Neutrophil Extracellular Traps - The Invisible Inflammatory Mediator in Fibrosis
Received: 14-May-2015 / Accepted Date: 08-Jul-2015 / Published Date: 12-Jul-2015 DOI: 10.4172/2161-0681.1000239
Abstract
Fibrosis is a dynamic process of abnormal connective tissue formation as a result of excessive wound healing and extracellular matrix deposition. It can occur in almost all tissues and it is a major contributor to clinical diseases, leading to organ failure and accounting for approximately one third of deaths worldwide [1,2]. It is proposed that fibrotic response may be distinguished in four major phases: 1) the initiation after tissue injury; 2) the activation of effector cells; 3) the deposition of extracellular matrix and 4) the progression to fibrosis with subsequent organ failure by excessive accumulation of extracellular matrix without sufficient degradation [2].
Introduction
Fibrosis is a dynamic process of abnormal connective tissue formation as a result of excessive wound healing and extracellular matrix deposition. It can occur in almost all tissues and it is a major contributor to clinical diseases, leading to organ failure and accounting for approximately one third of deaths worldwide [1,2]. It is proposed that fibrotic response may be distinguished in four major phases: 1) the initiation after tissue injury; 2) the activation of effector cells; 3) the deposition of extracellular matrix and 4) the progression to fibrosis with subsequent organ failure by excessive accumulation of extracellular matrix without sufficient degradation [2].
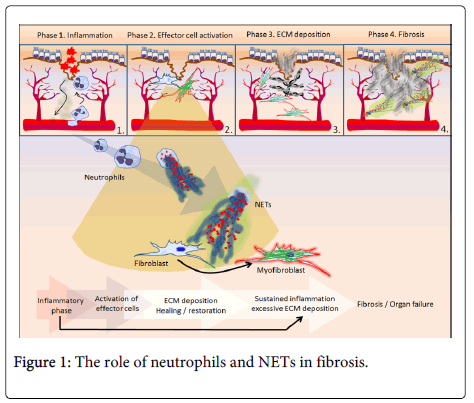
The first phase (local response to tissue injury) leads to inflammatory response in an effort to limit tissue damage and subsequently proceed with repair. However, when inflammation remains unresolved, fibrosis may be initiated. In particular, the inflammatory response leads to injury of epithelial/endothelial cells and enhances the release of inflammatory mediators, including chemokines, cytokines and inflammatory cells (mainly neutrophils and macrophages, but also eosinophils, basophils, mast cells). This inflammatory environment activates effector cells (such as fibroblasts and myofibroblasts) resulting in the production of matrix proteins and subsequent fibrotic process (second and third phases) [2]. The fibrotic changes can occur in different tissues, and are mediated by common pathogenic signaling such as transforming growth factor-β (TGF-β) [3], platelet-derived growth factor (PDGF) [4], connective tissue growth factor (CCN2) [5], endothelin-1 [6], angiotensin II [7], integrins [8] extracellular matrix-driven pathways. Among the mechanisms involved in fibrosis, a cornerstone for the progression of the fibrotic process is the differentiation of fibroblasts towards activated cells, namely myofibroblasts, which contribute in the production of abundant amount of extracellular matrix [2].
In a recent study [9] it was indicated for the first time that among various mediators involved in the inflammation-driven differentiation of fibroblast to an activated myofibroblast, a main architect is the neutrophil, a cell whose role in fibrosis was obscure up to now. Through a series of well-designed in vitro experiments it was demonstrated that agents involved in fibrotic process (i.e. cigarette smoke extract, magnesium silicate, bleomycin) were unable to affect fibroblasts directly, but their function had to pass through a neutrophil mechanism, neutrophil extracellular traps (NETs) [9]. A significant finding of this study was the observation that NETs were involved in the differentiation of fibroblasts into myofibroblasts irrespectively of the causative trigger of NET generation, suggesting that numerous inflammatory stimuli that can induce NET generation are candidate triggers of fibrogenesis. NETs have been first described in 2004 as a new anti-microbial function of neutrophils [10] and consist of extracellular chromatin fibres decorated with various neutrophil-derived granular, cytoplasmic and nuclear proteins. Since 2009, insightful studies demonstrated the involvement of this mechanism in the pathophysiology of various non-infectious disorders such as autoimmunity [11], autoinflammation [12], thrombosis [13,14] and cancer [15]. Kambas and colleagues provided the first evidence indicating a significant role for neutrophils in the pathophysiology of fibrosis [9].
Additionally, in the same report, Chrysanthopoulou et al. [9] have also indicated the presence of NETs' remnants in lung and skin biopsy specimens from human fibrotic diseases, which expressed cytokines involved in fibrotic process. This finding is consistent with the notion which suggests that pulses of neutrophilic migration are required during the chronic inflammation of many fibrotic disorders [2]. It is reasonable to suggest that NETs can act during the second phase of fibrotic response as activators of fibroblasts (Figure 1). Based on the above, we could also propose how fibrosis occurs after a prolonged period of time, where inflammatory flares lead to repeated neutrophilic infiltration pulses with subsequent NET-driven myofibroblast activation and excessive matrix deposition. Similarly, a very recent study indicated the key role of neutrophil elastase in the promotion of myofibroblast differentiation in experimental lung fibrosis, confirming indirectly but clearly the significance of NETs and their decorative components in fibrotic process [16]. Furthermore, at the same time when the critical role of NETs in inflammation-driven fibrosis was revealed [9], an independent clinical study demonstrated that decreased DNase I activity in dermatomyositis/polymyositis patients is significantly associated with interstitial lung disease, linking the abnormal clearance of NETs with a well-defined, severe fibrotic complication [17]. Considering that the lifespan of neutrophils is very short, and in many fibrotic cases the biopsies are performed when the fibrosis is established, it is rational to explain why neutrophils cannot be visualized in fibrotic tissues by conventional methods used in everyday pathology practice (e.g. eosin/hematoxylin staining). Monoclonal antibodies against neutrophil elastase and citrullinated H3 using modern technologies, such as immunofluorescence confocal microscopy or alkaline phosphatase anti-alkaline phosphatase (APAAP) immunocytochemistry offer the opportunity to confirm the presence of NET-derived neutrophilic remnants in archive histology samples of fibrotic disorders retrospectively, where there was an initially reported absence of intact neutrophils.
Additionally, NETs-bound components have various functions and their activity is greatly depended on the integrity of NET scaffold [13,15]. Several of these components are related with certain diseases and their specificity may define the role of NETs in each disorder. Particularly, there is a lot of evidence supporting the concept that some NET-bound molecules are different in various diseases and "all NETs are not equal", thus suggesting a specific role of their components in the pathophysiology of diseases. Hence, it is reasonable that NETs present during fibrosis carry mediators that could influence fibrotic mechanisms either positively or negatively. For example such components expressed on NETs are IL-17 [9], which is a key fibrotic cytokine, and metalloproteinases [18] which can play an important role in matrix turnover.
In the first phase of fibrotic process damaged tissue produces chemokines, cytokines and other inflammatory mediators which act as chemoattractants to cells, among them neutrophils, and subsequently under a suitable inflammatory environment neutrophils generate NETs. In the second phase, NETs can promote fibroblast differentiation into myofibroblasts. Following fibroblast differentiation, NETs carry components that can influence myofibroblastic activity either positively or negatively. During the development of fibrosis, neutrophils exist in the fibrotic tissue only in the form of "invisible" NET remnants, reminding their significant role.
Despite the above emerging findings the scientific field of NET-driven fibrosis is still an unwritten page. A lot of research effort is required in order to delineate which NET components influence pathways that are implicated in fibrotic process, while their presence in fibrotic tissue and their ability to affect a key stage of the fibrotic response constitute them a potential therapeutic target. Moreover, the inhibition of NET generation as a therapeutic approach could provide a more efficient and less immunosuppresive alternative [19]. Targeting NETs alone or in combined therapies could offer more efficient treatment against fibrosis.
References
- Zeisberg M, Kalluri R (2013) Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis.Am J Physiol Cell Physiol 304: C216-225.
- Rockey DC, Bell PD, Hill JA (2015) Fibrosis--a common pathway to organ injury and failure.NEngl J Med 372: 1138-1149.
- Massagué J (2012) TGFß signalling in context.Nat Rev Mol Cell Biol 13: 616-630.
- Pierce GF, Mustoe TA, Senior RM, Reed J, Griffin GL, et al. (1988) In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimericproteins.JExp Med 167: 974-987.
- Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, et al. (1999) Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model.J Cell Physiol 181: 153-159.
- Cambrey AD, Harrison NK, Dawes KE, Southcott AM, Black CM, et al. (1994) Increased levels of endothelin-1 in bronchoalveolar lavage fluid from patients with systemic sclerosis contribute to fibroblast mitogenic activity in vitro.Am J Respir Cell MolBiol 11: 439-445.
- Villarreal FJ, Kim NN, Ungab GD, Printz MP, Dillmann WH (1993) Identification of functional angiotensin II receptors on rat cardiac fibroblasts.Circulation 88: 2849-2861.
- Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, et al. (2013) Targeting of av integrin identifies a core molecular pathway that regulates fibrosis in several organs.Nat Med 19: 1617-1624.
- Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, et al. (2014) Neutrophil extracellular traps promote differentiation and function of fibroblasts.JPathol 233: 294-307.
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. (2004) Neutrophil extracellular traps kill bacteria.Science 303: 1532-1535.
- Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, et al. (2011) Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 187:538-552.
- Apostolidou E, Skendros P, Kambas K, Mitroulis I, Konstantinidis T, et al. (2014) Neutrophil extracellular traps regulate IL-1β-mediated inflammation in familial Mediterranean fever.Ann Rheum Dis .
- von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, et al. (2012) Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo.JExp Med 209: 819-835.
- Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, et al. (2015) Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction.Eur Heart J 36: 1405-1414.
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, et al. (2013) Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. pii: 67484.
- Gregory AD, Kliment CR, Metz HE, Kim KH, Kargl J, et al. (2015) Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis.JLeukocBiol .
- Zhang S, Shu X, Tian X, Chen F, Lu X, et al. (2014) Enhanced formation and impaired degradation ofneutrophil extracellular traps in dermatomyositis and polymyositis: a potential contributor to interstitial lung disease complications. ClinExpImmunol. 177:134-141.
- Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ (2014) Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 74: 1417-1424.
- Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, et al. (2015) PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemicshock.Blood 125: 1948-1956.
Citation: Ritis K, Kambas K (2015) Neutrophil Extracellular Traps - The Invisible Inflammatory Mediator in Fibrosis. J Clin Exp Pathol 5:239. DOI: 10.4172/2161-0681.1000239
Copyright: © 2015 Ritis K, et al. This is an open-access article` distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14985
- [From(publication date): 8-2015 - Dec 19, 2024]
- Breakdown by view type
- HTML page views: 10523
- PDF downloads: 4462