Research Article Open Access
Neuroprotective Activity of Asparagus racemosus Linn. Against Ethanol- Induced Cognitive Impairment and Oxidative Stress in Rats Brain: Auspicious for Controlling the Risk of Alzheimer's Disease
Md. Sahab Uddin1†*, Md. Asaduzzaman1†, Abdullah Al Mamun1, Mohammed Ashraful Iqbal2, Ferdous Wahid3and Ram Kamol Rony4
1Department of Pharmacy, Southeast University, Dhaka, Bangladesh
2Department of Chemistry, Fareast International University, Dhaka, Bangladesh
3Department of Pharmacy, University of Development Alternative, Dhaka, Bangladesh
4Department of Pharmacology and Clinical Pharmacy, North South University, Dhaka, Bangladesh
- *Corresponding Author:
- Md. Sahab Uddin
Department of Pharmacy, Southeast University
Dhaka, Bangladesh
Tel: +8801670760546
E-mail: msu-neuropharma@hotmail.com, msu_neuropharma@hotmail.com
Received date February 17, 2016; Accepted date June 27, 2016; Published date July 04, 2016
Citation: Uddin MS, Asaduzzaman M, Mamun AA, Iqbal MA, Wahid F, et al. (2016) Neuroprotective Activity of Asparagus racemosus Linn. Against Ethanol-Induced Cognitive Impairment and Oxidative Stress in Rats Brain: Auspicious for Controlling the Risk of Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 6:245. doi:10.4172/2161-0460.1000245
Copyright: ©2016 Uddin MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Background: Medicinal plants are superior gift of nature to human lives to support disease free healthy life. Neurodegenerative diseases especially Alzheimer’s disease (AD) affects the central nervous system causing progressive degeneration of neurons, which affect cognitive function. The plant Asparagus racemosus (AR) Linn. has been used traditionally by Ayurvedic practitioners for nervous disorders. In this consequence, the intention of this study was to evaluate the neuroprotective effects of ethanolic extract of Asparagus racemosus (EEAR) Linn. roots in ethanol-induced cognitive impairment and oxidative stress in rats brain. Methods: The learning and memory enhancing activity of EEAR roots extract were investigated in Swiss albino male rats for 21 days and its effects on learning and memory were examined using various behavioral studies such as elevated plus maze (EPM) test, passive avoidance (PA) test, morris water maze (MWM) test, novel object recognition (NOR) test and biochemical studies such as lipid peroxidation (TBARS) contents and acetylcholinesterase (AChE) activity. Results: In the EPM test, administration of EEAR (i.e., 100 and 200 mg/kg b.w.) significantly (P<0.05, P<0.01) decreased retention transfer latency (RTL) on 21st day with respect to the disease control group. EEAR at 200 mg/kg b.w. markedly (P<0.05, P<0.01) increased the retention latency (RL) on 11th and 21st day compared to disease control group for PA test. In the NOR test administration of EEAR (i.e., 200 mg/kg b.w.) considerably (P<0.05) increased DI of rats on 21st day with respect to disease control group. Both doses of EEAR (i.e., 100 and 200 mg/kg b.w.) markedly (P<0.05, P<0.01) decreased escape latency (EL) and highest dose of EEAR (i.e., 200 mg/kg b.w.) noticeably (P<0.05, P<0.001) increased time spent in the target quadrant (TSTQ) on successive days for acquisition trial of MWM test. In case of probe trial administration of EEAR (i.e., 200 mg/kg b.w.) considerably (P<0.05, P<0.01) increased TSTQ and TSA (time spent in the annuli) of rats as compared to that of disease control group. EEAR administration (i.e., 100 and 200 mg/kg b.w.) significantly (P<0.01) decreased the TBARS level in the brain tissue of rats with respect to disease control group. The lowest and highest dose of EEAR (i.e., 100 and 200 mg/kg b.w.) significantly (P<0.05, P<0.01) decreases the AChE activity in the brain tissue of rats as compared to disease control group. Conclusion: The existing study displays that EEAR roots possesses an outstanding source for natural nootropic and confirming the traditional uses of this plant which could be industrialized for enhancing learning and memory impairment associated with neurodegenerative disorders particularly AD.
Keywords
Neuroprotective; Asparagus racemosus; Cognitive impairment; Oxidative stress; Alzheimer’s disease
Abbreviations
AD: Alzheimer’s disease; AR: Asparagus racemosus; EEAR: Ethanolic extract of Asparagus racemosus; EPM: Elevated plus maze; PA: Passive avoidance; MWM: Morris water maze; NOR: Novel object recognition;TBARS: Th iobarbituric acid reactive species (TBARS); AChE: Acetylcholinesterase; ITL: Initial transfer latency; RTL: Retention transfer latency; EL: Escape latency; RL: Retention latency; TSTQ: Time spent in the target quadrant; TSA: Time spent in the annuli; DI: Discrimination index; PUFA: Polyunsaturated fatty acids; CNS: Central nervous system; Aβ: amyloid beta; WHO: World Health Organization; TCA: Trichloroacetic acid; TBA: Thiobarbituric acid; ATCI: Acetyl thiocholine iodide; DTNB: 5,5-dithiobis-2- nitrobenzoate ion; Tris-HCl: Trisfamino methane hydrochloride; BSA: Bovine serum albumin; NIH: National Institutes of Health; OECD: Organisation for Economic Cooperation and Development .
Introduction
Alzheimer’s disease (AD) is the most common form of dementia that results in memory impairment and cognitive dysfunction due to progressive neurodegeneration [1]. Worldwide at present 35 million people are affected by AD, including 5.5 million Americans and it is projected that in 2050 more than 115 million people will have dementia [2,3]. AD is currently ranked as the 6th leading cause of death in the United States, but recent estimates indicate that the disorder may rank 3rd , just behind heart disease and cancer, as a cause of death for older people [4]. This disease is characterized by deposition of amyloid β (Aβ) plaques, development of neurofibrillary tangles (NFTs), inflammation and neuronal loss in specific regions of the forebrain that slowly destroys memory and thinking skills and eventually the ability to carry out the simplest tasks. The most notable symptom of AD is progressive neurodegenerative disease in which dementia gradually worsen over time followed by an overall cognitive decline [5]. Alzheimer’s is not a normal part of aging, although the greatest known risk factor is increasing age and the majority of people with Alzheimer’s are 65 years and older [6]. But Alzheimer’s is not just a disease of old age. Up to 5% of people with the disease have early onset Alzheimer’s also known as younger-onset, which often appears when someone is in their 40s or 50s [7].
Ethanol is neurotoxic that able to alter behavioral and cognitive performance in experimental animals in addition to humans. It mainly impairs hippocampal-dependent learning and memory functions [8]. The mechanism of ethanol-induced neurotoxicity is not well understood. Several studies show that free-radical mediated oxidative stress play an imperative role [9]. The brain is extremely susceptible to oxidative stress due to high level of polyunsaturated fatty acids (PUFAs) and catecholamines, large amounts of oxygen (O2) in relatively small mass and in conjunction with low antioxidant activities. Furthermore, certain regions of the central nervous system (CNS), especially hippocampus and cerebellum, may be more sensitive to oxidative stress because of their low endogenous antioxidant, in relation to other brain regions [10]. Study showed that acetaldehyde dehydrogenase is responsible for the generation of reactive oxygen species (ROS) by converting cytotoxic acetaldehyde produced from oxidation of ethanol to acetate. It has been confirmed that ethanol induces the synthesis of CYP2E1 that lead to oxidative stress. It also increases the ratio of NADH/NAD, responsible for reduction of ferric ion (Fe3+) to ferrous ion (Fe2+)) which causes lipid peroxidation by generating hydroxyl radical [11].
Increasing evidence highlights the role played by oxidative stress in AD. It has been shown that free radical and oxidative stress induce memory deficits and enhance behavioral impairments in AD patients [12]. Several studies have suggested that Aβ induced the oxidative stress observed in neurodegenerative AD brain [13]. Alzheimer’s has no current cure. Although current Alzheimer’s treatments cannot stop Alzheimer’s from progressing, they can temporarily slow the worsening of dementia symptoms and improve quality of life for patients with Alzheimer’s [14]. Currently, the treatment for AD is acetylcholinesterase (AChE) inhibitors these are donepezil, galantamine, rivastigmine and tacrine. However, the resulting adverse effects associated with nootropic agents, such as piracetam, pramiracetam, aniracetam and donepezil have restricted their use for improving memory, mood and behavior [15]. The connection of oxidative stress within the forebrain cholinergic system has been recommended by several studies [16]. The drugs with antioxidant effects might be beneficial for preserving brain function. Today, there is a worldwide effort under way to find better ways to treat the disease, delay its onset and prevent it from developing [17].
Nature is the best combinatorial chemist and possibly has used to treat almost any medical problem of mankind. It is estimated that about 75% of useful bioactive plant derive pharmaceuticals used globally are discovered by systemic investigation of leads from traditional medicines [18]. The World Health Organization (WHO) estimates that 80% of the population of some Asian and African countries presently uses herbal medicine for some aspect of primary health care [19]. Complementary and alternative medicine, especially Ayurvedic, Unani and Siddha provide health care facility of more than 70% of people exist in the rural areas [20]. Studies in the United States and Europe have shown that their use is less common in clinical settings, but has become increasingly more common in recent years as scientific evidence about the effectiveness of herbal medicine has become more widely available [21]. Herbal medicine treatment for AD becomes very popular nowadays because of their activities against AD and slowing down the progression of it. Many herbal medicines have been researched and the benefits derived from using these medicines for AD and dementias are promising. Also, these herbs are inexpensive and can be easily available. The results observed for the treatment with herbal medicines are promising also with fewer adverse effects [22]. A variety of medicinal plants such as Ginkgo biloba, Panax ginseng, Withania somnifera, Huperzia serrata, Rhodiola rosea, Vinca minor, Bacopa monnieri, Centella asiatica, Eruca sativa, Marsilea quadrifolia, Terminalia chebula, Aloe vera, Alpinia galanga, Andrographis paniculata, Arnica montana and Bupleurum falcatum have been reported to exhibit anti-Alzheimer activity [23].
The plant Asparagus racemosus (AR) Linn. is known in Bengali as Shatamull belongs to Asparagaceae family [23]. This plant is common throughout Nepal, Sri Lanka, India and also widely distributed throughout the villages of the Bangladesh [24]. The plant is a spinous under-shrub, with tuberous, short rootstock bearing numerous succulent tuberous roots (30-100 cm long and 1-2 cm thick) that are silvery white or ash colored externally and white internally [25]. It has folk usage as a treatment for dyspepsia, gastric ulcers, constipating, galactogogue, aphrodisiac, diuretic, rejuvenating, carminative, immunostimulant, gastroprotective, nerve tonic and antiseptic effects [26]. The plant has phytochemical constituents such as alkaloids, triterpenes, saponins, glycosides, diosgenin, cytoesterol, stigmaesterol, isoflavonoids etc [27]. The important pharmacological actions of this plant are adaptogenic, hypolipidemic, immunomodulatory, antibacterial, antidepressant, antiulcerant, antidiabetic, antioxidant, cardio protective and memory enhancing activity [28].
A previous preliminary study has shown that the roots extract of this plant has memory enhancing activity in mice [29]. Therefore, the purpose of this study was to investigate the neuroprotective effect of ethanolic extract of Asparagus racemosus (EEAR) Linn. roots in ethanol-induced rats of cognitive impairment and oxidative stress by behavioral studies such as elevated plus maze (EPM) test, passive avoidance (PA) test, novel object recognition (NOR) test, morris water maze (MWM) test as well as the activity of antioxidant enzymes by biochemical studies such as estimation of lipid peroxidation (TBARS) and acetylcholinesterase (AChE) activity in rat brain tissue homogenates.
Materials and Methods
Chemicals and Drugs
Ethanol (98%); trichloroacetic acid (TCA); thiobarbituric acid (TBA); acetyl thiocholine iodide (ATCI); 5,5-dithiobis-2-nitrobenzoate ion (DTNB); trisfamino methane hydrochloride (Tris-HCl) and bovine serum albumin (BSA) were purchased from Sigma-Aldrich, USA. Unless otherwise stated, all other chemicals were of analytical grade and purchased from native sources. Donepezil hydrochloride powder was achieved from Incepta Pharmaceuticals Ltd. Dhaka, Bangladesh as gift.
Collection and Identification of Plant Materials
The fresh roots of AR were collected from Kurigram Sadar, Kurigram, Bangladesh, in the month of February, 2015. After collection the roots were identified by expert of Bangladesh National Herbarium, Mirpur, Dhaka, Bangladesh. Accession number: DACB-39527 for AR.
Drying and Grinding of Plant Materials
To remove adhering dirty materials from the collected roots of the plant these were washed properly. Then these were shade dried for 7 days with irregular sun drying. For facilitating grinding after that root were dried in an oven for 24 hrs at 50°C. The roots were crushed into the coarse powder with the help of a suitable grinder and stored in an airtight clean glass container for extraction.
Extraction of Plant Materials
For extraction about 500 g powdered plant materials were taken in an amber colored glass bottle and subjected to extraction by 2 liter of 98% ethanol for 7 days with occasional shaking. After that, the whole mixture was filtered through cotton and finally by Whatman No.1 filter paper. The collected filtrate after filtration was concentrated with a rotary evaporator under reduced pressure at 50°C temperature. The sticky concentrate was designated as crude extract (8.65 g) of ethanol and stored at 4ºC for further tests.
Experimental Animals
Fifty six healthy Swiss albino male rats weighing between 170-220 g were obtained from the animal resources outlet of ICDDR,B, Dhaka, Bangladesh. The obtained rats were housed 3 per cage and placed under standard environmental condition with a 12:12 hrs light-dark cycle. The rats were timely fed with standard laboratory food and water. As per the guide for laboratory animals of the National Institutes of Health (NIH) the care and usage of entire rats were completed [30]. The protocol of the study was approved by the animal ethics committee of the Department of Pharmacy, Southeast University, Dhaka, Bangladesh.
Administration of Drugs and Test Compounds
The solution of the standard drug donepezil hydrochloride was prepared by using normal saline (pH 7.4) and ingested orally to rats at 2.5 mg/kg body weight (b.w.). Ethanol was prepared as a 20% solution in sterile normal saline and treated subcutaneously at a dose of 4.5 g/ kg b.w. Normal saline (pH 7.4) was used to prepare the suspension of the EEAR and administered orally at 100 and 200 mg/kg b.w. to rats. According to the result of the literature searches the duration of the study and the doses of the donepezil hydrochloride, ethanol and EEAR were adjusted [31,32,33]. The standard drug, ethanol and the suspension of the extract were prepared newly everyday and ingested 30 min before the experiment.
Experimental Design
Rats were divided randomly into eight groups with 6 rats in each as follows:
Group 1: In case of this group standard laboratory food and water were administered for 21 days to rats (Con)
Group 2: In case of this group donepezil hydrochloride at a dose of 2.5 mg/kg b.w. was administered orally for 21 days to rats (Don)
Group 3: In case of this group ethanol at a dose of 4.5 g/kg b.w. was administered subcutaneously for 21 days to rats (Eth)
Group 4: In case of this group plant extract at a dose of 100 mg/kg b.w. was administered orally for 21 days to rats (EEAR 100)
Group 5: In case of this group plant extract at a dose of 200 mg/kg bw was administered orally for 21 days to rats (EEAR 200)
Group 6: In case of this group ethanol at a dose of 4.5 g/kg b.w and plant extract at a dose of 100 mg/kg b.w. were administered subcutaneously and orally respectively for 21 days to rats (Eth + EEAR 100)
Group 7: In case of this group ethanol at a dose of 4.5 g/kg b.w. and plant extract at a dose of 200 mg/kg b.w. were administered subcutaneously and orally respectively for 21 days to rats (Eth + EEAR 200)
Group 8: In case of this group only ethanol at a dose of 4.5 g/kg b.w. and donepezil hydrochloride at a dose of 2.5 mg/kg b.w. were administered subcutaneously and orally respectively for 21 days to rats (Eth+ Don)
Acute Toxicity Study
The acute toxicity of EEAR roots was determined by using 24 Swiss albino male rats were divided into 4 groups, with 6 rats per groups. The suspension of the extracts were prepared by using normal saline and administered to rats only once at a dose of 5, 50, 300 and 2000 mg/ kg b.w. The rats were observed for 24 hrs for signs of toxicity and 14 days for mortality. This test was performed as per the guidelines of the Organisation for Economic Cooperation and Development (OECD) [34].
Behavioral Study
Before starting the behavioral studies 1 week training was conducted. Only food and water was administered during this period. All the experiments were carried out between 10.00 am and 3.00 pm.
Elevated Plus Maze (EPM) Test
The apparatus of EPM test was consisted of two open arms (length 500 mm × width 100 mm) and two close arms (length 500 mm × width 100 mm × height of side walls 400 mm), with an open roof used for the measurement of spatial long-term memory of rats [35]. The arms were connected by a central square in the middle of the maze. The maze was elevated to a height of 500 mm from the floor [36]. At the start of the trial each rat was placed at the end of an open arm and the time it took to move from the end of open arm to either of the closed arms was recorded using a stopwatch as initial transfer latency (ITL). If the rat did not enter into one of the closed arm within 300 sec, was eliminated from the experiments. After that rat was allowed to explore the apparatus for 30 sec and returned to its home cage. Retention transfer latency (RTL) of this learned task (memory) was examined 24 hrs after the first day trial [37]. The apparatus was cleaned after each test with 70% ethanol to remove any olfactory clue [38].
Passive Avoidance (PA) Test
The apparatus of PA test was consisted of light compartment (depth 270 mm × width 370 mm × height 360 mm) and dark compartment (depth 270 mm × width 370 mm × height 360 mm) used for the measurement of emotional memory of rats [39,40]. The two compartments of this apparatus were connected by a sliding door having 90 mm of diameter in the middle part. The floor of this apparatus was consisted of a metal grid with spaced 0.9 cm apart and connected to a shock generator, able to generate shock in the range of 0.5 mA [40,41]. Each test consists of two distinct trials such as acquisition trial and retention trial. For the acquisition trial, each rat was placed in the light compartment and when the rat entered into the dark compartment, an electrical foot shock of 0.5 mA was administered for 3 sec. By the help of stopwatch, the latency times once the rat had entered the dark compartment was recorded as escape latency (EL). After that the rat was returned to its home cage. A retention trial was performed after 24 hrs of the acquisition trial, in which no shock was given when the rat entered the dark compartment and latency times to re-enter the dark chamber was measured as retention latency (RL) up to 300 sec [42]. The apparatus was cleaned after each test with 70% ethanol to remove any olfactory clue [38].
Novel Object Recognition (NOR) Test
The apparatus of NOR test was consisted of a box shaped (depth 70 cm × width 50 cm × height 40 cm) used for the measurement of the object recognition memory of rats [43]. Each test consists of two distinct trials such as training session and test session. In the beginning of the trial two identical objects were introduced into the apparatus. As object metal triangular (8.5 × 5 × 14 cm) and rectangular prisms (5 × 5 × 14 cm) were used. In the training sessions, a rat was placed in the apparatus and allows exploring the objects for a period of ≥ 10 sec. Object exploration was determined when the rat sniffed and or touched the object < 2 cm from its nose. Later the rat was returned to its home cage. After 24 hrs a test session was performed and before the test session one of the identical objects was replaced randomly by a novel object. In the test session each rat was allowed to explore the novel as well as familiar object for 4 min. Based on the investigation times of novel and familiar object discrimination index (DI) was determined [44]. The apparatus was cleaned after each test with 70% ethanol to remove any olfactory clue [38].
Morris Water Maze (MWM) Test
The apparatus of MWM test was consisted of tank shaped (diameter 1700 mm × depth 450 mm) used for the measurement of the spatial learning and memory of rats [40,45]. The water maze was divided into four quadrants in which a submerged platform (diameter 110 mm × height 250 mm) was placed in one compartment. During testing, the tank was filled to a depth of 260 mm with opaque water so that the platform was situated approximately 1 cm below the water surface and also hidden [46]. In acquisition trial, a rat was placed in the middle of a compartment of the tank and the time it took to mounted onto the submerged platform was recorded using a stopwatch as escape latency (EL). If the rat failed to find the platform within 300 sec, it was directed toward the platform. In both of this case a rat was allowed to stay on the platform for 30 sec [47]. Each day each rat was subjected to four consecutive trials remaining fixed the platform (East) with a different starting point, in random order as specified in (Table 1). The time spent in the target quadrant (TSTQ) was also recorded using a stopwatch. After the end of the fourth trial, the rat was gently dried with a soft cloth and kept warm under a bulb within the home case [48].
After the fourth trial of the last day, a probe trial was performed in which the platform was removed and the time the rat spent in the target quadrant (TSTQ) and time spent in the annuli (TSA) were measured for 60 sec. In the probe trial, all rats started from the same start position [49].
| 1st Day | 2nd Day | 3rd Day | 4th Day | 5th Day | 6th Day | 7th Day |
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 |
| Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 |
| Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 |
| Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 |
Table 1:The sequence of trials during the study period of WMM test.
Biochemical Study
The rats were sacrificed on 22nd day by using light anesthesia and whole brain was collected. From the whole brain the cerebellum and brainstem were removed and the remaining brain (i.e., portion without cerebellum and brainstem) was washed with ice cold sterile NaCl solution (0.9%). Then sodium phosphate buffer (30 mM, pH 7.4) was used to prepare 10% brain homogenate in a homogenizer. Finally the homogenate was subjected to centrifugation at 20,000 RPM for 2 hrs at 4ºC to eliminate cellular debris followed by collection of supernatant for further studies. The protein concentration was measured using bovine serum albumin [50].
Lipid Peroxidation (TBARS) Activity
The oxidative damage of lipid was estimated by the method of Iqbal et al., to measure the formation of thiobarbituric acid reactive species (TBARS) [51]. The total volume of the reaction mixture of this test was 1.0 ml includes 0.58 ml of 0.1 M phosphate buffer having pH 7.4, 0.2 ml of 100 mM ascorbic acid, 0.02 ml of 100 mM ferric chloride and 0.2 ml of 10% brain homogenate incubated at 37ºC in a shaking water bath for 1 hrs. To stop the reaction 1.0 ml of 10% TCA was added after that 1.0 ml 0.67% TBA was added, followed by boiling in a water-bath for 20 min. Then the test tubes were placing in a ice-bath followed by centrifuging at 2500 × g for 10 min. The amount of TBARS was measured at 535 nm by the help of spectrophotometer. The results of this test were expressed as nM TBARS/min/mg protein at 37°C by means of a molar extinction coefficient of 1.56 × 105 M-1 cm-1.
Acetylcholinesterase (AChE) activity
The cholinergic marker, acetylcholinesterase was estimated by the method of Ellman et al., [52]. In this test, 25 μl of 15 mM ATCI, 75 μl of 3 mM DTNB and 75 μl of 50 mM Tris–HCl having pH 8.0, containing 0.1% BSA were added in the 96 well plates. After that the absorbance of this reaction mixture was measured at 405 nm by the help of spectrophotometer. Then to the aforementioned reaction mixture 25 μl of 10% brain homogenates was added followed by incubation for 5 min at 25ºC. After that again absorbance was measured at 405 nm by the help of spectrophotometer. The results of this test were expressed as M/min/g protein.
Statistical Analysis
Results were expressed as mean ± SEM and analyzed using oneway analysis of variance (ANOVA). For behavioral studies Tukey’s post hoc test were carried out and in case of biochemical studies the least significant difference (LSD) was determined using post hoc testing for inter group comparisons at a probability level of 0.05% and 0.01%. Microsoft Excel 2010 (Roselle, IL, USA) was used for the statistical and graphical evaluations. A probability of P<0.05 was considered as significant with respect to disease control group.
Results
Determination of Acute Toxicity
The doses of EEAR did not exert any lethality consequently there were no behavioral, neurological or physical changes. In addition, to this body weight, skin, fur and eyes of the rats remained unchanged and no mortality was observed at the test doses. Thus, the doses of this study were within safe margin.
| Treatment | TBARS (nM/min/mg protein) |
|---|---|
| Con | 394.05 ± 5.36 |
| Don | 158.29 ± 4.69*** |
| Eth | 457.95 ± 6.92 |
| EEAR 100 | 216.25 ± 3.65 |
| EEAR 200 | 198.57 ± 4.68** |
| Eth+EEAR 100 | 274.39 ± 6.73** |
| Eth+EEAR 200 | 241.67 ± 5.67 |
| Eth+Don | 186.49 ± 6.95** |
The rats brain TBARS status was expressed as mean ± SEM values (n = 6/group).
**P<0.01, ***P<0.001 significant difference from the disease control group
Table 2: Neuroprotective effect of EEAR on TBARS status of rat brain.
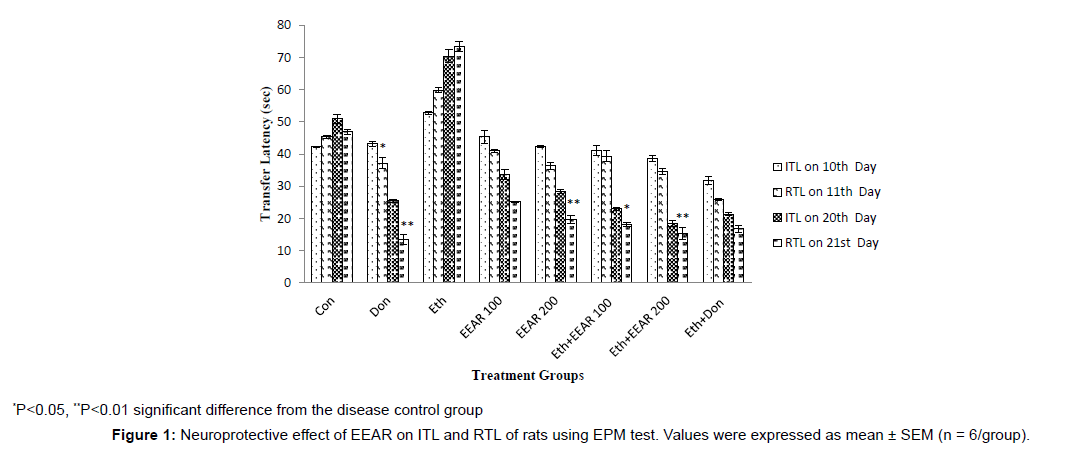
Neuroprotective Effect of EEAR on ITL and RTL of Rats using EPM test
In EPM test the ITL was measured on 10th and 20th days and RTL was measured on 11th (after 24 hrs of ITL) and 21st day (after 24 hrs of ITL) respectively presented in Figure 1. Donepezil significantly (P<0.05, P<0.01) reduced RTL of rats on 11th and 21st day as compared to disease control group on 10th and 20th day ITR. Ethanol-treated group exhibited increased in RTL with respect to other groups on successive days. Administration of EEAR resulted in decrease in RTL on 11th and 21st day when compared to disease control rats. However, administration of 100 and 200 mg/kg b.w. EEAR markedly (P<0.05, P<0.01) reversed ethanolinduced memory deficits, indicating considerable improvement of learning and memory of rats.
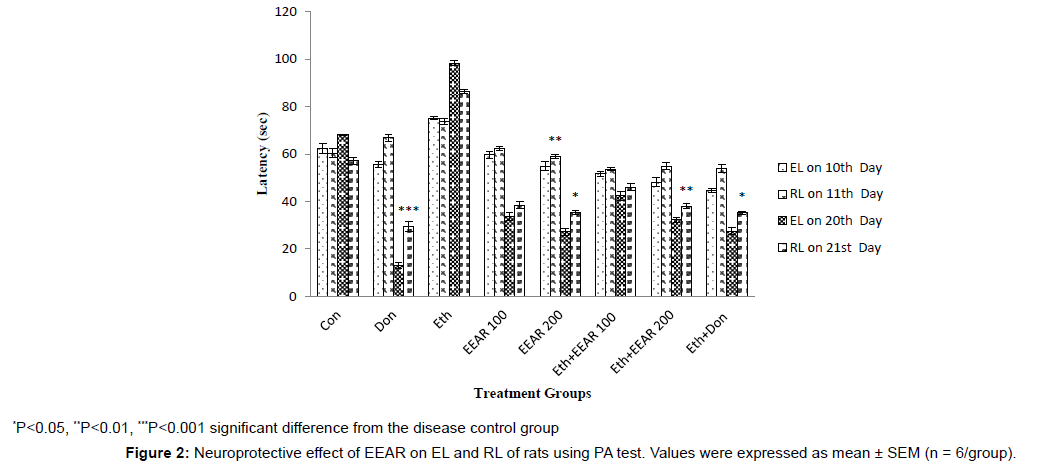
Neuroprotective Effect of EEAR on EL and RL of Rats using PA test
In PA test the EL was measured on 10th and 20th days and RL was measured on 11th (after 24 hrs of EL) and 21st day (after 24 hrs of EL) respectively given in Figure 2. In the ethanol treated group, significant decreased in RL was reported compared to other groups on successive days. Treatment with donepezil significantly (P<0.05, P<0.001) increased RL of rats on 21st day compared with disease control group. The highest and lowest doses of EEAR increased RL of rats as compared to that of disease control group on successive days. Particularly administration of 200 mg/kg b.w. EEAR markedly (P<0.05, P<0.01) increased the value of RL on 11th and 21st day thus showed significant cognition enhancing activity
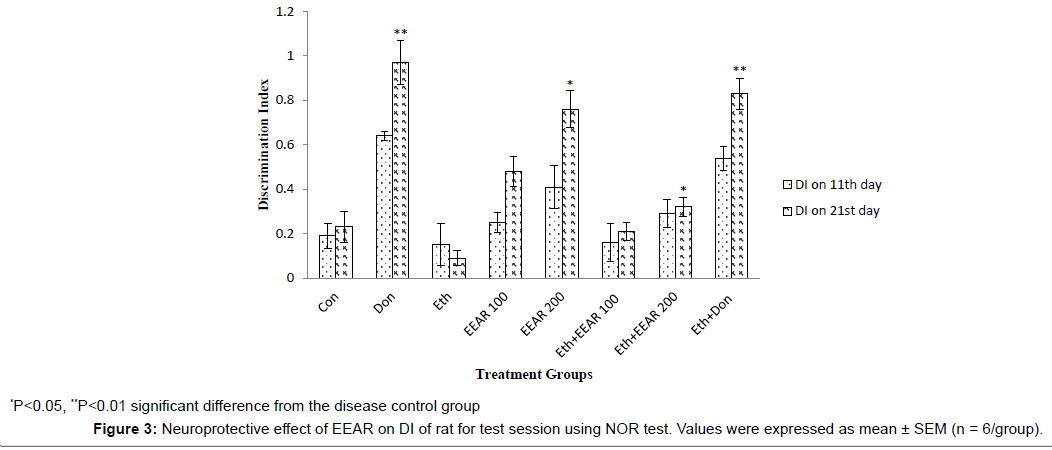
Neuroprotective Effect of EEAR on DL of Rats using NOR test
In NOR test the DI was measured on 11th (after 24 hrs of training session) and 21st (after 24 hrs of training session) day respectively assumed in Figure 3. The standard drug, donepezil hydrochloride significantly (P<0.01) increased the DI of rats on 21st day with respect to disease control group. Ethanol-treated rats showed a reduced in DI on 11th and 21st day as compared to other groups. This outcome was altered by the highest dose of EEAR. EEAR (i.e., 200 mg/kg b.w.) treated rats exhibited a significantly (P<0.05) increased in DI on 21st day compared with that of disease control rats.
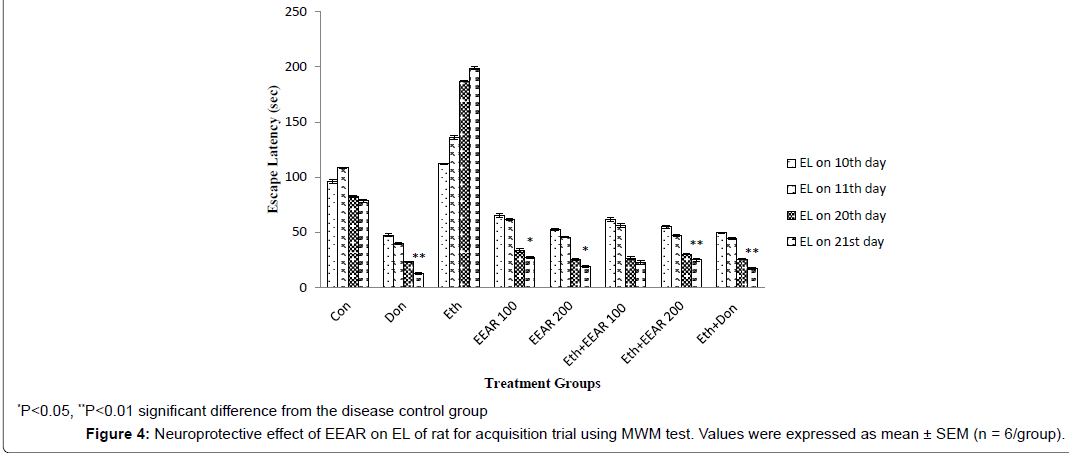
Neuroprotective Effect of EEAR on EL, TSTQ and TSA of Rats using MWM test
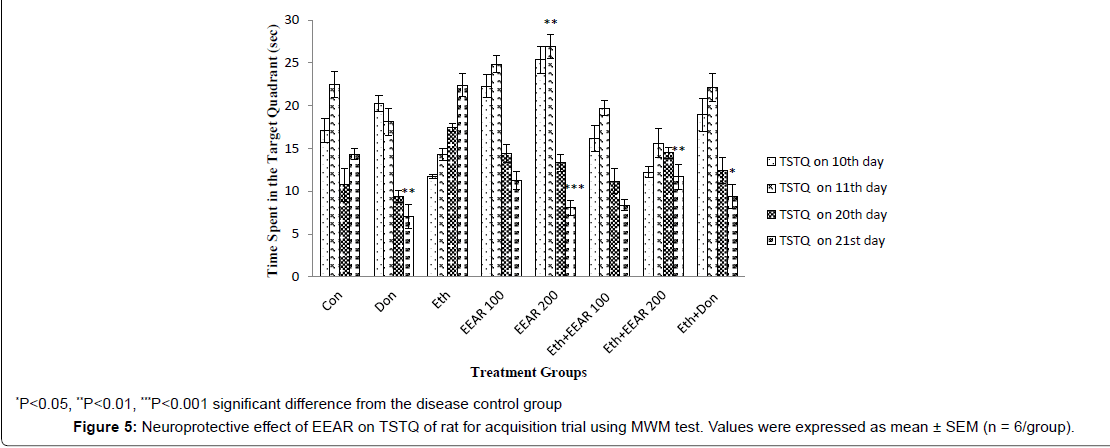
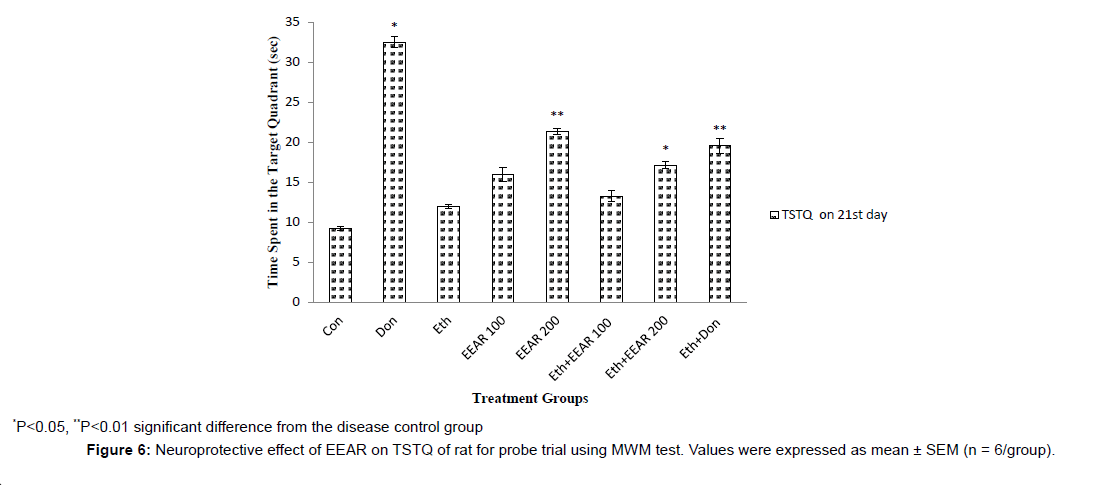
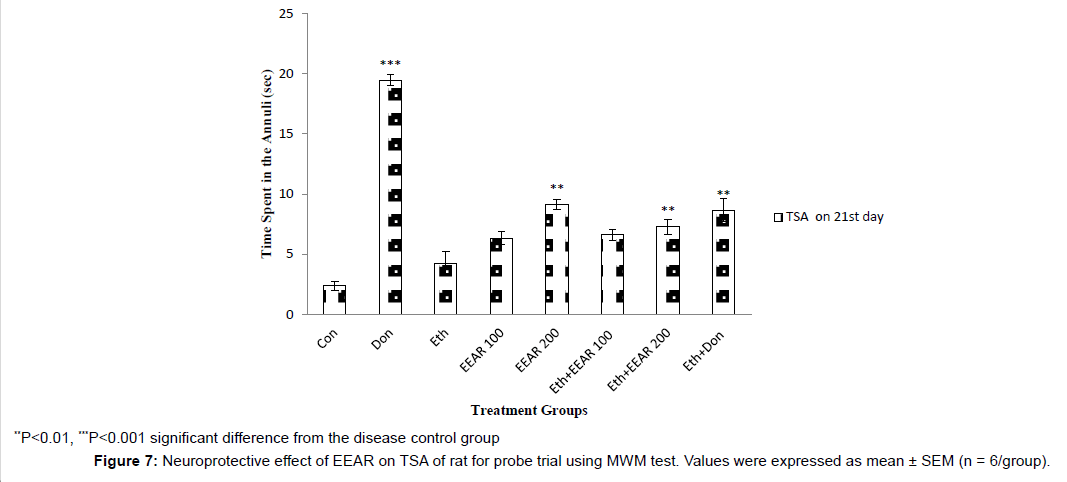
In MWM test the EL and TSTQ for acquisition trial was measured on 10th, 11th, 20th and 21st day (Figure 4 and Figure 5). In acquisition trial, ethanol-treated rats showed markedly increased EL and decreased TSTQ on successive days as compared to other groups. Donepezil administered rats for 21 days showed significantly (P<0.01) decreased in EL and markedly (P<0.05, P<0.01) increased in TSTQ of rats as compared to that of ethanol-induced group. Administration of EEAR decreases the EL and increased the TSTQ on successive days. Administration of EEAR (i.e., 100 and 200 mg/kg b.w.) resulted in markedly (P<0.05, P<0.01) decreased in EL on 21st day as compared to that of disease control group. Out of two doses highest dose of EEAR (i.e., 200 mg/kg b.w.) markedly (P<0.01, P<0.001) increased the TSTQ of rats on 11th and 21st day as compared to that of disease control group. In case of probe trial, like acquisition trial EEAR (i.e., 200 mg/kg b.w.) significantly (P<0.05, P<0.01) increased TSTQ and TSA of rats as compared to that of disease control group on 21st day thus showed significant improvement of learning and memory given in Figure 6 and Figure 7.
Neuroprotective Effect of EEAR on TBARS Status in Rat’s Brain
Treatment with ethanol increased the level of TBARS as compared to other groups specified in Table 2. Administration of both doses of EEAR (i.e., 100 and 200 mg/kg b.w.) significantly (P<0.01) decreased the TBARS level with respect to disease control group. The rats treated with donepezil markedly (P<0.01, P<0.001) decreased the TBARS level to that of disease control group.
Neuroprotective Effect of EEAR on AChE Activity in Rat’s Brain
Ethanol-treated rats showed a decreased in the brain AChE activity as compared to other groups. Donepezil treatment significantly (P<0.01) inhibited the brain AChE level compared to their corresponding disease control group. Brain AChE activity was considerably decreased in rats owing to EEAR administration. The lowest and highest dose of EEAR (i.e., 100 and 200 mg/kg b.w.) significantly (P<0.05, P<0.01) decreased the AChE activity in the brain tissue of rats as compared to disease control group (Table 3).
Discussion
| Treatment | AChE (M/min/g of tissue protein) |
|---|---|
| Con | 0.194 ± 0.001 |
| Don | 0.049 ± 0.004** |
| Eth | 0.307 ± 0.005 |
| EEAR 100 | 0.166 ± 0.003* |
| EEAR 200 | 0.129 ± 0.005** |
| Eth+EEAR 100 | 0.244 ± 0.006 |
| Eth+EEAR 200 | 0.201 ± 0.004** |
| Eth+Don | 0.102 ± 0.004** |
Table 3: Neuroprotective effect of EEAR on AChE activity in rat brain.
No cure for Alzheimer’s exists and the drugs currently available to treat the disease have limited effectiveness. The uses of complementary medicines such as plant extracts exert a substantial strategy for the treatment of neurological disorders such as AD [53]. Indeed, several scientific studies have described the use of various medicinal plants and their bioactive constituents for the treatment of AD [54]. Although the exact mechanism of their action is still not clear, phytochemical studies of the different parts of the plants have shown the presence of many valuable compounds, such as lignans, flavonoids, tannins, polyphenols, triterpenes, sterols and alkaloids, that show a wide spectrum of pharmacological activities, including anti-inflammatory, antiamyloidogenic, anti-cholinesterase, hypolipidemic and antioxidant effects [55]. In this study, EEAR roots extract were administration for 21 days showed significant neuroprotective activity in ethanol-induced rats of cognitive impairment and oxidative stress analyzed by using various behavioral and biochemical studies.
The EPM is a commonly used test for the determination of spatial long term memory by measuring ITL and RTL. In this test, after 24 hrs of ITL determination RTL was measured in which a decrease in RTL after the ILT determination indicated improvement of spatial longterm memory of rats. In this study, EEAR administration significantly reduced RTL of rats as compared to disease control group. In the study of protective effects of Punica granatum seeds extract against aging and scopolamine induced cognitive impairments in mice Kumar et al., reported analogous result in transfer latency of animals in this test [56]. The PA is a used test for the determination of emotional memory by measuring EL and RL. During EL determination an electric shock was given and after 24 hrs of electric shock RL was measured. An increased in RL was observed in rats treated by EEAR as compared to disease control rats. In this test an increase in RL after the EL determination indicated improvement of emotional memory of rats as compared to disease control rats. By using this test Bera et al., observed brain tonic activity of Shankhavali churna in Wistar albino rats [57]. The NOR test is used for the determination of recognition memory by measuring DI based on the exploration times of novel and familiar object. In this test, an increase in DI after the training session indicated improvement of recognition memory, observed in EEAR treated rats as compared to disease control rats. In term of this test Une et al., in the study of Ziziphus mauritiana leaves extract stated nootropic effect [58]. The MWM test is the most commonly test used to evaluate cognitive functions related to spatial learning and memory by measuring EL, TSTQ and TSA. EL is the time required to find the submerged platform. TSTQ and TSA are the time spends to stay in the target quarantine and annuli respectively. Herein in case of acquisition trial a decrease in EL and an increase in TSTQ indicated improvement of spatial learning and memory of rats. For probe trial an increase in TSTQ and TSA also means enhancement of spatial learning and memory of rats. In this test EEAR administration considerably decrease EL and increase TSTQ and TSA of rats with respect to disease control rats. In the study of Nyctanthes arbor-tristis leaves extract against scopolamine-induced cognitive impairment in rats, neuroprotective effect was obserbed by Raju et al., [59].
The main target for lipid peroxidation is the CNS due to high lipid content (PUFA), high consumes of the body’s oxygen (20%) and high in redox transition metal ions. Lipid peroxidation is the hallmark of AD [60]. Increasing evidence demonstrates abnormal lipid peroxidation, cerebrospinal fluid and plasma in the brain of AD patients. In addition, lipoproteins in the AD patient’s brain are more susceptible to oxidation damage [61]. As per this study EEAR roots administration in rat’s markedly decreased brain TBARS activity. For the study of neuroprotective effect of Phyllanthus acidus fruits extract on learning and memory impairment in scopolamine-induced animal model of dementia and oxidative stress, Uddin et al., reported similar finding [62]. The foremost enzyme of the cholinergic nervous system is the AChE. Higher level of AChE is found in the brain of AD patients [63]. Study suggested that AChE is linked with the formation of Aβ plaques and NFTs, both are pathological features of AD [64]. Numerous studies recommend that in the expression of AChE, Aβ and atypically hyperphosphorylated tau (P-tau) can impact [65]. In this study treatment with EEAR roots widely decreased AChE level. In the study on nootropic effects of aerial part of Persicaria flaccida on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats by Uddin et al., showed beneficial effects of this plant extracts for the management of AD [66].
The outcome of the aforementioned studies recommends that EEAR roots extract has neuroprotective activity and gifted to improve learning, memory impairments and hippocampus, cerebellum oxidative stress.
Conclusion
From the present study it was clearly demonstrated that EEAR root has significant neuroprotective activity and showed marked constructive effects for improving the learning and memory. Therefore, this root extract can be used for the treatment of various cognitive dysfunctions connected with neurodegenerative disorders predominantly AD. Regardless of these findings, further investigations will be necessary to illustrate the active nootropic compound(s).
Ethical Approval
The protocol of the experiment was approved by the animal ethics committee of the Department of Pharmacy, Southeast University, Dhaka, Bangladesh.
Authors’ Contributions
This work was carried out in collaboration among all authors. MSU and MA designed the study, wrote the protocol, managed the analyses of the study and prepared the draft of the manuscript. MSU, AAM and MAI performed the laboratory experiments. FW participated in literature searches. RKR participated in data analysis and interpretation. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to thank the Department of Pharmacy, Southeast University, Dhaka, Bangladesh for providing research facilities.
Competing Interests
The authors proclaim that they have no competing interests.
References
- Uddin MS, Mamun AA, Iqbal MA, Islam A, Hossain MF, Khanum S, Rashid M (2016) Analyzing nootropic effect of Phyllanthus reticulatus Poir. on cognitive functions, brain antioxidant enzymes and acetylcholinesterase activity against aluminium-induced Alzheimer’s model in rats: Applicable for controlling the risk factors of Alzheimer’s Disease. Adv Alzheimer's Dis 5: 87-102.
- Querfurth HW, LaFerla FM (2010) Alzheimer's dis. N Engl J Med 362: 329-344.
- Wimo A1, Winblad B, Aguero-Torres H, von Strauss E (2003) The magnitude of dementia occurrence in the world. Alzheimer Dis Assoc Disord 17: 63-67.
- Anonymous (Accessed 20 January 2016) Alzheimer's Disease Fact Sheet.
- Mamun AA, Uddin MS, Wahid F, Iqbal MA, Rahman MM (2016) Neurodefensive effect of Oleaeuropaea L. inalloxan-induced cognitive dysfunction and brain tissue oxidative stress in mice: Incredible natural nootropic
- Burns A1, Iliffe S (2009) Alzheimer's disease. Bri Med J 338: b158.
- Anonymous (Accessed 20 January 2016)Younger/ Early Onset Alzheimer's & Dementia.
- Vetreno RP, Hall JM, Savage LM (2011) Alcohol-related amnesia and dementia: Animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol Learn Mem 96: 596-608.
- Hernández JA, López-Sánchez RC, Rendón-Ramírez A (2016) Lipids and oxidative stress associated with ethanol-induced neurological damage. Oxidative Medicine and Cellular Longevity 2016: 1543809.
- Ramezani A, Goudarzi I, Lashkarboluki T, Ghorbanian MT, Abrari K, et al. (2012) Role of Oxidative Stress in Ethanol-induced Neurotoxicity in the Developing Cerebellum. Iran J Basic Med Sci 15: 965-974.
- Lu Y, Cederbaum AI (2008) CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med 44: 723-738.
- Massaad CA (2011) Neuronal and vascular oxidative stress in Alzheimer's disease. Curr Neuropharmacol 9: 662-673.
- Mao P, Reddy PH (2011) Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer's disease: implications for early intervention and therapeutics. Biochim Biophys Acta 1812: 1359-1370.
- Anonymous (Accessed 20 January 2016) What Is Alzheimer's?
- Giri M, Swamy BMV, Jayaveera KN (2012) Evaluation of nootropic activity of leaves of boragoofficinalis. Research Journal of Pharmaceutical, Biological and Chemical Sciences 3: 405-406.
- Nade VS, Kanhere SV, Kawale LA, Yadav AV (2011) Cognitive enhancing and antioxidant activity of ethyl acetate soluble fraction of the methanol extract of Hibiscus rosasinensis in scopolamine-induced amnesia. Indian J Pharmacol 43: 137-142.
- Yiannopoulou KG, Papageorgiou SG (2013) Current and future treatments for Alzheimer's disease. Ther Adv Neurol Disord 6: 19-33.
- Elufioye TO, Oladele AT, Cyril-Olutayo CM, Agbedahunsi JM, Adesanya SA (2012) Ethnomedicinal study and screening of plants used for memory enhancement and antiaging in Sagamu, Nigeria. Eur J Medi Plants 2: 262-275.
- Uddin MS, Mamun AA, Khanum S, Begum Y, Alam MS (2016)
- Bhattacharjee SK (2001) Handbook of Medicinal Plants. Jaipur: Pointer Pub.
- Jayakumar K, SatheesKannan TM, Vijayarengan P (2015) Leucasaspera L. – Medicinal Herb. Int J Trad Nat Med 5: 1-5.
- Patel KC, Pramanik S, Patil VC (2014) Ayurvedic approach with a prospective to treat and prevent Azheimer’s and other cognitive diseases: a review. Wor J Pharm Pharmace Sci 3: 234-236.
- Rao RV, Descamps O, John V, Bredesen DE (2012) Ayurvedic medicinal plants for Alzheimer's disease: a review. Alzheimers Res Ther 4: 22.
- Hannan JMA, Ali L, Khaleque J, Akhter M, Flatt PR, et al. (2012) Antihyperglycaemic activity of Asparagus racemosus roots is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of cellular insulin action. British J Nutri107: 1316-1323.
- Wani JA, Achur RN, Nema RK (2011) Phytochemical Screening and Aphrodisiac Activity of Asparagus racemosus. Int J Pharma Sci Drug Rese 3: 112-115.
- Sharma PC, Yelne MB, Dennis TJ (2000) Database on medicinal plants used in Ayurveda. New Delhi: Central Council for Research in Ayurveda and Siddha.
- Negi JS, Singh P, Joshi GP, Rawat MS, Bisht VK (2010) Chemical constituents of Asparagus
- Diwanay S, Chitre D, Patwardhan B (2004) Immunoprotection by botanical drugs in cancer chemotherapy. J Ethnopharmacol 90: 49-55.
- Saxena G, Singh M, Meena P, Barber S, Sharma D, et al. (2007) Neuroprotective effects of Asparagus racemosus linn root extract: an experimental and clinical evidence. Annals Neurosci 14: 59-63.
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals. Washington: National Academies Press.
- Goverdhan P, Sravanthi A, Mamatha T (2012) Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int J Alzheimers Dis 2012: 974013.
- Shirpoor A, Minassian S, Salami S, Khadem-Ansari MH, Ghaderi-Pakdel F, et al. (2009) Vitamin E protects developing rat hippocampus and cerebellum against ethanol-induced oxidative stress and apoptosis. Food Che 113: 115-120.
- Choudhary D, Sharma D, (2014) A phytopharmacological review on Asparagus racemosus. Int J Sci Res 3: 742-745.
- Organisation for Economic cooperation and Development (2002) OECD guidelines for the testing of chemicals: acute oral toxicity – acute toxic class method. Paris: OECD.
- Reddy DS, Kulkarni SK (1998) Possible role of nitric oxide in the nootropic and antiamnesic effects of neurosteroids on aging- and dizocilpine-induced learning impairment. Brain Res 799: 215-229.
- Hlinak Z, Krejci I (2002) MK-801 induced amnesia for the elevated plus-maze in mice. Beh Brain Rese 131: 221-225.
- Swaroop TVSS, Banerjee S, Handral M (2014) Neuroprotective evaluation of leaf extract of Dalbergiasissoo in 3-nitropropionic acid induced neurotoxicity in rats. Int J Pharma Sci Drug Res 6: 41-47.
- Ozkay UD, Can OD, Ozkay Y, Ozturk Y (2012) Effect of benzothiazole/piperazine derivatives on intracerebroventricularstreptozotocin-induced cognitive deficits. Pharmaco Rep 64: 834-847.
- Ogren SO, Stone WS, Altman HJ (1987) Evidence for a functional interaction between serotonergic and cholinergic mechanisms in memory retrieval. Beh Neural Bio 48: 49-62.
- van der Staay FJ, Schuurman T, van Reenen CG, Korte SM (2009) Emotional reactivity and cognitive performance in aversively motivated tasks: a comparison between four rat strains. Beha Brain Fun 5: 1-24.
- Wang J, Wang X, Lv B, Yuan W, Feng Z, et al. (2014) Effects of Fructus Akebiae on learning and memory impairment in a scopolamine-induced animal model of dementia. Exp Ther Med 8: 671-675.
- Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47-60.
- Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13: 93-110.
- Ennaceur A, Neave N, Aggleton JP (1997) Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experime Brain Rese 113: 509-519.
- D'Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36: 60-90.
- Dhingra D, Kumar V (2012) Memory-enhancing activity of palmatine in mice using elevated plus maze and morris water maze. Adv Pharmacol Sci 2012: 357368.
- Taati M, Alirezaei M, Moshkatalsadat MH, Rasoulian B, Moghadasi M , et al. (2011) Protective effects of Ziziphus jujube fruit extract against ethanol-induced hippocampal oxidative stress and spatial memory impairment in rats. J Medi Plants Res 5: 915-921.
- Blokland A, Geraerts E, Been M (2004) A detailed analysis of rats' spatial memory in a probe trial of a Morris task. Behav Brain Res 154: 71-75.
- Das A, Dikshit M, Nath C (2005) Role of molecular isoforms of acetylcholinesterase in learning and memory functions. Pharmacol Biochem Behav 81: 89-99.
- Kameyama T, Nabeshima T, Kozawa T (1986) Step-down-type passive avoidance- and escape-learning method. Suitability for experimental amnesia models. J Pharmacol Methods 16: 39-52.
- Iqbal M, Sharma MD, Zadeh HR, Hasan N, Abdulla M, et al. (1996) Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate (Fe-NTA) mediated hepatic injury. Redox Repo 2: 385-391.
- Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7: 88-95.
- Singhal AK, Naithani V, Bangar OP (2012) Medicinal plants with a potential to treat Alzheimer and associated symptoms. Int J Nutri, Pharmaco, Neuro Dis 2: 84-85.
- NahidJivada, Rabieib Z (2014) A review study on medicinal plants used in the treatment of learning and memory impairments. Asi Paci J Tro Biomed 4: 780-781.
- Rao RV, Descamps O, John V, Bredesen DE (2012) Ayurvedic medicinal plants for Alzheimer's disease: a review. Alzheimers Res Ther 4: 22.
- Kumar S, Maheshwari KK, Singh V (2008) Protective effects of Punicagranatum seeds extract against aging and scopolamine induced cognitive impairments in mice. Afr J Tradit Complement Altern Med 6: 49-56.
- Bera KM, Ghule RS, Kapdiya NS, Shah MB, Upadhyay UM (2011) Brain tonic activity of shankhavali churna. Res J Pharma, Bio Che Sci 2: 791-797.
- Dureshahwar K, Mubashir M, Une HD, Hundekari GI, Dehghan MH (2012) Nootropic activity of n-butanolic fraction of methanolic extract of leavesof Ziziphus mauritiana Lam. in mice. Der Pharmacia Sinica 3:193-198.
- Phanindhra B, Raju AB, Vikas G, Anusha R, Deepika D (2014) Effect of nyctanthes arbor tristis leaf extract against scopolamine-induced cognitive impairment in rats. Herba Polonica 60: 34-49.
- Adibhatla RM, Hatcher JF (2010) Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antiox Red Sig 12: 125-169.
- Maynard CJ, Bush AI, Masters CL, Cappai R, Li QX (2005) Metals and amyloid-beta in Alzheimer's disease. Int J Exp Pathol 86: 147-159.
- Uddin MS, Mamun AA, Hossain MS, Ashaduzzaman M, Noor MA, et al.(2016) Neuroprotective effect of Phyllanthus acidus L. on learning and memory impairment in scopolamine-induced animal model of dementia and oxidative stress: Natural wonder for regulating the development and progression of Alzheimer’s Disease. Adv Alzheimer's Dis 5: 53-72.
- Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM (2013) Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr Neuropharmacol 11: 315-318.
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1: a006189.
- Golde TE, Petrucelli L, Lewis J (2010) Targeting Abeta and tau in Alzheimer's disease, an early interim report. Exp Neurol 223: 252-266.
- Uddin MS, Nasrullah M, Hossain MS, Rahman MM, Sarwar MS, et al. (2016) Evaluation of nootropic activity of Persicaria flaccida on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: Implication for the management of Alzheimer’s disease. Ame J Psy Neurosci 4: 26-37.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15750
- [From(publication date):
August-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 14498
- PDF downloads : 1252