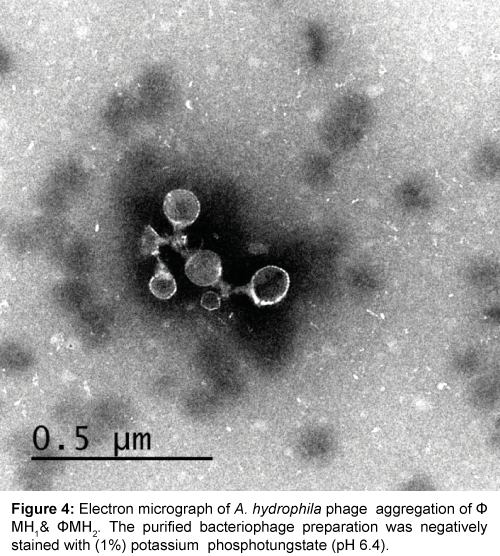
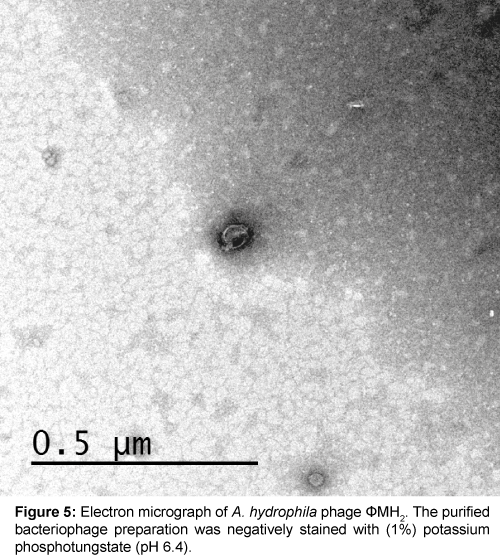
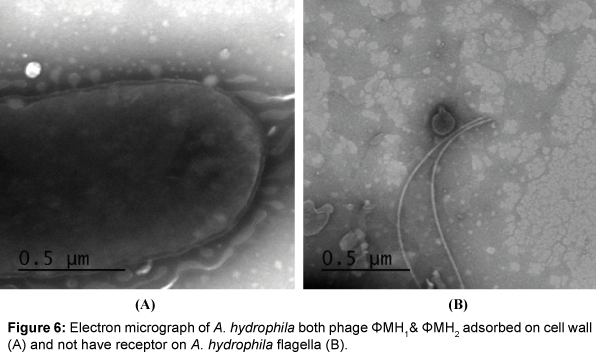
Five successive transfer plaques with clear area and center were selected to prepare height titer phage stocks (4.8×1011 pfu/ml) and (5.0×1011 pfu/ml). Each phage stock viewed under electron microscope after staining by (1%) potassium phosphotungstate at pH 6.4. Results in Table 1 and Figures 3-5 showed that, two phages were appeared. Icosahedral heads of these phages (φZH1 and φZH2) measured 100 and 50 nm respectively. Also these phages had very short non-contractile tail measured 30 and 7 nm respectively. Phages (φZH1 and φZH2) adsorbed on cell wall and not have receptor on flagella (Figure 6). On the basis of phage morphology, the phages φZH1 and φZH2 belongs to the family Podoviridae [22].
Host range
The host range of isolated phages (ΦZH11 and ΦZH2) was determined against isolates of Aeromonas bacteria and 4 strains of non-Aeromonas bacteria. Results in Table 2 revealed that the isolated phages ΦZH1 and ΦZH2 was very specific to infect Aeromonas and does not have the ability to infect any isolates of non-Aeromonas bacteria.
| Hosts | Sources | Formation of lytic area by spot test | |
|---|---|---|---|
| Aeromonashydrophilabacteria | 1 2 3 4 5 6 |
Ponds of Aquaculture Research Center of Abbassa, Abo-Hammad, Sharkia | - +++ - - - - |
| Non Aeromonashydrophilabacteria | Escherichia coli Listeria meningitis Staphylococcus aureus P. aeruginosa 62 |
Central Laboratory of aquaculture Research Center of Abbassa, Abo-Hammad, Sharkia Central Laboratory of aquaculture Research Center of Abbassa, abo-Hammad, Sharkia Faculty of Scince , zagazig University (Accession number KR270348) Faculty of Pharmacy, Zagazig University (Gen bank Bio project 219845) |
- - - - |
Table 2: Host range of A.hydrophila phages φZH1 and φZH2.
Effect of thermal inactivation
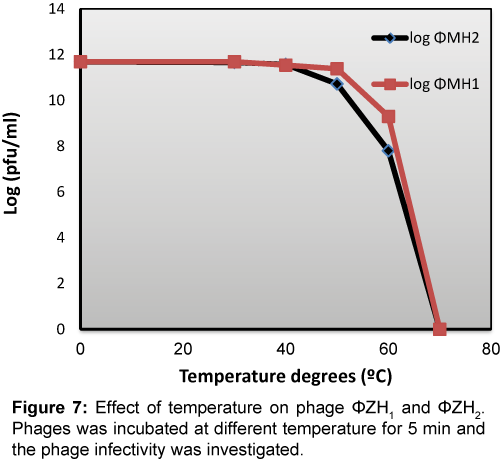
The infectivity of both phages was highly sensitive to temperature above 40°C. Whereas both phages lost its infectivity by percentage reached to 88% and 50% for ΦZH1 and ΦZH2 respectively (Figure 7).
Effect of irradiation by ultraviolet light on the isolated phages
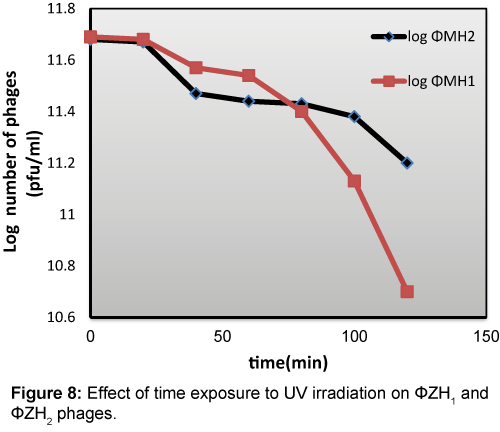
The exposure of 1011 pfu/ml purified phages suspensions to UV irradiation (at high 20 cm) for different periods of time (0-120 min) illustrated by Figure 8 from these results, isolated phages (ΦZH1 and ΦZH2) are resistant to UV irradiation. Whereas the infectivity of this phage still active after exposure to UV (40w) for 120 min. ΦZH1 lost 50% of its infectivity after exposure to UV irradiation for 100 min While, phage ΦZH2 reached to this percentage after exposure to UV for 80 min.
Adsorption rates
The two phages in current studies ΦZH11 and ΦZH2 exhepted to different rates. The maximum adsorption and percentage of adsorption are presented in Table 3. Adsorption rate were fast since the maximum phages adsorbed reached 51% and 66.8% of phages ΦZH11 and ΦZH2 were adsorbed after 20 and 30 min respectively the adsorption constant (K) were (2.7×10-13 ml/min) and (2.2×10-13 ml/min) for ΦZH11 and ΦZH2 respectively as determined by the formula K=2.3/ (B) t × log (Po/P), where Po= phage assay at zero time, P= phage not adsorbed at time t min, (B) = concentration of bacteria as number of cells/ml and K=velocity constant expressed as ml/min. Adsorption rate constant for both phages were similar.
| Incubation time (min) | φZH1 | φZH2 | ||
|---|---|---|---|---|
| pfu/ml | % | PFU/ML | Log | |
| 0 10 20 30 40 50 60 70 |
4.8×1011 4.0×1011 2.45×1011 2.55×1011 2.71×1011 4.0×1011 3.8×1011 5.8×1011 |
100 20 51 49 46 20 20 6 |
5.0×1011 2.11×1011 4.0×1011 1.66×1011 1.75×1011 4.10×1011 6.4×1011 6.9×1011 |
100 57.8 20 66.8 65 18 - - |
Table 3: Adsorption rate of A.hydrophila phages φZH1 and φZH2 in TS broth at 37°C MOI >1.0.
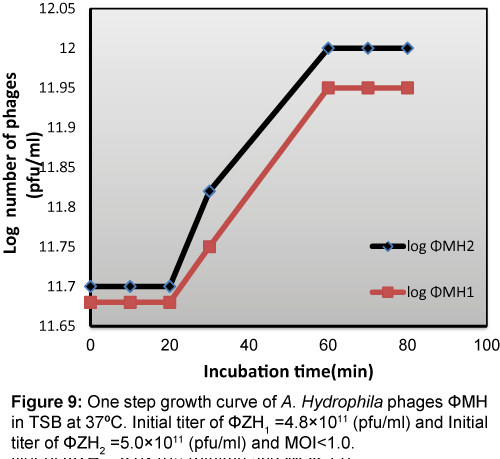
One-step growth experiment
One step growth curve (Figure 9) shows that the latent period was about 20 min, the rise period was 60 min, and the mean burst size was about 113 and 114 pfu per infected cell for ΦZH1 and ΦZH2 respectively.
Effect of different concentration of phages on growth of A. hydrophila
Each phage was used at MOI = 10, 1 and 0.1 over a time from 0-24 hrs. Data in Table 4 showed that, the highest reduction in bacterial count was observed when phage ΦZH1 and ΦZH2 added separately to A. hydrophila at M.O.I = 10 and incubated at 37°C for 12 hrs. On other hand, addition of phage ΦZH1 or ΦZH2 to A. hydrophila at M.O.I less than 10 (1.0 or 0.1) not gave efficient in reduction of bacterial growth.
| Time | A. hydrophila | φZH1 | φZH2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOI=0.1 | MOI = 1 | MOI =10 | MOI=0.1 | MOI = 1 | MOI =10 | |||||||||
| Bacterial count | OD | pfu | cfu | pfu | cfu | pfu | cfu | pfu | cfu | Pfu | cfu | pfu | cfu | |
| 6 | 1.8×1013 | 0.324 | 4.1×1011 | 1.9×1013 | 5.5×1016 | 2.1×1013 | 7.3×1017 | 1.7×1013 | 4.5×1013 | 1.8×1013 | 2.8×1013 | 2.2×1013 | 3.8×1013 | 2.2×1013 |
| 12 | 2.5×1013 | 0.450 | 4.0×1013 | 2.3×1013 | 6.1×1016 | 2.4×1013 | 6.8×1017 | 3.1×1011 | 4.0×1013 | 4.6×1013 | 2.1×1013 | 3.8×1013 | 9.5×1014 | 2.1×109 |
| 18 | 3.6×1013 | 0.648 | 3.8×1013 | 3.1×1013 | 6.8×1016 | 2.1×1013 | 5.4×1017 | 3.4×1011 | 3.9×1013 | 4.1×1013 | 1.7×1013 | 3.4×1013 | 4.9×1016 | 1.8×109 |
| 24 | 4.2×1013 | 0.756 | 3.0×1013 | 2.8×1013 | 7.1×1013 | 2.0×1013 | 3.8×1017 | 3.3×1011 | 3.2×1013 | 4.2×1013 | 1.8×1013 | 2.9×1013 | 6.0×1016 | 1.1×109 |
Table 4: Effect of incubation of several different multiplicity of infection (M.O.I) on growth curve of the host A.hydrophila.
The challenges to motile Aeromonas Septicemia (MAS) causing bacteria by both ΦZH1 and ΦZH2
Result in Table 5 showed that the addition of A. hydrophila to fishes in aquaria containing Nile water increases the mortality compared to it control. 2nd aquaria mortality of this treatment reached 68%. Addition of phage to such treatment reduced the mortality to 18% with reduction efficiency reached above 50%. Also phage reduced total count of bacteria from 4.8×1013 cfu/ml to 7.3×107 cfu/ml. on other hand, phages titer in fourth treatments increased above the initial titer of phages it changed from 8.1×109 to 8.1×1013 pfu/ml.
| Treatment | Mortality% | Total bacterial count after 72 hrs. | Phages titer after 72 hrs. |
|---|---|---|---|
| Nile water | 0.00 | 5.2×1011 | 0.00 |
| Nile water + fishes | 5.00 | 3.9×1011 | 0.00 |
| Nile water + fishes + A. hydrophila (3.73×109 cfu/ml) | 68.00 | 4.8×1013 | 0.00 |
| Nile water + fishes + A. hydrophila + phages (8.1×109 pfu/ml) (MOI=2.1) | 18.00 | 7.3×107 | 8.1×1013 |
Table 5: The challenges to Motile Aeromonas Septicemia (MAS) causing bacteria by both φZH1 and φZH2.
Discussion
Fish diseases are major problem for fish farming industry and among those bacterial infections are considered to be a major cause of mortality in fish [23]. These awaited drawbacks enforced the fish pathologists to seek for other alternatives; the use of natural immunostimulants in fish culture for the prevention of diseases was a promising new development and could solve the problems of massive antibiotic use. Natural immunostimulants were biocompatible, biodegradable and safe for both the environment and human health. Moreover, they possess an added nutritional value [24].
Aeromonas hydrophila was described as the dominant infectious agent of ‘fish-bacterial-septicemia’ in freshwater cultured finfish all over the world [25]. A hydrophila was also associated with EUS, which was a major problem in different countries [26]. The observed clinical signs in the examined fish suffering from motile Aeromonas Septicemia (MAS) were previously reported by Samal [25]. They reported that septicemia, ascitis, erosion, ulceration, detachment of scale, exophthalmia and muscular necrosis were the most predominant clinical signs of MAS in Nile tilapia.
In this study, the results showed that two bacteriophages ΦZH1 and ΦZH2 possess infection on its specific host A.hydrophila, isolated from Nile water. According to the electron micrograph; the two phages were characterized as podoviruses. The dimensions of the isolated podoviruses were similar or semi-similar to each other and also resembled those which were previously isolated for A.hydrophila [27]. A hydrophila phages ΦZH1 and ΦZH2 infected some Ahydrophila strains, but none of other genera or species tested. These results are in accordance with those obtained by Mitchell Sc [28].
Temperature is a crucial factor for bacteriophage survivability [29]. The results in showed that ΦZH1 and ΦZH2 phages were thermos table, between a temperature ranges of 30-60°C where it still remained active after 10 min exposure at 60°C. Interestingly, ΦZH1 and ΦZH2 phages survived at 37°C, with no significant loss in phage particle number, which is a very important parameter for phages considered for therapeutic application.
Phages examined here were tolerant to UV irradiation with distinct rate of inactivation where ΦZH1 and ΦZH2 lost 50% of their infectivity after exposure time reach to 100 and 80 min respectively. These result are in accordance with those obtain by Ramanandan [29]. This finding indicated that phages are suitable to use in field experiment where their infectivity not affected by UV in solar reached to water in aquarium.
The data obtained in one- step growth experiment were comparable with data presented by Cheng [30]. These authors conducted a similar growth experiment with Aeromonas species. It as reported by those authors that the latent periods of phage DH1 were 90 min which was much longer than Aeromonas hydrophila phages Aeh1 and Aeh2 [28] on other hand in our studies the latent period of phages ΦZH1 and ΦZH2 were 20 min. the average burst size of phage DH1 were about 125 PFU/cell, which was also bigger than Aeh1 and Aeh2 [28], but the latent period of phages ΦZH1 and ΦZH2 were 113 and 114 pfu per infected cell. The isolated phages (ΦZH1 and ΦZH2) administered via injection was found to be effective in treating fish infected with Aeromonas hydrophila shown through the significant decrease in number of A.hydrophila found in the water of treated fish. Where our results showed that the addition of phages ΦZH1 and ΦZH2 (M.O.I=2.1) to Nile water in aquaria. Which was inoculated by A.hydrophila (3.37×109 CFU/ml) reduced the percentage of mortality from 68% to 18% after treatment for 15 days. Also total number of bacteria in polluted aquarium changed from 4.18×1013 cfu/ml to 7.5×107 Cfu/ml after three days of treatments. The efficiency of isolated phages reduction of A.hydrophila in Nile water more than that founded by Donn Cruz-Papa [15].
References
- Lau SK, Woo PC, Fan RY, Lee RC, Teng JL, et al. (2007) Seasonal and tissue distribution of Laribacterhongkongensis, a novel bacterium associated with gastroenteritis, in retail freshwater fish in Hong Kong. Intern J of Food Microbiology 113: 62-66.
- Gonzalez Serrano CJ, Santos JA, Garcia Lopez ML, Atero A (2002)Virulence markers in Aeromonashydrophila and Aeromonasveroniibiovarsobria isolates from freshwater fish and from a diarrhoea case. J ApplMicrobiol 93: 414-9.
- Mitchell AJ, Plumb JA (1980) Toxicity And Efficacy Of Furanace On Channel Catfish IctalurusPunctatus (Rafinesque) Infected Experimentally With Aeromonashydrophila. J of Fish Diseases 3: 93-99.
- Madigan MT, Martinko JM, Parker J (1997) Brock Biology of Microorganisms. Upper Saddle River, NJ: Prentice Hall.
- Watson JD, Hopkins NH, Roberts JW, Steitz JA, Weiner AM (1987) Molecular Biology of the Gene. Menlo Park: Benjamin Cummings Company, Inc, CA.
- Nakai T, Park SC (2002) Bacteriophage therapy of infectious diseases in aquaculture. Res Microbiol 153: 13-18.
- Talledo M, Rivera IN, Lipp EK, Neale A, Karaolis D, et al. (2003) Characterization of a Vibrio cholerae phage isolated from the coastal water of Peru. Environ Microbiol 5: 350-354.
- Seed KD, Bodi KL, Kropinski AM, Ackermann HW, Calderwood SB (2011) Evidence of a dominant lineage of Vibrio cholera specific lytic bacteriophages shed by cholera patients over a 10-year period in Dhaka, Bangladesh. BioMed 2: e00334.
- Goode D, Allen VM, Barrow PA (2003) Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by Applica.
- Leverentz B, Conway WS, Janisiewich W, Camp MJ (2004) Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J Food Prot 67: 1682-1686.
- Atterbury RJ, Connerton PL, Dodd CER, Rees CED, Connerton IF (2003) Application of host-specific bacteriophages to the surface of chicken leads to reduction in the recovery of Campylobacter jejuni. Appl Environ Microbiol 69: 6302-6306.
- Imbeault S, Parent S, Lagace M, Uhland CF, Blais JF (2006) Using bacteriophages to prevent furunculosis caused by Aeromonassalmonicida in farmed brook trout. J AquatAnim Health 18: 203-214.
- Shivu MM, Rajeeva BC, Girisha SK, Karunasagar I, Krohne G, et al. (2007) Molecular characterisation of Vibrio harveyibacteriophages isolated from aquaculture environments along the coast of India. Environ Microbiol 9: 322-331.
- Nakai T, Sugimoto R, Park KH, Mori Kl, Nishioka T, et al. (1999) Protective effects of bacteriophage on experimental Lactococcusgarvieaeinfection in yellowtail. Dis AquatOrga 37: 33-41.
- Cruz-Papa DMAD, Candare CMG, Cometa GLS, Gudez DEG, Guevara AMIT, et al. (2014)Aeromonashydrophila Bacteriophage UP87: An Alternative to Antibiotic Treatment for Motile Aeromonas Septicemia in Nile Tilapia (Oreochromis niloticus). PHILIPP AGRIC SCIENTIST 97: 96-101.
- Eisenstark A (1967) Bacteriophage techniques. Meth Virol 1:449-524.
- Adams MH (1959) Bacteriophages. Inter science Publishers, New York, NY, USA.
- Clokie MR, Kropinski AM (2009): Bacteriophages Methods and Protocols. Volume 1: Isolation, Characterization and Interactions. New York: Human Press p: 307.
- Birge EA (2000) Bacterial and bacteriophage Genetics. 4thed Springer, New York.
- Carraschi SP, Cruz DAC, Neto JGM, De Morges FR, Junior ODR, et al. (2013) Fish Pathology (3rdedtn) WB SAUNDERS London, Edinburgh, New York, Philadelphia St. Louis, Sydney, Toronto.
- Yardimic B, Aydin Y (2011) Pathological findings of experimental Aeromonashydrophila infection in Nile tilapia (Oreochromisniloticus) Ankara Univ Vet FakDerg 58: 47-54.
- Matthews REF (1982) Classification and nomenclature of viruses. Fourth report of the International Committee on Nomenclature of Viruses. Intervirology 17: 1-200.
- Gomez-Gil B, Roque A, Turnbull JF (2000) The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms. Aquaculture 191: 259-270.
- Ortuno J, Cuesta A, Rodriguez A, Eesteban MA, MeseguerJ (2002)Oral administration of yeas,Saccharomycescerevisiae, enhances the celluler innate immune response of gillheadseabream,Sparusaurata LJ. Veterinary immunology and immunopathology 85: 41-50.
- Samal KS, Das KB, Pal BB (2014) Isolation, biochemical characterization, antibiotic susceptibility study of Aeromonashydrophila isolated from freshwater fish, nt J CurrMicrobiol App Sci 3: 259-267.
- Iqbal MM, Tajima K, Ezura Y (1999) Pathogenicity of Motile Aeromonas Species Isolated from Fishes with Epizootic Ulcerative Syndrome (EUS) in Southeast Asian Countries. Bull Fac Fish Hokkaido Univ 50: 93-100.
- Lavigne R, Burkal’tseva MV, Robben J (2003) The genome of bacteriophage w KMV, a T7-like virus infecting Pseudomonas eruginosa . Virology 312: 49-59.
- Mitchell SC , Rouf MA (1983) Isolation and partial characterization of two Aeromonashydrophila bacteriophages ApEnviMicrobiol p: 1670-1676.
- Ramanandan P, Shiivapriya C, Xuhua X (2015) E. coli and Staphylococcus phages: Effect of translation initiation efficiency on differential codon adaptation mediated by virulent and temperature lifestyles. J Gen Virol: vir 0.000050vl- vir 0.000050.
- Cheng K, Zhang D, Deng J, Zhao Y (2015): Isolation and characterization of an Aeromonaspuncatata Bacteriophage, the open biomed Eng J 9: 185-187.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 13927
- [From(publication date):
June-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 12802
- PDF downloads : 1125