Neuroprotective and Anti-Inflammatory Effects of Sonlicraomanol in Rat Cerebral Ischemia/Reperfusion Injury Is Achieved Through Improving Mitochondrial Function
Received: 06-Dec-2021 / Manuscript No. CMB-21-49080 / Editor assigned: 08-Dec-2021 / PreQC No. CMB-21-49080 (PQ) / Reviewed: 08-Jan-2022 / QC No. CMB-21-49080 / Revised: 13-Jan-2022 / Manuscript No. CMB-21-49080 (R) / Accepted Date: 13-Jan-2022 / Published Date: 20-Jan-2022 DOI: 10.4172/1165-158X.1000222
Abstract
Background: Mitochondrial improvement is the central player of neuroprotection following cerebral ischemia/ reperfusion injury (I/RI). The present study evaluated the neuroprotective and anti-inflammatory effects of a new mitochondrial-acting drug, sonlicraomanol (SONL), in rats with cerebral I/RI, by focusing on the role of mitochondrial ATP-sensitive potassium (mK-ATP) channels and mitochondrial biogenesis.
Methods: Cerebral I/RI was modeled in Sprague Dawley rats (n=36) through induction of two hours of local ischemia via middle cerebral artery occlusion, followed by 24 hours of reperfusion. SONL at the concentrations of 10 and 50μM was intraperitoneally administered to rats for one week before the onset of occlusion. Cerebral infarcted areas, brain activity, mitochondrial function and biogenesis, and the levels of pro-inflammatory cytokines were quantified by triphenyl-tetrazolium chloride, behavioral tests, fluorometry, immunoblotting, and ELISA, respectively.
Results: Administration of SONL significantly reduced cerebral infarct volume and neurological activity in a dosedependent manner, as compared with the untreated control group (p<0.01). SONL (50μM) significantly reversed the I/RI-induced changes in mitochondrial membrane depolarization, mitochondrial reactive oxygen species (mitoROS), superoxide dismutase (mnSOD), and pro-inflammatory cytokines TNF-α, IL-1β, IL-6 (p<0.01). As well, the expression of mitochondrial biogenesis proteins PGC-1α, NRF1, and TFAM was upregulated following SONL 50 μM treatment. Importantly, the inhibition of mK-ATP channels through 5-hydoxydecanoate significantly eliminated the neuroprotective, anti-inflammatory, and mitochondrial impacts of SONL.
Conclusion: SONL post-conditioning had a significant neuroprotective effect which was mediated through increasing mK-ATP channels activity and subsequent improvement of mitochondrial biogenesis and function, and reduction of inflammatory responses.
Keywords
Stroke; Ischemia/Reperfusion injury; Mitochondria; Inflammation; Neuroprotection
Introduction
Cerebrovascular ischemic disorders are the most common disorders worldwide, leading to high morbidity and mortality rates. Acute ischemic stroke and the subsequently delayed reperfusion are the main cause of many pathophysiological changes and relevant disabilities following brain damage in adults [1]. Cerebral ischemia/ reperfusion injuries (I/RI) include a variety of pathophysiological consequences that upsurge oxidative stress, inflammatory reactions, mitochondrial dysfunction, releasing of endogenous toxic substances, microcirculation abnormalities, and apoptotic cell death [1,2]. The higher number of cerebral I/RI pathologies is linked with mitochondrial dysfunction, which can arise from malfunctioning pathways disturbing mitochondrial homeostasis and biogenesis [3].
Previous studies discovered that mitochondrial biogenesis is suppressed following cerebral I/RI and thus its upregulation would be neuroprotective [3,4]. The transcriptional coactivator, peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α), plays a critical role in maintaining mitochondrial biogenesis and function [5]. In collaboration with nuclear respiratory factor-1 (NRF1), PGC1α also controls the expression of a nucleus-encoded protein, mitochondrial transcription factor-A (TFAM), which regulates the copy number of mitochondrial DNA [6]. As such, the entire mitochondrial biogenesis network is highly regulated. Nonetheless, ATP-dependent potassium (mK-ATP) channels located in the mitochondrial membrane also play a critical role in maintaining this harmony in mitochondrial biogenesis [7,8]. However, these channels are blocked during I/R damage, disrupting mitochondrial integrity and increasing the production of reactive oxygen species (ROS) and inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukins [9,10]. As a result, mitochondria and mK-ATP channels are considered as the central pharmacological targets for neuroprotection and effective inhibition of cerebral I/RI.
Sonlicromanol (SONL) is a new mitochondrial-acting drug that has strong antioxidative properties and enhances the physiological features of mitochondria in vital cells [11,12]. Its safety and efficacy have been confirmed recently in patients with mitochondrial diseases in phase 1 and 2 clinical trials [11,13]. A recent study reported that SONL enhances prostaglandin E2 (PGE2) activity and may increase its antioxidative and inflammatory actions [14]. Owing to the protective potentials of SONL, there is an opportunity to evaluate its protective effects and underlying mechanisms on stroke pathophysiology. Consequently, considering the involvement of mitochondrial dysfunction and inflammatory responses in the expansion of cerebral I/RI, we aimed to evaluate the protective impacts of SONL on cerebral infarction outcomes, mitochondrial function and biogenesis, and the levels of pro-inflammatory cytokines, and explore the role of mK-ATP channels in these effects.
Materials and Methods
Animals and ethics
Thirty-six Sprague-Dawley rats between 250 and 300g for each experiment were obtained from the University Laboratory Animal Center and kept at standard animal house temperature of about 24℃ and humidity of 55% in a 12-h cycle of lightness/darkness. All procedures of animal research and handling were performed based on the approval of the University Animal Ethics Committee.
Modeling of cerebral ischemia/reperfusion injury and animal grouping
The middle cerebral artery occlusion (MCAO) model was performed to establish cerebral I/RI based on the previous study [15]. At a brief, the rats were anesthetized with chloral hydrate (400 mg/kg, intraperitoneally). After exposing the right carotid arteries, a monofilament thread with 0.234 mm in diameter and a rounded tip was guided into the internal carotid artery through the trunk of the external carotid artery and mildly advanced for a length of 18-20 mm from the bifurcation to block the middle cerebral artery origin. The thread was kept in place for 2 hours and then it was withdrawn to start reperfusion. A heating pad was utilized to keep body temperature at 37- 37.5°C throughout the experiment. Rats were sacrificed 24 hours after postoperative recovery under anesthesia for sampling. Sham-operated rats went through the same surgery except for artery occlusion. Rats with considerable bleeding and hemorrhage and premature death were excluded from the study.
Rats were randomly allocated into the following six groups (n=6): 1) Sham; 2) I/R; 3) I/R + SONL10; 4) I/R + SONL50; 5) I/R + 5HD; and 6) I/R + 5HD + SONL50. In the SOLN-receiving groups, sonlicromanol was intraperitoneally administered to rats at the dosages of 10 μM (SONL10) and 50 μM (SONL50), for seven days before the onset of occlusion [16]. The drug was prepared in 1% DMSO (Sigma- Aldrich, USA) and the non-treated I/R animals received the same volume of DMSO intraperitoneally. To inhibit mK-ATP channels, 5-Hydroxydecanoate (5HD) at a concentration of 5 μM was injected intraperitoneally five minutes before injection of SONL at 50 μM.
Neurological deficit score
Neurological deficit scoring was carried out after 30 minutes of the onset of occlusion, for confirmation of successful development of MCAO, and 24 hours after occlusion immediately before rats being sacrificed. Higher scores in this system indicate more severe cerebral injury. A 5-scale scoring system was employed to score neurological findings, including No symptoms of neurological deficit = 0, failure to extend left paw fully = 1, circling to left = 2, falling to left = 3, did not walk spontaneously, and has depressed levels of consciousness = 4 [15].
Measurement of infarct volume
Under anesthesia, the brain tissues from 6 rats in each group were immediately collected and samples were frozen at -20°C. The brain samples were cut into coronal slices with two millimeters of thickness and stained with 2% (w/v) 2,3,5-triphenyl tetrazolium chloride (TTC) (Sigma-Aldrich, USA) for 30 min at 37°C followed by overnight immersion in 4% (w/v) paraformaldehyde. The slices were photographed and analyzed by Image J software (National Institutes of Health, Bethesda, USA). White zones of each slice were representative of infarct areas. The infarct volumes of brains were measured as infarct area percentage and normalized to the brain volume of the animals [17].
Measurement of brain water content
The brain water content was determined based on previous work [17]. Briefly, all groups of rats were deeply anesthetized and their brains were quickly harvested after reperfusion, and the wet tissue was weighed, and then dried in an oven for 48 hours at 105°C to measure its dry weight. The calculation of brain water content was done according to the following formula: [(wet weight − dry weight) / wet weight] × 100%.
Measurement of mitochondrial activity indices
For isolation of the mitochondrial fraction, the samples taken from the peripheral penumbra of the ipsilateral cortex of all groups of rats were homogenized in the presence of an isolation buffer containing proteases inhibitor (Sigma-Aldrich, USA), and centrifuged at 10,000 rpm for 10 minutes. The resulting pellets were then suspended again in the isolation solution and centrifuged at 21,000 rpm to obtain mitochondrial fraction. Total proteins of the cortex were extracted and quantified using a BCA kit (Beyotime, Jiangsu, China). To measure mitochondrial ROS level, the supernatant was incubated at 37°C for 30 minutes in a 2 μmol DCFDA dye-containing phosphate buffer solution (Sigma-Aldrich, USA). Thereafter, using the excitation and emission absorbance was detected fluorometrically at 480 nm and 530 nm, respectively. The resultant values were adjusted based on the samples’ protein concentration. For deterring mitochondrial membrane potential depolarization, 100 μl mitochondrial supernatant was incubated in a 2 μl JC-1 dye-containing phosphate buffer solution (Sigma-Aldrich, USA) at 37°C for 30 minutes in at dark. Then the red and green fluorescence intensities of JC-1 in each sample were read fluorometrically and the red to green ratio was calculated to estimate the degree of mitochondrial membrane depolarization. Additionally, mitochondrial manganese superoxide dismutase (MnSOD) was measured from the supernatants using an ELISA kit, according to instructions of the kit (Sigma-Aldrich, USA). The relative absorbance was read at 450 nm spectrophotometrically.
Measurement of pro-inflammatory cytokines
After 24 hours of reperfusion, the samples of the ipsilateral cortex were homogenized in lysis buffer solution (Beyotime, Jiangsu, China), and then centrifuged at 10,000 rpm. After taking sample supernatants, the pro-inflammatory cytokines contents including TNF-α, IL-1β, and IL-6 were quantified using specific ELISA kits according to manufacturer instruction (MyBioSource, Inc., USA). The cytokines contents in each sample were adjusted by protein contents of the samples and the values were reported as mg of samples protein.
Western blotting for mitochondrial biogenesis proteins
After subjecting the samples to polyacrylamide gel electrophoresis, the proteins were transferred onto a PVDF membrane. Then, the skim milk-blocked membranes were incubated in primary antisera including rabbit polyclonal antibodies against PGC-1α, NRF-1, and TFAM (1:1000, Santa Cruz, USA), or β-actin (1:2000, Santa Cruz, USA). The specific antibody-antigen reactions were visualized by an enhanced-chemiluminescence detection system (Keygen Biotech, Nanjing, China). The bands' intensity of target proteins was calculated using Quantity One 1-D Analysis Software (Bio-Rad, USA), and normalized to β-actin intensities.
Statistical analysis
The data were reported as mean ± SD. One-way analysis of variance (one-way ANOVA) and Tukey post hoc test was employed to analyze the statistical differences between the groups. The minimum level of significance was considered at the alpha level of 0.05.
Result
The neuroprotective action of SONL
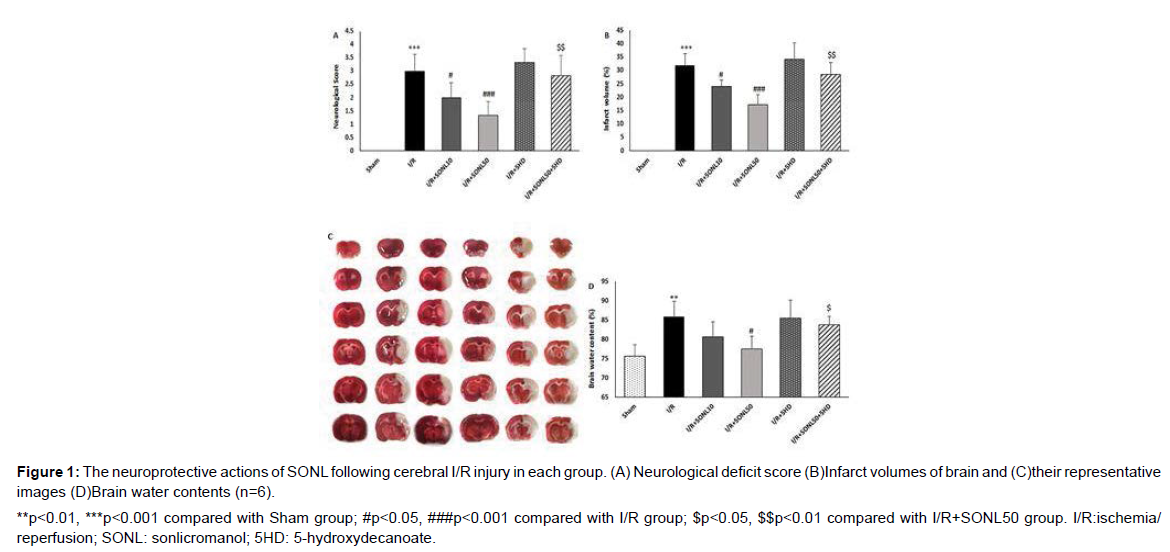
Neurological deficit was assessed using a 5-degree scoring system in rats with MCAO-induced cerebral I/R injury. A greater neurological deficit was observed in MCAO-receiving rats in comparison to the Sham group, indicating the successful induction of cerebral I/R injury. After 24 hours of reperfusion, SONL-treated rats showed lower degrees of neurological deficit in comparison to the I/R group, but the effect of the higher dose of SONL (p<0.001) was greater than that of the low dose (p<0.05) (Figure 1). In addition, inhibition of mK-ATP channels by 5HD significantly abolished the protective effect of the drug on neurological findings as compared with I/R rats receiving SONL 50 μM (p<0.01). To confirm SONL-neuroprotective effects, cerebral infarct volumes were quantified using the TTC staining method, and the results showed that administration of SONL at both doses significantly reduced I/R-induced cerebral infarct volumes in a dose-dependent manner (p<0.05 and p<0.001) (Figure 2A). Also, SONL at 50 μM significantly decreased the brain water content as compared with the I/R group (p<0.05) (Figure 2C). Blocking of mK-ATP channels significantly abolished the SONL effects on infarct volume (p<0.01) and brain water content (p<0.05), compared to the I/R+SONL50 group.
**p<0.01, ***p<0.001 compared with Sham group; #p<0.05, ###p<0.001 compared with I/R group; $p<0.05, $$p<0.01 compared with I/R+SONL50 group. I/R:ischemia/ reperfusion; SONL: sonlicromanol; 5HD: 5-hydroxydecanoate.
Figure 1: The neuroprotective actions of SONL following cerebral I/R injury in each group. (A) Neurological deficit score (B)Infarct volumes of brain and (C)their representative images (D)Brain water contents (n=6).
***p<0.001 compared with Sham group; #p<0.05, ###p<0.001 compared with I/R group; $$p<0.01 compared with I/R+SONL50 group. I/R:ischemia/reperfusion; SONL: sonlicromanol; 5HD: 5-hydroxydecanoate.
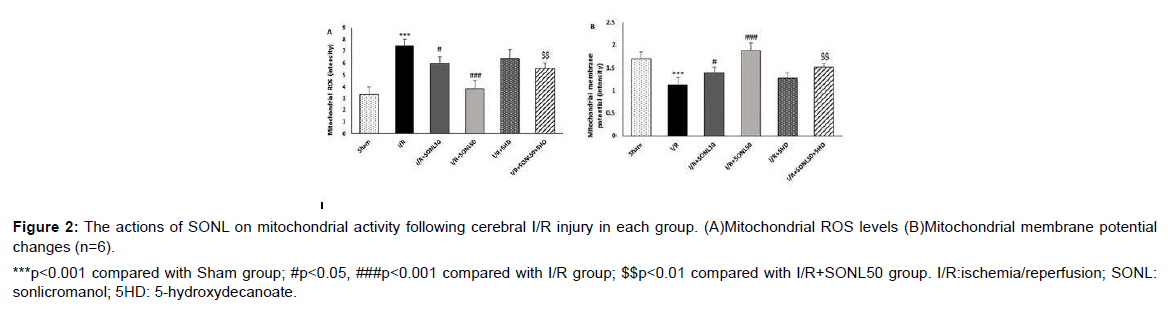
Figure 2: The actions of SONL on mitochondrial activity following cerebral I/R injury in each group. (A)Mitochondrial ROS levels (B)Mitochondrial membrane potential changes (n=6).
Effect of SONL on the mitochondrial activity
Mitochondrial activity was estimated through the assessment of mitochondrial ROS levels and mitochondrial membrane potential changes. Cerebral I/R injury significantly augmented the levels of mitochondrial ROS and depolarization of mitochondrial membrane potential (reduced potential) as compared with the Sham group (p<0.001) (Figure 2A and 2B). Nevertheless, SONL administration in rats prior to I/R injury significantly inverted I/R-induced changes in mitochondrial parameters, dose-dependently (p<0.05 and p<0.001). Furthermore, concomitant administration of 5HD significantly abolished the beneficial effects of SONL on the I/R-induced changes of mitochondrial ROS and membrane potential (p<0.01) (Figure 2).
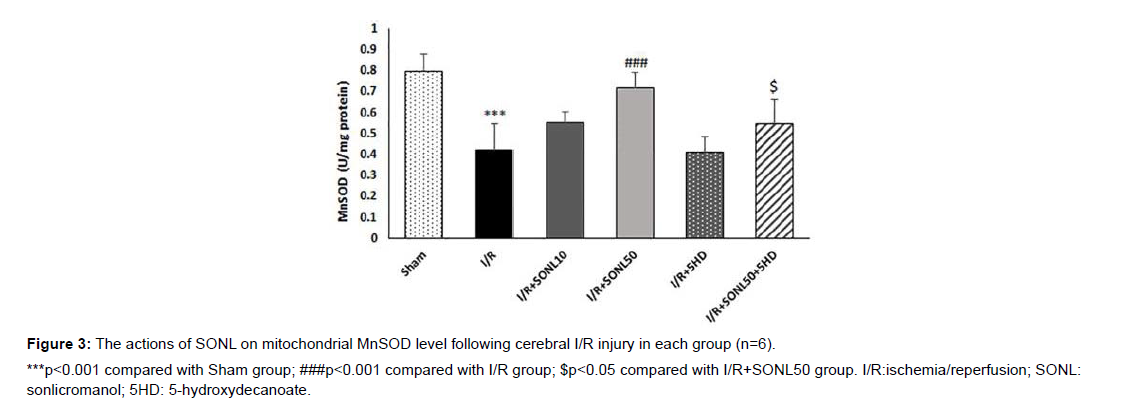
Effect of SONL on the MnSOD levels
The cerebral levels of MnSOD, as an endogenous potent antioxidative enzyme, in the I/R group was significantly lower than that of the Sham group (p<0.001) (Figure 3). SONL at 50 μM but not 10 μM significantly elevated I/R-induced reduction of MnSOD (p<0.001). However, administration of 5HD to inhibit mK-ATP channels significantly hindered the effect of the drug on the MnSOD level, as compared with the IR+SONL50 group (p<0.05).
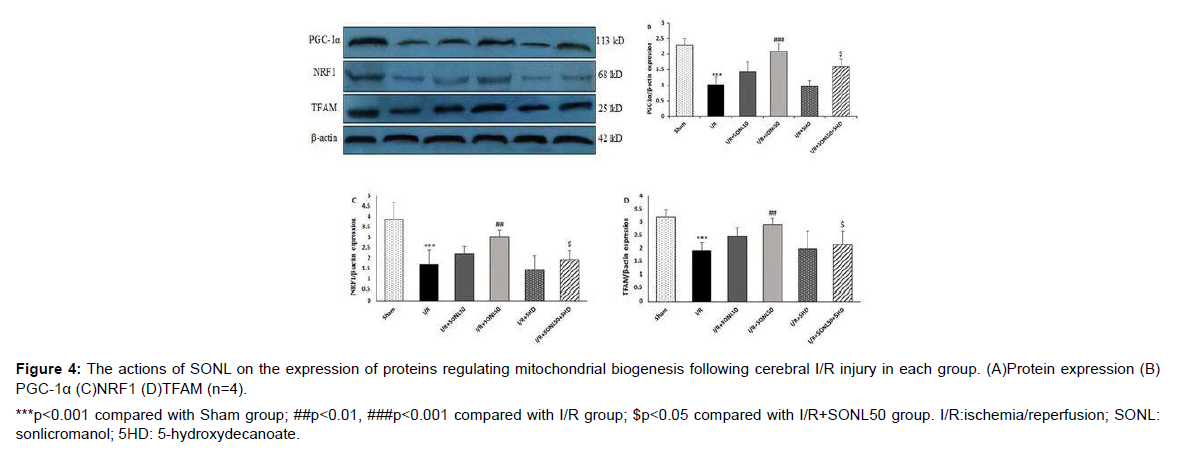
Effect of SONL on the mitochondrial biogenesis proteins
Following in vivo cerebral I/R injury in rats, the protein expression of proteins regulating mitochondrial biogenesis (PGC-1α, NRF1, and TFAM) decreased significantly (p<0.001). Administration of SONL at 10 μM was not capable to reduce I/R-induced reduction of proteins expression. However, the expression levels of PGC-1α (p<0.001), NRF1, and TFAM (p<0.01) were significantly upregulated after administration of SONL at 50 μM, compared to the I/R group. Blocking of mK-ATP channels by 5HD significantly prevented the effects of SONL at 50 μM on the expression of these proteins as compared with the I/R+SONL50 group (p<0.05) (Figure 4).
***p<0.001 compared with Sham group; ##p<0.01, ###p<0.001 compared with I/R group; $p<0.05 compared with I/R+SONL50 group. I/R:ischemia/reperfusion; SONL: sonlicromanol; 5HD: 5-hydroxydecanoate.
Figure 4: The actions of SONL on the expression of proteins regulating mitochondrial biogenesis following cerebral I/R injury in each group. (A)Protein expression (B) PGC-1α (C)NRF1 (D)TFAM (n=4).
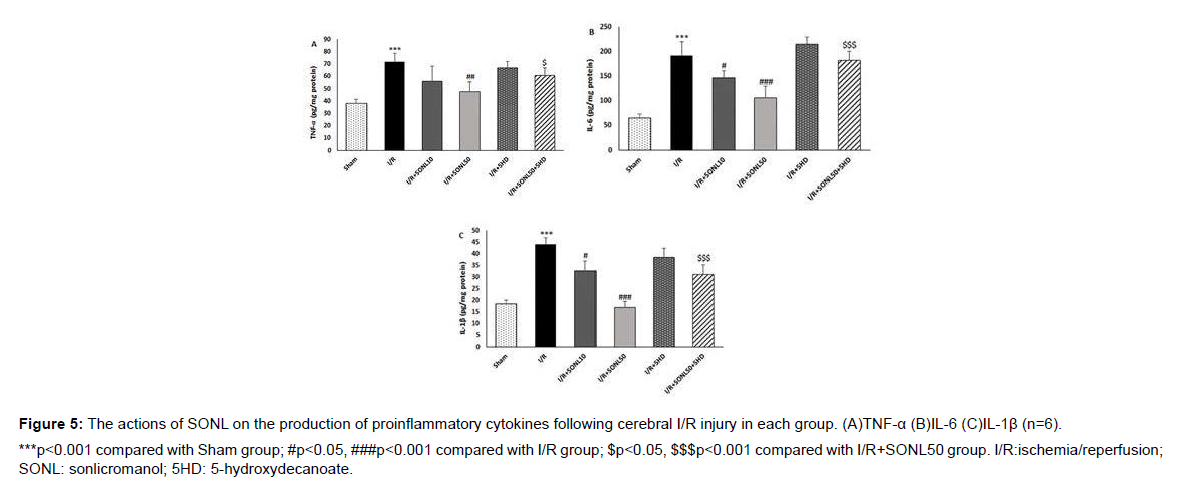
Effect of SONL on the proinflammatory cytokines
As shown in Figure 5, the production of proinflammatory cytokines TNF-α, IL-6, and IL-1β was significantly amplified after induction of cerebral I/R injury in rats compared with that in the sham group (P < 0.001). SONL at 10 μM did not affect the level of TNF-α, but it significantly reduced the production of IL-6 and IL-1β, in comparison to the I/R group (p<0.05). Compared with the I/R group, a significant downregulation of the proinflammatory cytokines was observed in brain tissues of the rats after administration of SONL at 50 μM group (p<0.01 and p<0.001). Furthermore, blocking of mK-ATP channels remarkably eliminated the antiinflammatory actions of SONL 50 μM in the cerebral I/R setting (p<0.05 and p<0.001).
***p<0.001 compared with Sham group; #p<0.05, ###p<0.001 compared with I/R group; $p<0.05, $$$p<0.001 compared with I/R+SONL50 group. I/R:ischemia/reperfusion; SONL: sonlicromanol; 5HD: 5-hydroxydecanoate.
Figure 5: The actions of SONL on the production of proinflammatory cytokines following cerebral I/R injury in each group. (A)TNF-α (B)IL-6 (C)IL-1β (n=6).
Discussion
Post-conditioning of rat brains with SONL significantly diminished the I/RI-induced cerebral infarcted volumes and neurological deficits in a dose-dependent manner. SONL at 50 μm markedly improved mitochondrial function and upregulated the expression of PGC-α, NRF1, and TFAM biogenesis proteins. It also reduced the production of cerebral inflammatory cytokines and inhibited mitochondrial oxidative stress. Blockade of mK-ATP channels by 5HD significantly suppressed the neuroprotective, anti-inflammatory, and mitochondrial boosting effects of SONL. Our findings provided evidence that strengthening mitochondrial activity in cerebral cells by SONL may be a promising strategy to prevent cerebral I/RI.
Owing to extensive function, mitochondria in cerebral tissues have a special role in protecting them against fatal I/RI outcomes [4]. Under normal activity of mitochondria, cellular oxygen is used mostly for mitochondrial reduction-oxidation processes, reducing the likelihood of high mitochondrial ROS production and increasing the expression of endogenous antioxidative enzymes like MnSOD [18]. Otherwise, mitochondria become dysfunctional with the sudden onset of reperfusion, and now they act as the main producer of ROS and free radicals in the cells [4]. Following amplification of mitochondrial oxidative stress and disruption of mitochondrial function, the production of pro-inflammatory cytokines is also accelerated [19]. The result of this situation is the exacerbation of brain I/R damages. In the present study, administration of SONL limited the production of pro-inflammatory TNF-α and other interleukins and suppressed mitochondrial oxidative stress (as assessed by the changes of mitochondrial ROS and MnSOD). These effects of the drug as well as its inhibitory effect against mitochondrial membrane depolarization indicate that this drug is able to modify mitochondrial internal homeostasis to a high degree in I/R conditions. Similarly, it has been reported that SONL has beneficial impacts in patients with mitochondrial diseases including mitochondrial encephalomyopathy, neuropathy, and Leigh syndrome [11,20,21]. It also interacts with peroxiredoxins, increasing their peroxidase activity and reducing ROS-induced cell death [22]. The active metabolite of this drug also reduced I/R-induced cardiac damages and prevented inflammatory responses by inhibiting the production of PGE2 [16]. All of these findings, together with our results, suggest that it suggested that the mitochondrial-directed pathway is an important target for this drug and it is a good candidate for reducing cerebral I/RI.
Mitochondrial dysfunction and subsequent inflammatory and oxidative responses are associated with alterations in mitochondrial biogenesis [19]. Accumulating evidence shows that promoting mitochondrial biogenesis contributes to neuroprotection [6,11]. In the present study, we found that SONL was capable of inducing mitochondrial biogenesis, thereby providing adequate neuroprotection against cerebral I/RI. Here, expressions of TFAM, NRF1, and PGC-1α were increased following SONL treatment in the I/R brain. PGC-1α is the initiating factor of mitochondrial biogenesis that induces the expression of TFAM in collaboration with NRF1, as its transcriptional partner [5,6,23]. The interplay between NRF1 and TFAM induces the transcription of mtDNA and stimulates mitochondrial oxidative/ phosphorylation [24]. Although these findings imply that SONL neuroprotective effects may be achieved through stimulating the entire mitochondrial biogenesis program, the correlation between SONL-induced neuroprotection and PGC-1α-activated mitochondrial recovery requires further study.
To further explore the role of SONL in controlling mitochondrial activity and biogenesis as well as inflammatory responses under cerebral I/R condition, mK-ATP channels were blocked using 5HD to examine the involvement of these channels in the neuroprotective effect of the drug. Blocking of mK-ATP channels not only abolished the neuroprotective effects of SONL but also reversed its anti-inflammatory and mitochondrial actions. These findings highlight that mK-ATP channels opening at the beginning of reperfusion mediates the beneficial effects of SONL in cerebral I/RI. In agreement with our results, it has been documented that mK-ATP channels are critically involved in the regulation of PGC-1α to influence mitochondrial homeostasis [7,8]. The density of these channels in the brain is greater than other organs [7] and this strongly emphasizes the importance of these mitochondrial channels in SONL actions on mitochondrial biogenesis and homeostasis (preventing mitochondrial swelling and membrane potential collapse) as well as anti-inflammation. These channels are considered as one of the main arms of mitochondria in their role as the end effector of neuroprotection [25] and thereby this main cellular component mediates the protective influences of SONL in reducing cerebral I/R outcomes. Therefore, the neuroprotective effect of SONL in cerebral I/RI was achieved through modulation of the mK-ATP/ mitochondrial biogenesis/inflammatory pathway. However, the contribution of other important mediators including PKG/cGMP and PI3K/AKT pathways in this effect requires further experimentation.
Conclusion
SONL, as a new mitochondrial-acting drug, displayed strong neuroprotection in cerebral I/RI through anti-inflammatory, anti-oxidative, and mitochondrial activity improving effects. Increased activity of mK-ATP channels markedly mediated the beneficial actions of SONL. This study highlighted that this drug can be one of the most promising pharmacological approaches to target mitochondrial dysfunction in I/R conditions.
Conflicts of Interest
The authors have no conflict of interest to declare.
Authors' contributions
All authors designed the project, performed the experimentations, analyzed, and interpreted the data. XX was the major contributor in writing the manuscript. All authors read and approved the final manuscript.
References
- Kuriakose D, Xiao Z (2020) Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int J Mol Sci 21:7609.
- Ansari J, Gavins FNE (2019) Ischemia-Reperfusion Injury in Sickle Cell Disease: From Basics to Therapeutics. Am J Pathol. 189:706-718.
- Liu F, Lu J, Manaenko A, Tang J, Hu Q (2018) Mitochondria in Ischemic Stroke: New Insight and Implications. Aging Dis 9:924-937.
- Carinci M, Vezzani B, Patergnani S, Ludewig P, Lessmann K, et al. (2021) Different Roles of Mitochondria in Cell Death and Inflammation: Focusing on Mitochondrial Quality Control in Ischemic Stroke and Reperfusion. Biomedicines 9:169.
- Xie Y, Li J, Fan G, Qi S, Li B (2014) Reperfusion promotes mitochondrial biogenesis following focal cerebral ischemia in rats. PLoS One 9:e92443.
- Gureev AP, Shaforostova EA, Popov VN (2019) Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction between the Nrf2 and PGC-1α Signaling Pathways. Front Genet 10:435.
- Peng K, Hu J, Xiao J, Dan G, Yang L, et al. (2018) Mitochondrial ATP-sensitive potassium channel regulates mitochondrial dynamics to participate in neurodegeneration of Parkinson's disease. Biochim Biophys Acta Mol Basis Dis 1864:1086-1103.
- Nikbakht F, Khanizadeh AM, Golab F, Baluchnejadmojarad T, Vazifehkhah S, et al. (2021) Mitochondrial ATP-sensitive potassium channel, MitoKATP, ameliorates mitochondrial dynamic disturbance induced by temporal lobe epilepsy. J Chem Neuroanat 113:101808.
- Vishwakarma VK, Upadhyay PK, Chaurasiya HS, Srivasatav RK, Ansari TM, et al. (2019) Mechanistic Pathways of ATP Sensitive Potassium Channels Referring to Cardio-Protective Effects and Cellular Functions. Drug Res (Stuttg) 69:365-373.
- Szeto V, Chen N, Sun H (2018) The role of KATP channels in cerebral ischemic stroke and diabetes. Acta Pharmacol Sin 39:683-694.
- Bottani E, Lamperti C, Prigione A, Tiranti V, Persico N, et al. (2020) Therapeutic Approaches to Treat Mitochondrial Diseases: "One-Size-Fits-All" and "Precision Medicine" Strategies. Pharmaceutics 12:1083.
- Gunnewiek TMK, Verboven AHA, Pelgrim I, Hogeweg M, Schoenmaker C, et al. (2021) Sonlicromanol improves neuronal network dysfunction and transcriptome changes linked to m.3243A>G heteroplasmy in iPSC-derived neurons. Stem Cell Reports 16:2197-2212.
- Koene S, Spaans E, Van Bortel L, Van Lancker G, Delafontaine B, et al. (2017) KH176 under development for rare mitochondrial disease: a first in man randomized controlled clinical trial in healthy male volunteers. Orphanet J Rare Dis 12:163.
- Jiang X, Renkema H, Pennings B, Pecheritsyna S, Schoeman JC, et al. (2021) Mechanism of action and potential applications of selective inhibition of microsomal prostaglandin E synthase-1-mediated PGE2 biosynthesis by sonlicromanol's metabolite KH176m. Sci Rep 11:880.
- Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
- Xiao Y, Yim K, Zhang H, Bakker D, Nederlof R, et al. (2021) The Redox Modulating Sonlicromanol Active Metabolite KH176m and the Antioxidant MPG Protect Against Short-Duration Cardiac Ischemia-Reperfusion Injury. Cardiovasc Drugs Ther 35:745-758.
- Cheng X, Zhang F, Li J, Wang G (2019) Galuteolin attenuates cerebral ischemia/reperfusion injury in rats via anti-apoptotic, anti-oxidant, and anti-inflammatory mechanisms. Neuropsychiatr Dis Treat 15:2671-2680.
- Shields HJ, Traa A, Van Raamsdonk JM (2021) Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front Cell Dev Biol 9:628157.
- Patergnani S, Bouhamida E, Leo S, Pinton P, Rimessi A (2021) Mitochondrial Oxidative Stress and "Mito-Inflammation": Actors in the Diseases. Biomedicines 9:216.
- de Haas R, Das D, Garanto A, Renkema HG, Greupink R, et al. (2017) Therapeutic effects of the mitochondrial ROS-redox modulator KH176 in a mammalian model of Leigh Disease. Sci Rep 7:11733.
- Frambach SJCM, van de Wal MAE, van den Broek PHH, Smeitink JAM, Russel FGM, et al. (2020) Effects of clofibrate and KH176 on life span and motor function in mitochondrial complex I-deficient mice. Biochim Biophys Acta Mol Basis Dis 1866:165727.
- Beyrath J, Pellegrini M, Renkema H, Houben L, Pecheritsyna S, et al. (2018) KH176 Safeguards Mitochondrial Diseased Cells from Redox Stress-Induced Cell Death by Interacting with the Thioredoxin System/Peroxiredoxin Enzyme Machinery. Sci Rep 8:6577.
- Liu L, Zhang W, Wang L, Li Y, Tan B, et al. (2014) Curcumin prevents cerebral ischemia reperfusion injury via increase of mitochondrial biogenesis. Neurochem Res 39:1322-1331.
- Kang I, Chu CT, Kaufman BA (2018) The mitochondrial transcription factor TFAM in neurodegeneration: emerging evidence and mechanisms. FEBS Lett 592:793-811.
- Wrzosek A, Augustynek B, Żochowska M, Szewczyk A (2020) Mitochondrial Potassium Channels as Druggable Targets. Biomolecules 10:1200.
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Google Scholar Cross Ref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Google Scholar Cross Ref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Citation: Wu W, Wang Y (2022) Neuroprotective and Anti-Inflammatory Effects of Sonlicraomanol in Rat Cerebral Ischemia/Reperfusion Injury Is Achieved Through Improving Mitochondrial Function. Cell Mol Biol, 68: 222. DOI: 10.4172/1165-158X.1000222
Copyright: © 2021 Wu W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3648
- [From(publication date): 0-2022 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 3104
- PDF downloads: 544