Research Article Open Access
Neurotoxin-Induced Catecholaminergic Loss in the Colonic Myenteric Plexus of Rhesus Monkeys
Jeanette M Shultz1, Henry Resnikoff2, Viktorya Bondarenko2, Valerie Joers2, Andres Mejia2, Heather Simmons2 and Marina E Emborg3*1Wisconsin National Primate Research Center (WNPRC) and Cellular and Molecular Pathology (CMP) Graduate Program, University of Wisconsin-Madison, Madison, WI, USA
2WNPRC, University of Wisconsin-Madison, Madison, WI, USA
3WNPRC, CMP Graduate Program and Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, USA
- *Corresponding Author:
- Marina Emborg
Wisconsin National Primate Research Center
University of Wisconsin–Madison, 1220 Capitol Court
Madison, WI, USA, 53715
Tel: 608-262-9714
E-mail: emborg@primate.wisc.edu
Received date: August 10, 2016; Accepted date: October 27, 2016; Published date: November 03, 2016
Citation: Shultz JM, Resnikoff H, Bondarenko V, Joers V, Mejia A, et al. (2016) Neurotoxin-Induced Catecholaminergic Loss in the Colonic Myenteric Plexus of Rhesus Monkeys. J Alzheimers Dis Parkinsonism 6:279. doi:10.4172/2161-0460.1000279
Copyright: © 2016 Shultz JM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Objective: Constipation is a common non-motor symptom of Parkinson’s disease (PD). Although pathology of the enteric nervous system (ENS) has been associated with constipation in PD, the contribution of catecholaminergic neurodegeneration to this symptom is currently debated. The goal of this study was to assess the effects of the neurotoxin 6-hydroxydopamine (6-OHDA) on the colonic myenteric plexus and shed light on the role of catecholaminergic innervation in gastrointestinal (GI) function. Methods: Proximal colon tissue from 6-OHDA-treated (n=5) and age-matched control (n=5) rhesus monkeys was immunostained and quantified using ImageJ software. All animals underwent routine daily feces monitoring to assess for constipation or other GI dysfunction. Results: Quantification of tyrosine hydroxylase (TH) and aromatic L-amino acid decarboxylase (AADC)- immunoreactivity (-ir) revealed significant reduction in myenteric ganglia of 6-OHDA-treated animals compared to controls (TH-ir: 87.8%, P<0.0001; AADC-ir: 61.7% P=0.0034). Analysis of pan-neuronal markers (PGP9.5, HuC/D), other neurochemical phenotypes (VIP, nNOS), PD-associated pathology proteins (α-synuclein, phosphorylated α-synuclein), glial marker GFAP and neuroinflammation and oxidative stress (HLA-DR, CD45, Nitrotyrosine) did not show significant differences. Monitoring of feces revealed frequent (>30% days) soft stool or diarrhea in 2 of the 5 6-OHDA-treated animals and 0 of the 5 control animals during the 2 months prior to necropsy, with no animals exhibiting signs of constipation. Conclusion: Systemic administration of 6-OHDA to rhesus monkeys significantly reduced catecholaminergic expression in the colonic myenteric plexus without inducing constipation. These findings support the concept that ENS catecholaminergic loss is not responsible for constipation in PD.
Keywords
Colon; Catecholaminergic; 6-hydroxydopamine; Parkinson’s disease; α-synuclein; myenteric plexus; Inflammation; Oxidative stress; Tyrosine hydroxylase
Introduction
Parkinson’s disease (PD) is traditionally defined as a movement disorder associated with loss of dopaminergic neurons in the substantia nigra pars compacta and the presence of Lewy bodies (LBs), intraneuronal aggregates composed largely of α-synuclein. However, PD is increasingly recognized as a complex disorder involving debilitating non-motor symptoms (NMS) such as mood disorders, cognitive impairment and autonomic dysfunction in addition to its hallmark motor symptoms [1,2]. Autonomic dysfunction in PD, including sleep disorders, orthostatic hypotension, urinary dysfunction and constipation, often begins before motor symptoms and greatly impacts patients quality of life [3-7]. Constipation is especially common, occurring in 80-90% of PD cases, and is typically identified by patient reports of fewer than three bowel movements per week and hard stools [8,9]. Current anti-parkinsonian dopamine replacement therapies are not effective and can worsen gastrointestinal (GI) dysfunction [10]. The etiology of constipation in PD is still unclear; loss of catecholaminergic innervation in the colon [11] or the presence of LBs in autonomic neurons regulating GI motility [12] have been proposed as contributing factors. Recently, findings of colonic catecholaminergic neuron loss in PD patients and the role of this neurodegeneration in PD-related constipation have been challenged [13,14]. Studies in animal models can help unravel the cause of constipation in PD and identify targets for future therapeutic intervention.
The enteric nervous system (ENS) is the intrinsic nervous system of the GI tract and consists of groups of neuronal cell bodies, or ganglia, arranged in two neuronal plexuses. The submucosal plexus is located between the mucosa and circular muscle and primarily serves to regulate secretion. The myenteric plexus is located between the circular and longitudinal muscle layers and is principally involved in controlling smooth muscle motility [15]. Each plexus contains a complex, heterogeneous neuronal population, with neurons expressing and often co-expressing, a variety of transmitters and neuropeptides including acetylcholine, nitric oxide, vasoactive intestinal peptide (VIP), and dopamine [16]. GI function is controlled by ENS activity in combination with extrinsic input from the autonomic nervous system, of which the ENS is part [15,17]. Extrinsic autonomic innervation of the colon includes parasympathetic cholinergic input originating from the vagus and pelvic nerves and sympathetic catecholaminergic (noradrenergic) post-ganglionic input.
The neurotoxin 6-hydroxydopamine (6-OHDA) is specifically uptaken by catecholamine transporters [18] and induces catecholaminergic neuronal loss by increasing oxidative stress and eliciting inflammation, mechanisms reported to also be involved in PD-related neurodegeneration [18,19]. When delivered systemically, 6-OHDA does not cross the blood-brain barrier and therefore neither induces nigral dopaminergic cell loss nor the cardinal motor symptoms of PD [20-22], yet its toxic effects mimic peripheral catecholaminergic neuronal loss. Our research group used systemic 6-OHDA to develop a nonhuman primate model of PD cardiac dysautonomia, which typically presents cardiac postganglionic sympathetic denervation, and performed a battery of imaging, clinical and behavioral tests for in vivo characterization [23,24]. Our daily monitoring of the 6-OHDAtreated monkeys showed either normal or soft feces [23]. This finding prompted us to question whether systemic 6-OHDA affected the colonic ENS and, if so, what is the role of ENS catecholaminergic loss in GI dysfunction. Here, we report our analysis of the colonic ENS of the 6-OHDA-treated monkeys compared to age- and sex- matched controls.
Materials and Methods
Ethics statement
The experiment was performed in strict accordance with the recommendations in the National Research Council Guide for the Care and Use of Laboratory Animals (2011) in an AAALAC accredited facility, the Wisconsin National Primate Research Center (WNPRC) at the University of Wisconsin-Madison. Experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University at the Wisconsin-Madison (protocol G00538). All efforts were made to minimize the number of animals used and to ameliorate any distress. The control monkey tissues were obtained from the WNPRC tissue bank. These monkeys were originally assigned to and euthanized under the University of Wisconsin- Madison IACUC experimental protocols G00101, G00591and G00423.
Subjects
Proximal colon sections from 10 rhesus monkeys (Macaca mulatta, 5 male, 5 female, 5.4-10.4 years old) were utilized in this study. The animals from which the tissue was obtained were single housed in Group 3 or Group 4 enclosures (cage floor area 4.3 foot2 or 6.0 foot2 per animal, height 30 or 32 inch) in accordance with the Animal Welfare Act and its regulations and the Guide for the Care and Use of Laboratory Animals with a 12 h light/dark cycle. Throughout the study, the animals were monitored twice daily by an animal researcher or veterinary technician for evidence of disease or injury (e.g. inappetance, dehydration, diarrhea, lethargy, trauma, etc.). Body weight was monitored as a health measure and to ensure animals remained in properly sized cages. Animals were fed commercial nonhuman primate chow (2050 Teklad Global 20% Protein Primate Diet, Harlan Laboratories, Madison, WI) twice daily, supplemented with fruits or vegetables and a variety of environmental enrichment and forage items and received ad libitum water. Nonhuman primate chow soaked in a protein-enriched drink (Ensure ©, Abbott Laboratories, Abbott Park, IL) was offered to stimulate appetite as needed.
The 6-OHDA-treated proximal colon sections (n=5; 7.0 ± 1.57 years old; 7.35 ± 1.65 kg; 2 male) were obtained from previously published studies [20,23,24]. Dosing of 6-OHDA to these monkeys was done in sterile surgical conditions under isoflurane anesthesia, as a sequence of 7-9 intravenous injections totaling a final dose of 50 mg/ kg. The animals were necropsied 3 months post-6-OHDA. No animal died due to experimental procedures or needed to be euthanized prior to the end of the experiment due to pre-determined humane clinical endpoints (e.g.: loss of >15% body weight) or WNPRC clinical veterinarian’s determination that an animal developed an untreatable or incurable condition that would have caused significant stress or pain. If needed, discomfort, distress, pain or injury were minimized by the appropriate use of analgesics (e.g.: buprenorphine 0.01-0.03 mg/kg im) under veterinary direction. The normal proximal colon sections (n=5; 8.67 ± 1.94 years. old; 6.92 ± 2.42 kg; 3 male) were obtained from the tissue bank at the WNPRC; the donors were selected by matching them to the baseline condition of the 6-OHDA-treated monkeys. As described in Joers et al. [23], all animals underwent routine daily feces monitoring including reports of no stool, very little stool, firm stool, soft feces, diarrhea and mucus. Based on this scale, constipation is diagnosed by records of no or very little stool, and/or firm stool for more than 3 days during a period of a week. Comparison of this data revealed frequent (>30% days) soft stool or diarrhea in 2 of the 5 6-OHDA-treated animals and 0 of the 5 control animals during the 2 months prior to necropsy; no stool, very little stool, firm stool and mucus were either not observed or very infrequently present during this time (Supplementary Figure 1). The number of subjects per group was defined by our previous cardiac dysautonomia study [20,23,24] in which n=5 found a statistically significant loss of cardiac sympathetic innervation. Tissues from both groups were processed in parallel for each immunostaining to minimize bias.
Necropsy and tissue preparation
Following previous methods [23,24], all animals were anesthetized with ketamine hydrochloride (10 mg/kg, im) followed by pentobarbital sodium (25 mg/kg, iv) and perfused through the ascending aorta or left atrium (6-OHDA-treated) or left ventricle (normal controls) with heparinized phosphate-buffered saline, followed by 4% paraformaldehyde. Proximal colon sections approximately 3 cm in length were post-fixated in 4% paraformaldehyde for 24-48 h and further preserved with 70% ethanol at 4°C. Colon sections were trimmed and blocked in paraffin. All blocked tissue was cut on a standard rotary microtome in 5 μm section thickness and mounted on positively charged slides.
Anatomical evaluation
A representative proximal colon section from each subject was stained with hematoxylin and eosin (H&E) and blindly evaluated by board certified veterinary pathologists (HS & AM) for general histological changes and presence of inflammation. Assessment of the submucosal plexus was not performed because tissue processing and immunohistochemistry resulted in inconsistent presence of the submucosa across animals.
General immunohistochemistry
Immunohistochemistry (IHC) against markers of neurochemical phenotypes, α-synuclein, inflammation and oxidative stress in the myenteric plexus of the proximal colon was performed using a previously published protocol, modified as needed [24] (Supplementary Table 1 for full list of antibodies and specific protocol details). Colon sections were deparaffinized and treated with heat antigen retrieval. The sections were then washed and endogenous peroxidase activity blocked by incubation with 30% H2O2 and methanol for 20 min. Non specific binding sites were blocked with a 20% serum and 0.5% BSA solution for 60 min at room temperature and incubated overnight with a primary antibody (Supplementary Table 1) diluted in blocking buffer plus 0.1% triton-X. The sections were than incubated in appropriate biotinylated secondary antibody (1:200), followed by avidin-biotinperoxidase complex (ABC Standard, Vector Laboratories, Burlingame, CA), and visualized with either a commercial 3,3’-diaminobenzidine kit (DAB; colon tissue) (Vector Laboratories, Burlingame, CA) or a NovaRED kit (brain tissue) (Vector Laboratories, Burlingame, CA). All sections were counterstained with hematoxylin, dehydrated, and coverslipped (Cytoseal mounting media, Thermo Scientific, Waltham, MA).
Negative controls were performed in parallel by omitting the primary antibodies in the immunostaining procedures. Human substantia nigra tissue from a PD patient (courtesy of the Wisconsin Alzheimer’s Disease Research Center) was used as a positive control for α-synuclein and phosphorylated α-synuclein immunostaining.
Immunofluorescence
Double label immunofluorescence stainings were performed to verify neuronal localization of tyrosine hydroxylase (TH), validate TH colocalization with aromatic L-amino acid decarboxylase (AADC) and assess changes in α-synuclein expression (Supplementary Table 2) following previously validated methods [24]. Slides were deparaffinized and treated for heat antigen retrieval in a microwave for 6 min at 100% power followed by 6 min at 80% power and left to cool for 30 min at room temperature. Tissue was blocked with 5% donkey serum and 2% BSA solution and incubated in primary antibodies (Supplementary Table 2) overnight at 4°C. The sections were then incubated with alexafluor-conjugated secondary antibody (1:1000) against the appropriate species and cover slipped with mounting media with DAPI (Vector Laboratories, Burlingame, CA). Negative controls were performed in parallel by omitting the primary antibodies.
Immunohistochemistry quantification
Immunostainings in myenteric ganglia were quantified using a Zeiss Axioimager M2 equipped with a Qimaging camera and NIH ImageJ software. Each immunostained colon tissue section was first divided into 3 regions of approximately equal size. Per region, three individual myenteric ganglia (area >30 μm2) were identified and a photomicrograph captured at 40x (9 ganglia per colon tissue section). For each immunohistochemical marker, 45 ganglia were evaluated per treatment group (one tissue section per animal per immunostaining). In each photomicrograph, DAB color was separated from hematoxylin counterstain with the ImageJ Colour Deconvolution filter. ImageJ was calibrated using a step tablet and grey scale values were converted to optical density (OD) units using the Rodbard function. The ganglia were outlined and percent area above threshold (%AAT) measured. The threshold was calculated by averaging the optimal individual threshold that best represented the immunoreactivity (-ir) for 2-4 photomicrographs of immunostained tissue sections. Thresholds used were 0.37 for TH, protein gene product 9.5 (PGP9.5), VIP, AADC, nNOS (neuronal nitric oxide synthase); 0.45 for glial fibrillary acidic protein (GFAP); and 0.29 for CD45, human leukocyte antigen DR (HLA-DR), and nitrotyrosine. α-Synuclein-ir was evaluated using two different thresholds per ganglion, 0.42 and 1.27 to quantify light and dark immunoreactivity respectively and, therefore, identify potential protein aggregation. As phosphorylated α-synuclein-ir was minimal, %AAT analysis was not performed; instead if a ganglion presented any visible phosphorylated α-synuclein-ir, it was scored as positive. The total number of phosphorylated α-synuclein-ir ganglia was reported per treatment group.
To validate the results of PGP9.5-ir, neuron number per mm2 ganglion area was quantified in colon sections immunostained against human neuronal protein HuC/HuD (HuC/D). Twenty-40 ganglia (area >30 μm2 and any HuC/D-ir) per animal were photomicrographed using a Zeiss Axioimager M2 equipped with a Qimaging camera. The area of each ganglion was measured using NIH Image J software, the number of cells with nuclear HuC/D-ir was counted and then the neuron density per ganglion calculated. Abercrombie correction [25] was not applied, as this methodology overcorrects cell counts in tissue sections thinner than the object being counted [26-28].
Statistical Analysis
Data collection and analysis were performed by investigators blind to the treatment groups. Statistical analysis was performed using GraphPad Prism (version 5.0f, GraphPad Software). A P value<0.05 was accepted as significant. Group averages for colonic myenteric ganglia evaluations were compared by independent sample t-test. Potential relationships between data sets were analyzed with Pearson correlations.
Results
Colon anatomy of experimental animals
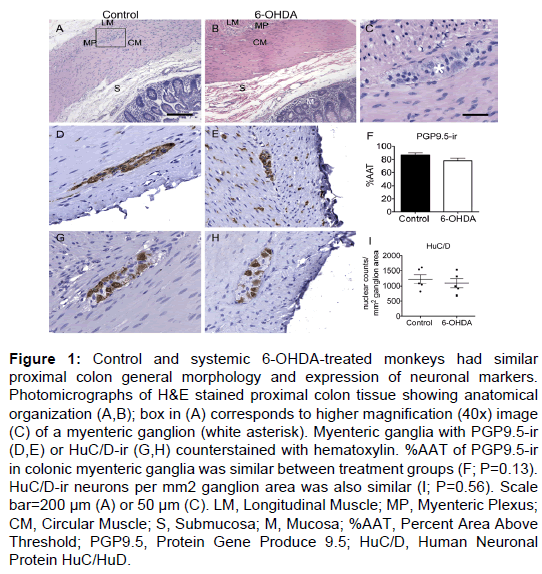
General evaluation of H&E stained tissue revealed normal proximal colon morphology in all animals (Figure 1). Ganglia in the myenteric plexus contained neurons with large, round euchromatic nuclei with prominent nucleoli. Tightly packed around these neurons were a large number of presumed enteric glial cells, with smaller cell bodies mostly filled by the nucleus. Some tissue separation and loss of the submucosa (including the submucosal plexus) were observed in both groups.
PGP9.5 immunostaining had a similar pattern in all animals, with immunoreactivity uniformly present throughout all neuronal cell bodies and processes in the myenteric plexus and in nerve fibers running between muscle fibers and mucosa (Figure 1). Quantification of PGP9.5-ir in myenteric ganglia (Supplementary Table 3) showed similar %AAT values in both control and 6-OHDA-treated animals (P=0.13).
HuC/D immunostained colon tissue sections were used to assess neuronal number in the myenteric plexus of all animals, as PGP9.5-ir throughout neuronal processes makes identifying individual neurons unreliable. HuC/D-ir was present in the neuronal cell bodies and nuclei of neurons in the myenteric plexus (Figure 1). Nuclear counts of HuC/D-ir myenteric neurons per mm2 ganglion area (Supplementary Table 3) showed no statistically significant difference between groups (P=0.56).
TH expression
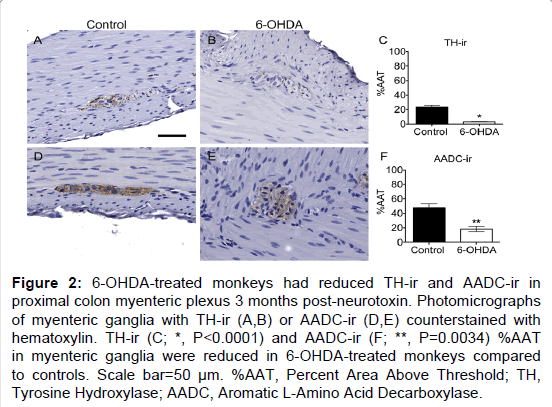
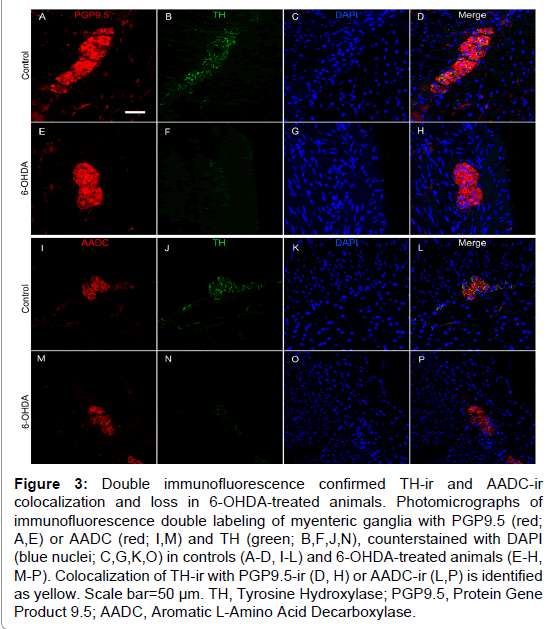
TH is the rate-limiting enzyme in catecholamine biosynthesis and is present in dopaminergic, adrenergic, and noradrenergic neurons [29]. TH positive neurons appeared to be a minority of those present in the myenteric plexus, with only 23% of average ganglion area exhibiting TH-ir in normal control animals (Figure 2 and Supplementary Table 3), similar to previous descriptions in humans and rhesus monkeys [13,25]. In the myenteric plexus of all animals TH-ir was present mainly in neuronal processes, appearing morphologically punctate or strand like, representing processes cut at transverse or longitudinal orientations. TH-ir was also found as a light, diffuse pattern in the neuronal somata in a minority of myenteric ganglia. Strikingly, less TH-ir in neuronal cell bodies and processes was visible in systemic 6-OHDA-treated animals compared to controls. Quantification of myenteric plexus ganglia confirmed catecholaminergic loss, revealing an 87.8% decrease in TH-ir %AAT in animals treated with 6-OHDA compared to controls (P<0.0001). Double label immunofluorescence confirmed TH-ir neuronal colocalization with PGP9.5-ir, TH-ir presence in a small minority of total ganglion area, and near complete loss of TH-ir in 6-OHDA treated monkeys (Figure 3).
To further assess the loss of catecholaminergic innervation, AADC immunohistochemistry was performed in proximal colon sections. AADC is an enzyme that catalyzes the decarboxylation of L-3,4-dihydroxyphenylalanine (L-DOPA) to dopamine and 5-hydroxytryptophan (5-HTP) to serotonin, therefore it is a marker of catecholaminergic and serotonergic innervation. AADC-ir was present in myenteric neurons, in nerve fibers in the muscle layers, and in enterochromaffin cells in the mucosal crypts (Figure 2). In the myenteric plexus, AADC-ir was visible in about 50% of ganglion area, mainly in neuronal processes with punctate or strand-like morphology, and occasionally as light, diffuse somatic immunoreactivity. As with TH-ir, AADC-ir appeared to be present in a greater percent area of ganglia in control animals compared to those treated with 6-OHDA. Analysis in myenteric plexus ganglia (Supplementary Table 3) confirmed a 61.7% loss of AADC-ir in 6-OHDA-treated animals compared to controls (P=0.0034). Double label immunofluorescence revealed that all TH-ir cells also expressed AADC, while only a subpopulation of AADC-ir neurons colocalized with TH-ir (Figure 3).
VIP and nNOS expression
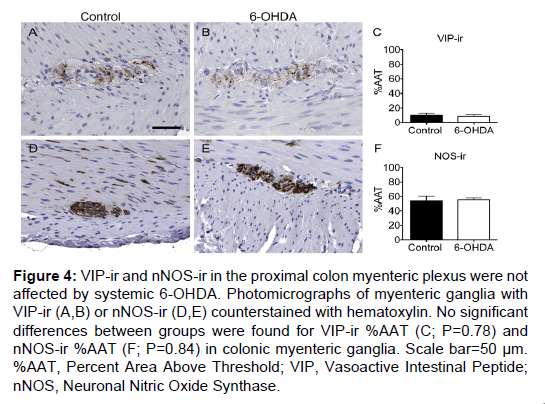
VIP and nNOS immunohistochemistry were performed to assess whether non-catecholaminergic neurochemical phenotypes were affected by 6-OHDA treatment in proximal colon sections. VIP-ir was present in a small percentage of the total ganglion area in the myenteric plexus and in nerve fibers in the mucosa (Figure 4). A punctate pattern was observed in neuronal processes, and occasionally light, granular immunoreactivity was present in neuronal cell bodies. VIP-ir %AAT did not show a difference between groups (P=0.78; Supplementary Table 3). nNOS-ir was present throughout the myenteric ganglia as a diffuse pattern of immunostaining in neuronal somata and processes, and in fibers in the muscle and mucosa. nNOS-ir %AAT was not significantly different between groups (P=0.84; Figure 4 and Supplementary Table 3).
Figure 1: Control and systemic 6-OHDA-treated monkeys had similar proximal colon general morphology and expression of neuronal markers. Photomicrographs of H&E stained proximal colon tissue showing anatomical organization (A,B); box in (A) corresponds to higher magnification (40x) image (C) of a myenteric ganglion (white asterisk). Myenteric ganglia with PGP9.5-ir (D,E) or HuC/D-ir (G,H) counterstained with hematoxylin. %AAT of PGP9.5-ir in colonic myenteric ganglia was similar between treatment groups (F; P=0.13). HuC/D-ir neurons per mm2 ganglion area was also similar (I; P=0.56). Scale bar=200 µm (A) or 50 µm (C). LM, Longitudinal Muscle; MP, Myenteric Plexus; CM, Circular Muscle; S, Submucosa; M, Mucosa; %AAT, Percent Area Above Threshold; PGP9.5, Protein Gene Produce 9.5; HuC/D, Human Neuronal Protein HuC/HuD.
Figure 2: 6-OHDA-treated monkeys had reduced TH-ir and AADC-ir in proximal colon myenteric plexus 3 months post-neurotoxin. Photomicrographs of myenteric ganglia with TH-ir (A,B) or AADC-ir (D,E) counterstained with hematoxylin. TH-ir (C; *, P<0.0001) and AADC-ir (F; **, P=0.0034) %AAT in myenteric ganglia were reduced in 6-OHDA-treated monkeys compared to controls. Scale bar=50 µm. %AAT, Percent Area Above Threshold; TH, Tyrosine Hydroxylase; AADC, Aromatic L-Amino Acid Decarboxylase.
Figure 3: Double immunofluorescence confirmed TH-ir and AADC-ir colocalization and loss in 6-OHDA-treated animals. Photomicrographs of immunofluorescence double labeling of myenteric ganglia with PGP9.5 (red; A,E) or AADC (red; I,M) and TH (green; B,F,J,N), counterstained with DAPI (blue nuclei; C,G,K,O) in controls (A-D, I-L) and 6-OHDA-treated animals (E-H, M-P). Colocalization of TH-ir with PGP9.5-ir (D, H) or AADC-ir (L,P) is identified as yellow. Scale bar=50 µm. TH, Tyrosine Hydroxylase; PGP9.5, Protein Gene Product 9.5; AADC, Aromatic L-Amino Acid Decarboxylase.
α-Synuclein expression
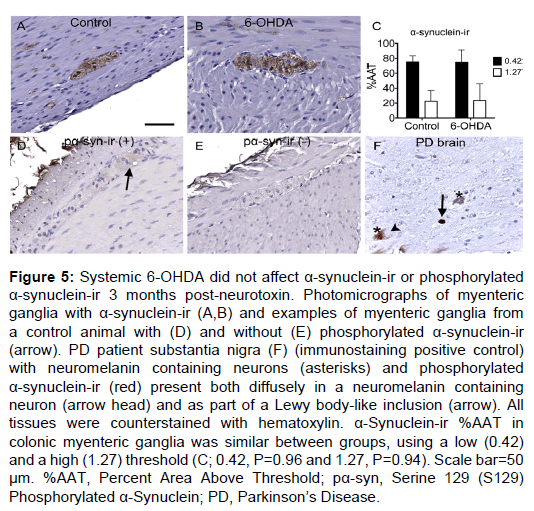
α-Synuclein-ir was found in nearly the entire myenteric ganglion area and in fibers running through the muscle and mucosal layers. In all myenteric ganglia, α-synuclein expression was present as both light, diffuse immunostain and as small, dark, granular spots, matching previous reports [30] (Figure 5). %AAT quantification did not reveal significant differences between treatment groups for light (P=0.96) or dark (P=0.94; Supplementary Table 3) α-synuclein-ir. Double label immunofluorescence of α-synuclein with PGP9.5, TH or VIP (Supplementary Figure 2) showed similar colocalization in all animals.
Phosphorylated (serine 129) α-synuclein was rarely detected in the myenteric plexus of all subjects. When present, it was observed as light, diffuse immunostaining in neuronal somata of the ganglia (Figure 5). No immunoreactivity was seen in nerve fibers in the muscle or mucosal layers, similar to previous descriptions [30]. Phosphorylated α-synuclein-ir was detected in a total of 3 ganglia in 2 control animals and 0 ganglia in any 6-OHDA-treated animals.
Inflammation and oxidative stress
Evaluation of H&E stained sections revealed variable amounts of inflammation in animals of both groups (Supplementary Table 4), consistent with previous findings in captive rhesus monkeys [31]. In the control group, two animals had histopathologically normal proximal colon tissue, one had moderate proliferation of Peyer’s patches and two displayed mild to moderate colitis. Of the 5 animals that received systemic 6-OHDA, one animal displayed moderate proliferation of Peyer’s patches and 4 had mild to moderate colitis.
Immunostainings against CD45, HLA-DR, nitrotyrosine and GFAP were performed to corroborate the above-described findings. CD45-ir and HLA-DR-ir were observed on the cell surface or cytoplasm of cells with circular/ovoid shape in all animals with varying amounts between subjects; HLA-DR-ir was also visible in macrophage-like cells with visible processes (Supplementary Figure 3). In each treatment group, three animals had minimal CD45-ir and HLA-DR-ir mainly in the mucosa, while two subjects displayed more robust immunoreactivity in the mucosa, large reactive Peyer’s patches in the submucosa and occasional staining within the muscle layers and myenteric plexus. No significant differences between groups were found for CD45-ir %AAT (P=0.24) or HLA-DR-ir %AAT (P=0.70) in the myenteric ganglia. Nitrotyrosine-ir was detected in all animals as diffuse, heterogeneous staining in myenteric ganglia and sometimes as a lighter immunoreactivity in muscle layers (Supplementary Figure 3). No significant difference was found between groups for nitrotyrosine-ir %AAT in the myenteric plexus (P=0.97). Pearson correlations between TH-ir %AAT and CD45-ir %AAT (P=0.20) or HLA-DR-ir %AAT (P=0.64) did not show an association between catecholaminergic loss and inflammatory markers.
Figure 4: VIP-ir and nNOS-ir in the proximal colon myenteric plexus were not affected by systemic 6-OHDA. Photomicrographs of myenteric ganglia with VIP-ir (A,B) or nNOS-ir (D,E) counterstained with hematoxylin. No significant differences between groups were found for VIP-ir %AAT (C; P=0.78) and nNOS-ir %AAT (F; P=0.84) in colonic myenteric ganglia. Scale bar=50 µm. %AAT, Percent Area Above Threshold; VIP, Vasoactive Intestinal Peptide; nNOS, Neuronal Nitric Oxide Synthase.
Figure 5: Systemic 6-OHDA did not affect a-synuclein-ir or phosphorylated a-synuclein-ir 3 months post-neurotoxin. Photomicrographs of myenteric ganglia with a-synuclein-ir (A,B) and examples of myenteric ganglia from a control animal with (D) and without (E) phosphorylated a-synuclein-ir (arrow). PD patient substantia nigra (F) (immunostaining positive control) with neuromelanin containing neurons (asterisks) and phosphorylated a-synuclein-ir (red) present both diffusely in a neuromelanin containing neuron (arrow head) and as part of a Lewy body-like inclusion (arrow). All tissues were counterstained with hematoxylin. a-Synuclein-ir %AAT in colonic myenteric ganglia was similar between groups, using a low (0.42) and a high (1.27) threshold (C; 0.42, P=0.96 and 1.27, P=0.94). Scale bar=50 µm. %AAT, Percent Area Above Threshold; pa-syn, Serine 129 (S129) Phosphorylated a-Synuclein; PD, ParkinsonâÂ?Â?s Disease.
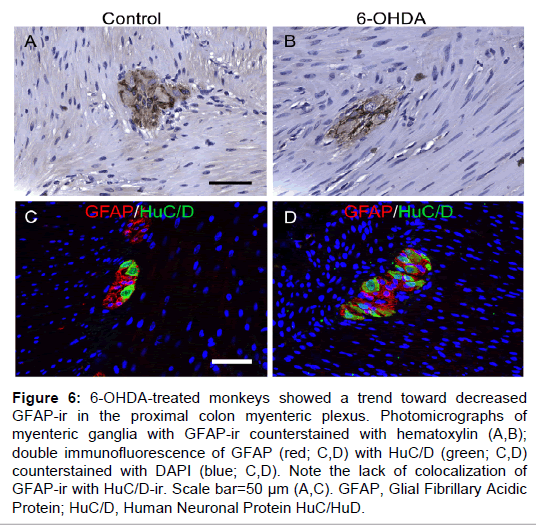
GFAP-ir was found throughout a majority of the myenteric plexus area, packed tightly around neuronal somata, and occasionally visible in the muscle and mucosal layers (Figure 6). In the myenteric plexus, GFAP-ir was expressed in enteric glial cell bodies and nuclei, and also abundantly present in glial projections of varying shapes and lengths, matching previous descriptions [32]. GFAP-ir %AAT in the myenteric plexus was not statistically different between groups, although there was a trend towards less expression in 6-OHDA-treated monkeys (P=0.093). Double GFAP and HuC/D immunofluorescence confirmed non-neuronal localization of GFAP (Figure 6).
Discussion
The present study in rhesus monkeys demonstrates that 3 months after systemic 6-OHDA administration nearly all TH-ir in the colonic myenteric plexus is lost. To the best of our knowledge, this is the first report of the effects of systemic 6-OHDA in the colon of nonhuman primates.
Myenteric neuronal numbers in the proximal colon were not affected by 6-OHDA as evaluated with PGP9.5-ir and HuC/D-ir. The lack of change in pan-neuronal markers is likely due to the low overall %AAT (23%) of TH-ir in the proximal colon myenteric plexus. It should be noted that TH-ir in the colonic myenteric ganglia is localized almost entirely in neuronal processes rather than cell bodies. As the unmyelinated neuronal processes of the ENS tend to be less than 1 μm in diameter [33] and the tissue used for this study was cut at 5 μm section thickness, even a complete loss of TH-ir neuronal processes would be difficult to detect by PGP9.5-ir.
Our finding of an 87.8% decrease in TH-ir in the colonic myenteric plexus following systemic 6-OHDA administration is in agreement with a previous publication by Chaumette et al. [34] on rhesus ENS changes following catecholaminergic neurotoxin challenge. The authors reported that 5 months after chronic, systemic 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment there was a 72% decrease in TH-ir in the colon myenteric plexus [34]. Loss of myenteric catecholaminergic innervation and not simply a downregulation in TH protein expression levels, was confirmed in our study by AADC immunostaining. Previous reports indicate that in mammals about 5-10% of total colonic myenteric plexus ENS neurons are catecholaminergic [13,25] while approximately 5% are serotonergic [35]. As AADC is a marker of both catecholaminergic and serotonergic neurons, the 61.7% loss of AADC-ir observed in the 6-OHDA-treated monkeys is in agreement with the near complete loss of catecholaminergic innervation.
Figure 6: 6-OHDA-treated monkeys showed a trend toward decreased GFAP-ir in the proximal colon myenteric plexus. Photomicrographs of myenteric ganglia with GFAP-ir counterstained with hematoxylin (A,B); double immunofluorescence of GFAP (red; C,D) with HuC/D (green; C,D) counterstained with DAPI (blue; C,D). Note the lack of colocalization of GFAP-ir with HuC/D-ir. Scale bar=50 µm (A,C). GFAP, Glial Fibrillary Acidic Protein; HuC/D, Human Neuronal Protein HuC/HuD.
Myenteric VIP-ir and nNOS-ir were not affected by systemic 6-OHDA treatment. In contrast, systemic MPTP dosing to nonhuman primates led to a significant increase in nNOS-ir in the myenteric plexus 5 months post-neurotoxin [34]. It could be argued that differences in neurotoxin administration and time of euthanasia after dosing of the neurotoxin affected the results. In the Chaumette et al. study, rhesus monkeys received a daily dose of MPTP following a semichronic dosing paradigm until they displayed parkinsonian symptoms (mean cumulative dose 3.7mg/kg iv; approximately 18 dosing days) and were euthanized 5 months post-MPTP. Compared to our acute neurotoxin dosing followed by necropsy at 3 months, the extended period during and after MPTP administration may have allowed time for compensatory ENS changes post-neurotoxin. Alternatively, differences in criteria for quantification of nNOS expression may have affected the results. The MPTP study quantified number of nNOSir cells colocalized with the somatic/nuclear pan-neuronal marker HuC/D. As nNOS subcellular localization can be affected by cell stress [36,37], inclusion of neuronal somata and pprocesses in our %AAT whole ganglion analysis may have produced differences between studies. Another explanation could be related to the ability of MPTP to cross the blood brain barrier and induce nigral dopaminergic loss, unlike systemic 6-OHDA [20]. A related key issue to keep in mind when comparing these results to previous publications is the method of neurotoxin delivery. Specifically, in rodents 6-OHDA is typically delivered directly into the brain, exerting central nervous system effects, while in our study it was delivered systemically via intravenous administration. In that regard, rats injected with 6-OHDA only in the medial forebrain bundle presented, in addition to dopaminergic nigrostriatal loss, a colonic increase in VIP [38] and dopamine [39] and decreased nNOS [38,40]. Collectively, these reports suggest that catecholaminergic loss may have differential effects on colonic enteric neurons depending on whether the subjects have central and/or peripheral lesions.
In PD patients, α-synuclein accumulation in the form of Lewy bodies has been found in VIP-ir neurons and some in TH-ir neurons of the myenteric and submucosal plexus, throughout the entire length of the GI tract [41,42] suggesting that α-synuclein may have a role in PD GI dysfunction. Evaluation in our 6-OHDA-treated monkeys failed to show changes in α-synuclein and phosphorylated α-synuclein expression. The lack of changes may be due to the acute dosing and neurotoxicity compared to the progressive neurodegeneration characteristic of PD or chronic PD models. For example, transgenic mice overexpressing human wild type α-synuclein had increased α-synuclein immunoreactivity surrounding cholinergic neurons in the colonic myenteric plexus and decreased fecal output at 8-10 months of age [43] and, in a separate study, displayed increased insoluble colonic myenteric α-synuclein and constipation (reduced fecal pellet output and fecal water content) at 12-15 months [44]. In addition, transgenic mice expressing human A53T α-synuclein, a mutation associated with familial, early-onset PD, had accumulation of insoluble phosphorylated α-synuclein in the ENS [45], decreased stool frequency and fecal water content [46,47] and increased α-synuclein protein and transcript levels in the colon [47]. Although changes in enteric α-synuclein were not reported for the nonhuman primate systemic MPTP model [34], analysis at varying timepoints in neurotoxin-induced rodent models of PD has produced diverse results. Systemic MPTP in mice increased α-synuclein-ir in the duodenum, with no change in the colon, one week post-neurotoxin [48]. In another rodent PD model, rotenone (a mitochondrial complex I inhibitor like MPTP and 6-OHDA) increased phosphorylation, accumulation, and aggregation of enteric α-synuclein when delivered via stomach tube 5 days a week for 3 months [49], but had no effect on colonic α-synuclein following 28 days of daily oral administration [50]. When rotenone was administered as five 2 mg/kg injections per week for 6 weeks, rats displayed slowing of gastrointestinal motility [51]. In these animals, an acute decrease in α-synuclein expression was observed at 3 days, while a moderate loss of small intestine myenteric neurons and an increase in Lewy bodylike neuronal cytoplasmic inclusions were found at 6 months [51]. Interestingly, rats injected unilaterally with 6-OHDA into the medial forebrain bundle had no changes in the α-synuclein mRNA levels in the substantia nigra and decreased levels in the striatum three weeks after neurotoxin, further suggesting that type of neurotoxin and paradigm of administration affect the impact on α-synuclein expression [52].
Inflammatory and oxidative stress markers in the colon were similar between groups 3 months post-neurotoxin and did not correlate with TH-ir. The presence of inflammatory cell infiltration or colitis found in varying degrees in animals of both groups may have affected the detection of 6-OHDA-induced inflammation. Colitis occurs in about 20% of monkeys in captive rhesus colonies [31]; therefore it is unsurprising that at least one animal in the 6-OHDAtreated and control groups had mild to moderate colitis. Another possibility is that a difference in inflammation and oxidative stress may not have been observed due to the length of time post-intoxication (3 months); it should be mentioned that the monkeys in this study did not have upregulation of inflammatory markers in the heart three months after 6-OHDA administration, despite extensive left ventricle catecholaminergic loss [23,24]. In that regard, rats treated with unilateral intrastriatal 6-OHDA showed temporarily increased levels of reactive nitrogen and oxygen species markers in the striatum, peaking between 25 min to 2 h after neurotoxin, and returning to near-baseline values within 7 days [53]. mRNA microarray and positron emission tomography (PET) with the radioligand [3H]PK11195 (a marker of microglial activation) confirmed these transient inflammatory changes, demonstrating a return to baseline values within 28 days post- 6-OHDA [54,55].
The trend toward decreased GFAP-ir in animals that received 6-OHDA contrasts with reported increases in GFAP mRNA [56] and protein [57] levels in colonic biopsies from PD patients. A possible explanation is that PD patients have chronic upregulation of proinflammatory cytokines in the colon [56], which has been proposed to induce enteric gliosis and increased GFAP expression [58]. In comparison, the acute effect of 6-OHDA may not be sufficient to produce a persistent effect in enteric glial cells. Moreover, sympathetic innervation of the myenteric plexus has been shown to specifically activate enteric glial cells in the guinea pig distal colon [59]. As systemic 6-OHDA induced extensive loss of colonic catecholaminergic innervation (Figure 2), it may have eliminated sympathetic neuron activation of enteric glial cells, perhaps contributing to decreased GFAP-ir in neurotoxin-treated animals.
Investigating the relationship between ENS pathology and GI dysfunction in animal models can help tease apart the complex and multivariate etiology of constipation in PD. In both systemic MPTP and 6-OHDA-treated rhesus monkeys, loss of catecholaminergic neurons in the colonic ENS was not associated with constipation. Systemic MPTP did not affect stool consistency as evaluated one week before necropsy (5 months after neurotoxin, n=6 rhesus monkeys) [34], while in our study, two 6-OHDA treated animals exhibited frequent soft feces or diarrhea post-neurotoxin and three showed no change [23]. Although we did not perform specific assays to quantify changes in colonic transit time, the daily feces observations performed throughout the duration of this study produced data similar to patient reports used in clinical evaluations [56]. In PD patients, the presence of ENS catecholaminergic loss and its role in producing constipation is currently debated [11,13,60]. A significant decrease in dopaminergic immunoreactivity was found in the colon of PD patients with extremely severe constipation (9 of the 11 PD patients had colectomies undertaken for intractable constipation) [11]. However, these findings have not been replicated; more recent publications by Annerino et al. and Corbille et al. did not find any changes in ENS neurochemical phenotypes in PD patients [13,14]. Although our data cannot clarify the debate regarding the presence of ENS neurodegeneration in PD patients, our results demonstrate that dopaminergic neuron loss in the ENS is not sufficient to induce constipation. The lack of association between colonic ENS catecholaminergic loss and constipation is also supported by reports demonstrating that enteric dopaminergic neurons exert an inhibitory effect on GI motility [15,61,62]. These findings implicate additional alterations, not observed in the rhesus ENS following systemic 6-OHDA, as contributors to constipation in PD.
Although in this study we have focused on the ENS, GI function is also regulated by input from the extrinsic autonomic nervous system, which is affected in PD. For example, PD patients can experience impaired supraspinal modulation of the sacral defecation reflex, which is hypothesized to lead to dyssynergic defecation, contributing to constipation [12,63]. Lewy body pathology found in paravertebral sympathetic ganglia and the dorsal motor nucleus of the vagus (which are involved in regulation of colonic function and motility [64-66]), may affect neuronal function and colonic transit time [12]. Cholinergic dysfunction has been suggested to contribute to constipation in PD [67]. A recent publication using positron emission tomography and 5-[11C]-methoxy-donepezil documented decreased acetylcholinesterase (AChE) in the small intestine, pancreas and myocardium of PD patients compared to controls [68]. The authors concluded that this represented parasympathetic denervation, rather than ENS cholinergic neurodegeneration, based on correlation between AChE density loss and the distribution of vagal innervation, in addition to previous findings of no ENS neuron loss in PD patients [13,14]. Rodent models of PD have presented mixed effects on GI tract acetylcholine content and ENS cholinergic neurons, with some studies showing decreased AChE, decreased choline acetyltransferease (ChAT) immunoreactivity [69-71] or no effect on cholinergic markers [38,72,73]. Nigral neuron density has also been reported to correlate with bowel movement frequency in PD patients [74]. Similarly, in rodents bilateral injection of 6-OHDA into the substantia nigra has led to delayed gastric emptying in association with decreased ChAT and increased TH immunoreactivity in the dorsal motor nucleus of the vagus [71]. Additionally, unilateral nigral 6-OHDA increased TH and decreased nNOS expression in the colonic myenteric plexus [72]. These studies support the concept that nigral cell loss induces changes in neurotransmitter expression in anatomical areas not classically linked.
The diverse inputs involved in control of GI function and lack of characteristic PD Lewy body pathology may partially explain why the 6-OHDA-treated animals evaluated in this study did not show signs of constipation [23]. This complexity should be considered when analyzing potential models of PD-related constipation, as it suggests that GI symptoms depend on a combination of central and enteric nervous system pathology, as well as other factors, which are beyond the scope of this paper (e.g.: diet, physical activity, microbiome) [75- 77].
Conclusion
Overall, our results indicate that systemic administration of 6-OHDA to rhesus monkeys significantly reduces TH-ir and AADCir in the colonic myenteric plexus without affecting α-synuclein expression, inflammation or oxidative stress markers as observed 3 months post-intoxication. The reduction in ENS TH-ir and AADC-ir was not associated with constipation in monkeys, suggesting that loss of catecholaminergic innervation in the colon is not solely responsible for decreased bowel movement in PD. Future animal studies assessing the independent contribution of various pathological factors to GI dysfunction will be key to elucidate the etiology of constipation in PD.
Acknowledgement
The authors are grateful to the Wisconsin Alzheimer’s Disease Research Center and Dr. Shahriar Salamat for providing PD brain tissue (P50-AG033514) and WNPRC-Nonhuman Primate Biological Materials Distribution Core (NHPBMD) services for providing control rhesus tissue. This research was supported by grants from the National Institutes of Health P51 P51OD011106, UL1TR000427, R21 NS084158, R24 OD019803 and funding from the UW-Madison Vice Chancellor for Research and Graduate Education.
References
- Chaudhuri KR, Healy DG, Schapira AH; National Institute for Clinical Excellence (2006) Non-motor symptoms of Parkinson's disease: Diagnosis and management. Lancet Neurol 5: 235-245.
- Muller B, Assmus J, Herlofson K, Larsen JP, Tysnes OB (2013) Importance of motor vs. Non-motor symptoms for health-related quality of life in early ParkinsonâÂ?Â?s disease. Parkinsonism Relat Disord 19: 1027-1032.
- Palma JA, Kaufmann H (2014) Autonomic disorders predicting Parkinson's disease. Parkinsonism Relat Disord 20 Suppl 1: S94-S98.
- Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF (2015) Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol 14: 625-639.
- Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, et al. (2007) Patient-reported autonomic symptoms in Parkinson disease. Neurology 69: 333-341.
- Micieli G, Tosi P, Marcheselli S, Cavallini A (2003) Autonomic dysfunction in Parkinson's disease. Neurol Sci 24 Suppl 1: S32-34.
- Asahina M, Vichayanrat E, Low DA, Iodice V, Mathias CJ (2013) Autonomic dysfunction in parkinsonian disorders: Assessment and pathophysiology. Journal of Neurology, Neurosurgery and Psychiatry 84: 674-680.
- Blekken LE, Nakrem S, Vinsnes AG, Norton C, Mørkved S, et al. (2016) Constipation and laxative use among nursing home patients: Prevalence and associations derived from the residentâÂ?Â?s assessment instrument for long-term care facilities (interRAI LTCF). Gastroenterol Res Pract 2016: 1215746.
- Edwards L, Quigley EM, Hofman R, Pfeiffer RF (1993) Gastrointestinal symptoms in Parkinson disease: 18 month follow-up study. Mov Disord 8: 83-86.
- Rahman MM, Uddin MJ, Chowdhury JH, Chowdhury TI (2014) Effect of levodopa and carbidopa on non-motor symptoms and signs of Parkinson's disease. Mymensingh Med J 23: 18-23.
- Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, et al. (1995) Dopaminergic defect of enteric nervous system in Parkinson's disease patients with chronic constipation. Lancet 346: 861-864.
- Rossi M, Merello M, Perez-Lloret S (2015) Management of constipation in Parkinson's disease. Expert Opin Pharmacother 16: 547-557.
- Annerino DM, Arshad S, Taylor GM, Adler CH, Beach TG, et al. (2012) Parkinson's disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol 124: 665-680.
- Corbillé AG, Coron E, Neunlist M, Derkinderen P, Lebouvier T (2014) Appraisal of the dopaminergic and noradrenergic innervation of the submucosal plexus in PD. J Parkinsons Dis 4: 571-576.
- Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286-294.
- Furness JB (2000) Types of neurons in the enteric nervous system. J Auton Nerv Syst 81: 87-96.
- Goldstein DS (2013) Differential responses of components of the autonomic nervous system. Handb Clin Neurol 117: 13-22.
- Rodriguez-Pallares J, Parga JA, Muñoz A, Rey P, Guerra MJ, et al. (2007) Mechanism of 6-hydroxydopamine neurotoxicity: The role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J Neurochem 103: 145-156.
- Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson's disease: A target for neuroprotection? Lancet Neurol 8: 382-397.
- Joers V, Vermilyea S, Dilley K, Emborg ME (2014) Systemic administration of 6-OHDA to rhesus monkeys upregulates HLA-DR expression in brain microvasculature. J Inflamm Res 7: 139-149.
- Laverty R, Sharman DF, Vogt M (1965) Action of 2, 4, 5-Trihydroxyphenylethylamine on the storage and release of nor-adrenaline. Br J Pharmacol Chemother 24: 549-560.
- Kostrzewa RM, Jacobowitz DM (1974) Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev 26: 199-288.
- Joers V, Seneczko K, Goecks NC, Kamp TJ, Hacker TA, et al. (2012) Non-uniform cardiac denervation observed by 11C-meta-hydroxyephedrine PET in 6-OHDA-treated monkeys. PLoS One 7: e35371.
- Joers V, Dilley K, Rahman S, Jones C, Shultz J, et al. (2014) Cardiac sympathetic denervation in 6-OHDA-treated non-human primates. PLoS One 9: e104850.
- Noorian AR, Taylor GM, Annerino DM, Greene JG (2011) Neurochemical phenotypes of myenteric neurons in the rhesus monkey. J Comp Neurol 519: 3387-3401.
- Hedreen JC (1998) What was wrong with the Abercrombie and empirical cell counting methods? A review. Anat Rec 250: 373-380.
- Clarke PG (1992) How inaccurate is the Abercrombie correction factor for cell counts? Trends Neurosci 15: 211-212.
- Guillery RW (2002) On counting and counting errors. J Comp Neurol 447: 1-7.
- Daubner SC, Le T, Wang S (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508: 1-12.
- Böttner M, Zorenkov D, Hellwig I, Barrenschee M, Harde J, et al. (2012) Expression pattern and localization of alpha-synuclein in the human enteric nervous system. Neurobiol Dis 48: 474-480.
- Ardeshir A, Oslund KL, Ventimiglia F, Yee J, Lerche NW, et al. (2013) Idiopathic microscopic colitis of Rhesus macaques: Quantitative assessment of colonic mucosa. Anat Rec (Hoboken) 296: 1169-1179.
- Bassotti G, Villanacci V, Fisogni S, Rossi E, Baronio P, et al. (2007) Enteric glial cells and their role in gastrointestinal motor abnormalities: Introducing the neuro-gliopathies. World J Gastroenterol 13: 4035-4041.
- Sri Paran T, Rolle U, Puri P (2008) Postnatal changes in enteric plexus axonal thickness. Pediatric surgery international 24: 1365-1367.
- Chaumette T, Lebouvier T, Aubert P, Lardeux B, Qin C, et al. (2009) Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental parkinsonism. Neurogastroenterol Motil 21: 215-222.
- Keating DJ, Peiris H, Kyloh M, Brookes SJ, Spencer NJ (2013) The presence of 5-ht in myenteric varicosities is not due to uptake of 5-ht released from the mucosa during dissection: Use of a novel method for quantifying 5-ht immunoreactivity in myenteric ganglia. Neurogastroenterol Motil 25: 849-853.
- Abulrob A, Tauskela JS, Mealing G, Brunette E, Faid K, et al. (2005) Protection by cholesterol-extracting cyclodextrins: A role for n-methyl-d-aspartate receptor redistribution. J Neurochem 92: 1477-1486.
- Takagi N, Logan R, Teves L, Wallace MC, Gurd JW (2000) Altered interaction between psd-95 and the nmda receptor following transient global ischemia. J Neurochem 74: 169-178.
- Colucci M, Cervio M, Faniglione M, De Angelis S, Pajoro M, et al. (2012) Intestinal dysmotility and enteric neurochemical changes in a parkinson's disease rat model. Autonomic neuroscience: Basic & Clinical 169: 77-86.
- Levandis G, Balestra B, Siani F, Rizzo V, Ghezzi C, et al. (2015) Response of colonic motility to dopaminergic stimulation is subverted in rats with nigrostriatal lesion: Relevance to gastrointestinal dysfunctions in ParkinsonâÂ?Â?s disease. Neurogastroenterol Motil 27: 1783-1795.
- Blandini F, Balestra B, Levandis G, Cervio M, Greco R, et al. (2009) Functional and neurochemical changes of the gastrointestinal tract in a rodent model of Parkinson's disease. Neurosci Lett 467: 203-207.
- Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1988) Parkinson's disease: The presence of Lewy bodies in Auerbach's and Meissner's plexuses. Acta Neuropathol 76: 217-221.
- Wakabayashi K, Takahashi H, Ohama E, Ikuta F (1990) Parkinson's disease: An immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol 79: 581-583.
- Wang L, Magen I, Yuan PQ, Subramaniam SR, Richter F, et al. (2012) Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil 24: e425-436.
- Hallett PJ, McLean JR, Kartunen A, Langston JW, Isacson O (2012) Alpha-synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol Dis 47: 258-267.
- Bencsik A, Muselli L, Leboidre M, Lakhdar L, Baron T (2014) Early and persistent expression of phosphorylated alpha-synuclein in the enteric nervous system of a53t mutant human alpha-synuclein transgenic mice. Journal of Neuropathology and Experimental Neurology 73: 1144-1151.
- Noorian AR, Rha J, Annerino DM, Bernhard D, Taylor GM, et al. (2012) Alpha-synuclein transgenic mice display age-related slowing of gastrointestinal motility associated with transgene expression in the vagal system. Neurobiol Dis 48: 9-19.
- Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, et al. (2010) Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Human molecular genetics 19: 1633-1650.
- Natale G, Kastsiushenka O, Fulceri F, Ruggieri S, Paparelli A, et al. (2010) MPTP-induced parkinsonism extends to a subclass of TH-positive neurons in the gut. Brain Res 1355: 195-206.
- Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, et al. (2010) Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 5: e8762.
- Tasselli M, Chaumette T, Paillusson S, Monnet Y, Lafoux A, et al. (2013) Effects of oral administration of rotenone on gastrointestinal functions in mice. Neurogastroenterol Motil 25: e183-193.
- Drolet RE, Cannon JR, Montero L, Greenamyre JT (2009) Chronic rotenone exposure reproduces Parkinson's disease gastrointestinal neuropathology. Neurobiol Dis 36: 96-102.
- Zeng BY, Dass B, Owen A, Rose S, Cannizzaro C, et al. (2002) 6-hydroxydopamine lesioning differentially affects alpha-synuclein mRNA expression in the nucleus accumbens, striatum and substantia nigra of adult rats. Neuroscience letters 322: 33-36.
- Henze C, Earl C, Sautter J, Schmidt N, Themann C, et al. (2005) Reactive oxidative and nitrogen species in the nigrostriatal system following striatal 6-hydroxydopamine lesion in rats. Brain Res 1052: 97-104.
- Maia S, Arlicot N, Vierron E, Bodard S, Vergote J, et al. (2012) Longitudinal and parallel monitoring of neuroinflammation and neurodegeneration in a 6-hydroxydopamine rat model of Parkinson's disease. Synapse 66: 573-583.
- Na SJ, DiLella AG, Lis EV, Jones K, Levine DM, et al. (2010) Molecular profiling of a 6-hydroxydopamine model of Parkinson's disease. Neurochem Res 35: 761-772.
- Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, et al. (2013) Colonic inflammation in Parkinson's disease. Neurobiol Dis 50: 42-48.
- Clairembault T, Kamphuis W, Leclair-Visonneau L, Rolli-Derkinderen M, Coron E, et al. (2014) Enteric GFAP expression and phosphorylation in Parkinson's disease. J Neurochem 130: 805-815.
- von Boyen GB, Steinkamp M, Reinshagen M, Schafer KH, Adler G, et al. (2004) Pro-inflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 53: 222-228.
- Gulbransen BD, Bains JS, Sharkey KA (2010) Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci 30: 6801-6809.
- Pfeiffer RF (2014) Parkinson's disease and the gut: 'The wheel is come full circle'. J Parkinsons Dis 4: 577-578.
- Walker JK, Gainetdinov RR, Mangel AW, Caron MG, Shetzline MA (2000) Mice lacking the dopamine transporter display altered regulation of distal colonic motility. American Journal of Physiology Gastrointestinal and Liver Physiology 279: G311-318.
- Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD (2006) Physiological modulation of intestinal motility by enteric dopaminergic neurons and the d2 receptor: Analysis of dopamine receptor expression, location, development and function in wild-type and knock-out mice. J Neurosci 26: 2798-2807.
- Cersosimo MG, Benarroch EE (2008) Neural control of the gastrointestinal tract: Implications for Parkinson disease. Mov Disord 23: 1065-1075.
- Wakabayashi K, Takahashi H (1997) Neuropathology of autonomic nervous system in Parkinson's disease. Eur Neurol 38 Suppl 2: 2-7.
- Takeda S, Yamazaki K, Miyakawa T, Arai H (1993) Parkinson's disease with involvement of the parasympathetic ganglia. Acta Neuropathol 86: 397-398.
- Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K (2007) Parkinson's disease: Lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 113: 421-429.
- Perez-Lloret S, Barrantes FJ (2016) Deficits in cholinergic neurotransmission and their clinical correlates in parkinsonâÂ?Â?s disease. NPJ Parkinson's Disease 2: 16001.
- Gjerløff T, Fedorova T, Knudsen K, Munk OL, Nahimi A, et al. (2015) Imaging acetylcholinesterase density in peripheral organs in Parkinson's disease with 11C-donepezil PET. Brain 138: 653-663.
- Fornai M, Pellegrini C, Antonioli L, Segnani C, Ippolito C, et al. (2016) Enteric dysfunctions in experimental ParkinsonâÂ?Â?s disease: Alterations of excitatory cholinergic neurotransmission regulating colonic motility in rats. The Journal of pharmacology and experimental therapeutics 356: 434-444.
- Zheng LF, Song J, Fan RF, Chen CL, Ren QZ, et al. (2014) The role of the vagal pathway and gastric dopamine in the gastroparesis of rats after a 6-hydroxydopamine microinjection in the substantia nigra. Acta Physiol (Oxf) 211: 434-446.
- Zheng LF, Wang ZY, Li XF, Song J, Hong F, et al. (2011) Reduced expression of choline acetyltransferase in vagal motoneurons and gastric motor dysfunction in a 6-ohda rat model of parkinson's disease. Brain research 1420: 59-67.
- Zhu HC, Zhao J, Luo CY, Li QQ (2012) Gastrointestinal dysfunction in a ParkinsonâÂ?Â?s disease rat model and the changes of dopaminergic, nitric oxidergic and cholinergic neurotransmitters in myenteric plexus. J Mol Neurosci 47: 15-25.
- Anderson G, Noorian AR, Taylor G, Anitha M, Bernhard D, et al. (2007) Loss of enteric dopaminergic neurons and associated changes in colon motility in an mptp mouse model of Parkinson's disease. Experimental neurology 207: 4-12.
- Petrovitch H, Abbott RD, Ross GW, Nelson J, Masaki KH, et al. (2009) Bowel movement frequency in late-life and substantia nigra neuron density at death. Mov Disord 24: 371-376.
- Ueki A, Otsuka M (2004) Life style risks of Parkinson's disease: Association between decreased water intake and constipation. J Neurol 251 Suppl 7: vII18-23.
- van der Kolk NM, King LA (2013) Effects of exercise on mobility in people with Parkinson's disease. Mov Disord 28: 1587-1596.
- Astarloa R, Mena MA, Sánchez V, de la Vega L, de Yébenes JG (1992) Clinical and pharmacokinetic effects of a diet rich in insoluble fiber on Parkinson disease. Clin Neuropharmacol 15: 375-380.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 7986
- [From(publication date):
November-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 7063
- PDF downloads : 923