Neutrophil CD64 Expression in CrohnâÃâ¬Ãâ¢s Disease following Anti-TNF-Îñ Therapy
Received: 29-Sep-2017 / Accepted Date: 09-Oct-2017 / Published Date: 17-Oct-2017 DOI: 10.4172/2576-3881.1000119
Abstract
Objective: Anti-TNF-α therapy is an effective therapy for Crohn’s disease (CD). Thus anti-TNF-α therapy may alter CD64 expression, one of the Fc receptors for IgG, in CD. We investigated CD64 expression on neutrophils before and after treatment with anti-TNF-α therapy in CD.
Methods: A total of 11 patients with active CD treated with anti-TNFα antibodies were enrolled. The severity of CD was assessed with the CD activity index (CDAI). Peripheral venous blood was obtained before and 2 weeks after the initial administration of anti-TNF-α antibody. CD64 expression on neutrophils was measured by FACS analysis of whole blood samples.
Results: CDAI, C-reactive protein value and CD64 expression decreased significantly after anti-TNF-α therapy. Both prior to and after anti-TNF treatment, there was a significant and positive correlation between CD64 expression and CDAI or CRP. Similarly, there was a significant and negative correlation between CD64 and albumin value.
Conclusion: Anti-TNF-α therapy suppresses CD64 expression of neutrophils, which may account for the mechanism underlying the efficacy of the medication in CD.
Keywords: CD64; Crohn’s disease; Anti-TNF-α therapy; Infliximab
Introduction
The chimeric anti-TNF-α monoclonal antibody infliximab (IFX) has been reported to be effective in induction and maintenance therapies for Crohn’s disease (CD). IFX binds to TNF-α with high affinity, thereby neutralizing the cytokine’s biologic activity. IFX treatment reduces serum levels of IL-6, IL-7, IL-8, IL-12, and MIP-1β in CD [1]. Serum IL-6 and IL-1β concentrations have been shown to decrease after IFX treatment in rheumatoid arthritis, and IFX decreases serum IL-18 levels in CD patients [2-4]. It has also been shown that IL-6 and MCP-1 levels were significantly decreased in all CD patients after adalimumab (ADA) treatment [5]. Finally, IFX treatment has been shown to significantly reduce plasma CD40 levels and reduce the number of activated Th-1 cells in the peripheral blood and intestinal mucosa [6-8].
CD64, one of the Fc receptors for IgG, plays a role in antibody-dependent cytotoxicity, clearance of immune complexes, and phagocytosis of targets opsonized with IgG. Fcγ receptors are expressed by innate immune cells including neutrophils and monocytes and serve to unite antibody specificity and effector cell functions. Quiescent neutrophils constitutively express Fcγ receptor II (CD32) and Fcγ receptor III (CD16), with minimal levels of Fcγ receptor I (CD64) expressed under basal conditions [9]. With active inflammation, neutrophil CD64 is upregulated early in the innate immune response by interferon (IFN)-γ and granulocyte colony stimulating factor (G-CSF) [10,11]. In the present study, we examined CD64 levels in CD patients before and after administration of IFX to investigate whether CD64 is included in the perpetuation of CD and the mechanism of action of IFX.
Patients and Methods
Patients
A total of 11 patients with active CD, 6 men and 5 women, mean age 32.0 years, were included in the study. Four patients had ileal disease, 6 patients ileo-colonic disease, and 1 patient colonic disease. Four patients had anal disease. Disease duration ranged from 4 to 19 years, with a mean of 10.0 years. The study was approved by the Human Ethics Review Committee of Iwate Medical University. Informed consent was obtained from each patient prior to enrollment.
Anti-TNF-α treatment
IFX was administered at a dose of 5 mg/kg intravenously. Treatment responses were followed by physical examination and Crohn’s disease activity index (CDAI) scoring before and 2 weeks after the initial administration of IFX. Peripheral blood samples were collected before and 2 weeks after treatment. White blood cell count (WBC) and serum levels of CRP and albumin were measured.
Quantitative measurement of CD64 expression
Expression of CD64 on neutrophils was measured by staining EDTA-3K whole blood with QuantiBrite™ CD64PE/CD45PerCP (Becton-Dickinson, San Jose, CA, USA) according to the manufacturer’s instructions. Briefly, 20 μL of QuantiBrite™ CD64PE/ CD45 Per CP was added to 50 μL of whole blood and incubated for 60 min in the dark at room temperature. This was followed by lysis of red blood cells with two mL of 1 × FACS™ lysing solution (Becton- Dickinson) without washing, followed by an additional 60 min incubation to reduce nonspecific background staining [12]. Specimens were analyzed using a FACSCalibur flow cytometer and CellQuest Pro software (Becton-Dickinson). The number of antibody-phycoerythrin (PE) binding sites per cell was computed with QuantiQuest software (Becton-Dickinson) using a linear regression curve (derived from data generated with QuantiBRITE™ PE beads, Becton-Dickinson) obtained in parallel for each sample. As each CD64-PE antibody is designed to bind one PE molecule, the mean number of CD64 molecules expressed on a cell can be calculated using the PE fluorescence quantification kit and QuantiBRITE™ PE beads.
Statistical analysis
CD64, CDAI, and CRP data are expressed as means ± standard deviation (SD). Statistical analyses comparing the CD64 data before and after IFX treatment were performed using the paired Student’s t-test. Correlation coefficients were assessed by linear regression analysis. A p value<0.05 was considered to be statistically significant.
Results
Efficacy of anti-TNF-αantibody
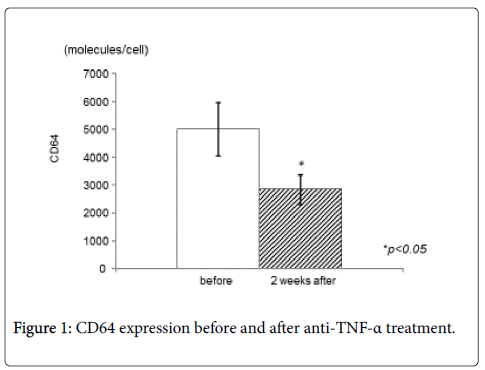
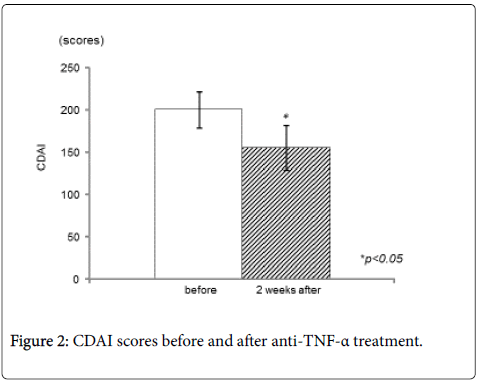
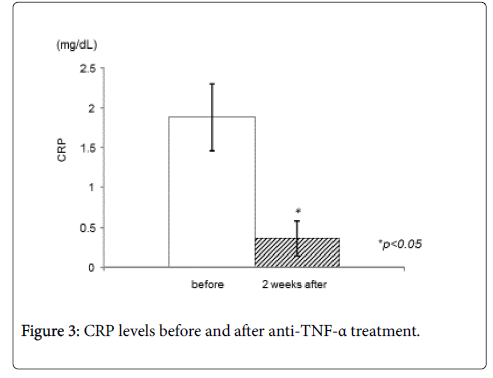
CD64 expression decreased significantly after IFX treatment (Figure 1). The mean CD64 expression before administration of IFX were 5021.8 ± 3171.6 molecules/cell, and the value decreased to 2847.2 ± 1767.0 molecules/cell two weeks after administration of IFX (p<0.05). CDAI decreased significantly 2 weeks after treatment (from 201.6 ± 70.7 to 156.1 ± 87.9, p<0.05) (Figure 2). In addition, serum CRP values decreased significantly (from 1.9 ± 1.4 to 0.3 ± 0.7, p<0.05) (Figure 3), while there was not any statistically significant difference in serum albumin or white blood cell count.
Correlations between CD64 and clinical parameters
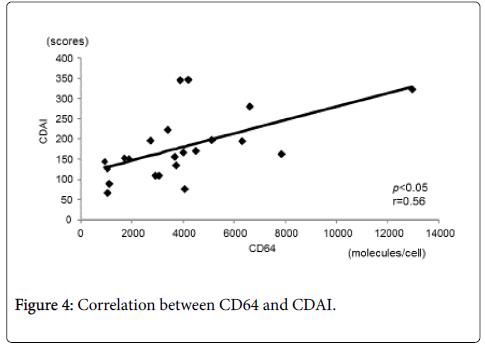
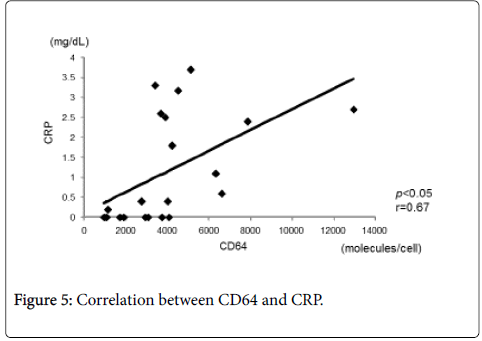
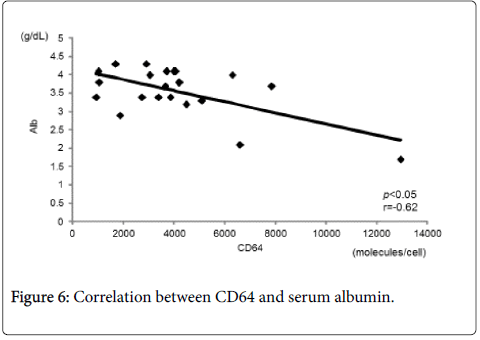
Figure 4 shows the correlation between CD64 and CDAI. CD64 showed a statistically significant and positive correlation with CDAI (r=0.56, p<0.05). There was also a statistically significant and positive correlation between CD64 and CRP (r=0.67, p<0.05) (Figure 5). In contrast, there was a statistically significant and negative correlation between CD64 and serum albumin (r=-0.62, p<0.05) (Figure 6). There was not any significant correlation between CD64 and WBC.
There was a statistically significant positive correlation between CD64 and CDAI (r=0.56, p<0.05).
Discussion
In this study, we observed a significant correlation between the CD64 expression and CDAI in patients with CD. Since an elevated CD64 expression has been shown to be associated with mucosal inflammation and an increase in the risk of clinical relapse in CD [13,14], CD64 expression, as well as endoscopic and histologic, severity may be one of the useful biomarkers for the assessment of disease activity, practical intestinal damage and the risk of relapse in patients with CD.
CD64 has been implicated as a modulator of the response to microbial challenge in intestinal mucosal inflammation [15]. Additionally, CD64 and toll like receptor 4 have been reported to be up-regulated in CD patients [16] and this correlates with clinical and biological parameters of inflammation in patients with inflammatory bowel disease (IBD) [17]. These observations suggest a role of Fc receptors as diagnostic markers and as potential, modulating factors for immunoglobulin-based medications [18]. Furthermore, there is a correlation between responsiveness to IFX and polymorphisms in FcRIII receptor isoforms [19,20]. These observations, together with a similar association found in rheumatoid arthritis [21,22] seem to support the current hypothesis that Fc receptors influence the outcome of IFX therapy.
Fcγ receptors are expressed by innate immune cells including neutrophils and monocytes and serve to unite antibody specificity and effector cell functions [23]. The central role of CD64, a high affinity receptor for IgG1 and IgG3, is recognition and clearance of immune complexes by phagocytosis as well as antibody-dependent cell-mediated cytotoxicity (ADCC) [23]. Upregulation of neutrophil CD64 has been shown to differentiate symptomatically active IBD from quiescent IBD [17]. CD64 positive neutrophils have been shown to infiltrate the colonic lamina propria in active CD [17], and FcγRIA mRNA expression has been shown to be significantly elevated in ileal and rectal tissue subjects with the disease. In IBD patients, who were refractory to anti-TNFα therapy, CD64 mRNA expression remained up-regulated even in post-treatment colonic biopsy specimens [24]. A correlation between the CD64 expression of the peripheral neutrophils and FcγRIA expression in the ileal mucosa has been demonstrated, thereby suggesting that CD64 expression can a marker for mucosal treatment response [13].
In this study, CD64 expression correlated significantly with CDAI and CRP. Furthermore, quantitative analysis of CD64 has been suggested to be applicable to the distinction of patients with chronic inflammatory conditions from healthy controls [17,25,26]. Hence, CD64 may be a candidate biomarker for CD patients receiving IFX therapy.
Our study has several limitations. First, since we did not enroll healthy controls, we could not determine reference values for CD64. Second, our investigation was a pilot study with a relatively small number of patients. These issues are being addressed in an ongoing prospective study.
In conclusion, we have shown that anti-TNF-α therapy reduced blood CD64 expression in patients with CD. CD64 expression correlated significantly with other parameters of disease activity. Based on these observations, we concluded that CD64 may play an important role in the perpetuation of disease in patients with CD.
Acknowledgements
A portion of this manuscript was presented at the Annual Meeting of the Japanese Society of Gastroenterology (JDDW 2013).
References
- Mizutani T, Akasaka R, Tomita K, Chiba T (2011) Serial changes of cytokines in Crohn’s disease treated with infliximab. Hepato-Gastroenterol 58: 1523-1526.
- Lorenz HM, Antoni C, Valerius T, Repp R, Grünke M, et al. (1996) In vivo blockade of TNF-alpha by intravenous infusion of a chimeric monoclonal TNF-alpha antibody in patients with rheumatoid arthritis. Short term cellular and molecular effects. J Immunol 156: 1646-1653.
- Minderhoud IM, Samsom M, Oldenburg B (2007) Crohn's disease, fatigue, and infliximab: is there a role for cytokines in the pathogenesis of fatigue? World J Gastroenterol 13: 2089-2093.
- Gustot T, Lemmers A, Louis E, Nicaise C, Quertinmont E, et al. (2005) Profile of soluble cytokine receptors in Crohn's disease. Gut 54: 488-495.
- Abiko Y, Mizutani T, Chiba T (2014) Serial changes of serum cytokines in Crohn's disease following treatment with adalimumab. Hepatogastroenterology 61: 357-362.
- Keating GM, Perry CM (2002) Infliximab: an updated review of its use in Crohn's disease and rheumatoid arthritis. BioDrugs 16: 111-148.
- Danese S, Sans M, Scaldaferri F, Sgambato A, Rutella S, et al. (2006) TNF-a blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn’s disease. J Immunol 176: 2617-2624.
- Ferkolj I, Ihan A, Markovic S, Veceric Z, Pohar M (2006) Infliximab reduces the number of activated mucosal lymphocytes in patients with Crohn’s disease. J Gastrointestin Liver Dis 15: 231-235.
- Hoffmeyer F, Witte K, Schmidt RE (1997) The high-affinity Fc gamma RI on PMN: regulation of expression and signal transduction. Immunology 92: 544-552.
- Buckle AM, Hogg N (1989) The effect of IFN –gamma colony-stimulating factors on the expression of neutrophil cell membrane receptors. J Immunol143: 2295-2301.
- Kerst JM, de Haas M, van der Schoot CE, Slaper-Cortenbach IC, Kleijer M, et al. (1993) Recombinant granulocyte colony-stimulating factor administration to healthy volunteers: induction of immuno-phenotypically and functionally altered neutrophils via an effect on myeloid progenitor cells. Blood 82: 3265-3272.
- Ng PC, Li K, Wong RP, Chui KM, Wong E, et al. (2002) Neutrophil CD64 expression: a sensitive diagnostic marker for late-onset nosocomial infection in very low birthweight infants. Pediatr Res 51: 296-303.
- Minar P, Haberman Y, Jurickova I, Wen T, Rothenberg ME, et al. (2014) Utility of neutrophil Fcγ receptor I (CD64) index as a biomarker for mucosal inflammation in pediatric Crohn's disease. Inflamm Bowel Dis 20: 1037-1048.
- Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, et al. (2010) Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 105: 162-169.
- Chen X, Feng BS, Zheng PY, Liao XQ, Chong J, et al. (2008) Fc gamma receptor signaling in mast cells links microbial stimulation to mucosal immune inflammation in the intestine. Am J Pathol 173:1647-1656.
- Kobayashi R, Okamura S, Ohno T, Saito H, Mori M, et al. (2007) Hyperexpression of FcgammaRI and Toll-like receptor 4 in the intestinal mast cells of Crohn's disease patients. Clin Immunol 125: 149-158.
- Tillinger W, Jilch R, Jilma B, Brunner H, Koeller U, et al. (2009) Expression of the high-affinity IgG receptor FcRI (CD64) in patients with inflammatory bowel disease: a new biomarker for gastroenterologic diagnostics. Am J Gastroenterol 104: 102-109.
- Davis PM, Abraham R, Xu L, Nadler SG, Suchard SJ (2007) Abatacept binds tothe Fc receptor CD64 but does not mediate complement-dependent cytotoxicity antibody-dependent cellular cytotoxicity. J Rheumatol 34: 2204-2210.
- Louis E, El Ghoul Z, Vermeire S, Dall'Ozzo S, Rutgeerts P, et al. (2004) Association between polymorphism in IgG Fc receptor IIIa coding gene and biological response to infliximab in Crohn’s disease. Aliment Pharmacol Ther 19: 511-519.
- Tomita K, Chiba T, Sugai T, Habano W (2010) Association between tumor necrosis factor-alpha and Fc-gamma receptor polymorphisms with infliximab in Crohn’s disease. Hepatogastroenterology 57: 535-539.
- Okuyama A, Nagasawa H, Suzuki K, Kameda H, Kondo H, et al. (2011) Fcγ receptor IIIb polymorphism and use of glucocorticoids at baseline are associated with infusion reactions to infliximab in patients with rheumatoid arthritis. Ann Rheum Dis 70: 299-304.
- Cañete JD, Suárez B, Hernández MV, Sanmartà R, Rego I, et al. (2009) Influence of variants of Fc gamma receptors IIA and IIIA on the American College of. Rheumatology and European League against Rheumatism responses to anti-tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis 68: 1547-1552.
- Hoffmeyer F, Witte K, Schmidt RE (1997) The high-affinity Fc gamma RI on PMN: regulation of expression and signal transduction. Immunology 92: 544-552.
- Wojtal KA, Rogler G, Scharl M, Biedermann L, Frei P, et al. (2012) Fc gamma receptor CD64 modulates the inhibitory activity of infliximab. PLoS One 7: e43361.
- Ureten K, Ertenli I, Oztürk MA, Kiraz S, Onat AM, et al. (2005) Neutrophil CD64 expression in Behçet's disease. J Rheumatol 32: 849-852.
- Hussein OA, El-Toukhy MA, El-Rahman HS (2010) Neutrophil CD64 expression in inflammatory autoimmune diseases: its value in distinguishing infection from disease flare. Immunol Invest 39: 699-712.
Citation: Chiba T, Endo M, Miura S, Hayashi Y, Asakura Y, et al. (2017) Neutrophil CD64 Expression in Crohn’s Disease following Anti-TNF-α Therapy. J Cytokine Biol 2: 119. DOI: 10.4172/2576-3881.1000119
Copyright: © 2017 Chiba T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5253
- [From(publication date): 0-2017 - Mar 29, 2025]
- Breakdown by view type
- HTML page views: 4357
- PDF downloads: 896