Neuropeptide Pharmacological Profiling on the Rabbit Vaginal Wall and Smooth Muscle of the Vaginal Arteries In Vitro
Received: 01-Nov-2023 / Manuscript No. wjpt-23-120197 / Editor assigned: 03-Nov-2023 / PreQC No. wjpt-23-120197(PQ) / Reviewed: 22-Nov-2023 / QC No. wjpt-23-120197 / Revised: 30-Nov-2023 / Manuscript No. wjpt-23-120197(R) / Accepted Date: 30-Nov-2023 / Published Date: 30-Nov-2023 QI No. / wjpt-23-120197
Abstract
Context and objective: Sexual arousal is centrally modulated by hypothalamic neuropeptides. The part neuropeptides play in peripheral arousal, however, has not received much attention. Female sexual arousal is mostly dependent on the relaxation of the vagina’s vascular and non-vascular smooth muscles. There have been no in vitro studies on vaginal arteries to date; instead, all research has been on vaginal strips. This study compared the effects of neuropeptides from the sexual hypothalamus on the arteries and vaginal wall strips of rabbits.
Experimental strategy: Isometric tension from strips and arteries was measured using tissue bath and wire myography procedures, respectively.
Important outcomes: Vasoactive Intestinal Peptide (VIP) and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) relaxed both preparations; these effects were only countered by the PAC1 antagonist PACAP 6-38 (1 nM) and the VIP/PACAP antagonist VIP6-28 (10 nM). mg). Both preparations were relaxed by the melanocortin agonist a-melanocortin-stimulating hormone (1 mM), but not by bremelanotide (1 mM). Vaginal preparations were contracted by oxytocin and vasopressin, which the V1A might exacerbate. Only arteries were constricted by neuropeptide Y (NPY) and the NPY Y1 agonist Leu31, Pro34; NPY was inhibited by the NPY Y1 receptor antagonist BIBP 3226. Arteries were constricted by melanin-concentrating hormone (MCH; 1 mM).
Final thoughts and ramifications: Hypothalamic neuropeptides have the ability to relax and contract the arteries and vaginal strips. Both central and peripheral female sexual arousal may benefit from the use of NPY Y1, V1A, MCH1, and VIP/PAC1 agonists. Which preparation is crucial for female sexual arousal is a question that is raised by variations in the neuropeptide effects of the preparations.
Keywords
Female sexual arousal; Vagina; Arteries; Neuropeptides; Contraction; Relaxation
Abbreviations: aMSH, a-melanocortin-stimulating hormone; AVP, vasopressin; IVA, intra-vaginal artery; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; PACAP, pituitary adenylate cyclaseactivating polypeptide; V1A, vasopressin 1A; VIP, vasoactive intestinal peptide.
Introduction
Numerous preclinical in vivo investigations have demonstrated the primary regulation of female sexual behavior by hypothalamic neuropeptides. Female receptive copulatory behavior, also known as lordosis or receptivity, has been demonstrated to be inhibited by neuropeptide Y [1,2] and vasopressin [3], while oxytocin [4-9] pituitary adenylate cyclase-activating polypeptide (PACAP; ApostolakisThe paraventricular nucleus, medial preoptic area, or ventromedial nucleus have all been shown to enhance female sexual receptivity when injected with melanin-concentrating hormone [10], a-melanocortinstimulating hormone [10-14]. Remarkably, whereas the majority of research has concentrated on the function of neuropeptides in centrally mediated sexual arousal, the neuropeptides underlying the peripheral regulation of female sexual arousal have received less attention up to this point. Deficits in these systems during sexual arousal may be the pathophysiology of female sexual arousal disorder. Vascular and nonvascular smooth muscle relaxation plays a vital role during sexual arousal. Blood flow to the external genital organs, clitoris, and vagina. Due to an increase in plasma transudation, the flow increases and lubricates the vagina more. Although the underlying mechanisms are unclear, it is believed that genital sensitivity rises during sexual arousal. Vaginal blood flow has been studied as a physiological indicator of peripheral sexual arousal using rabbits as a preclinical in vivo model [15- 21]. The majority of research has examined how the nitric oxide-cGMP pathway regulates the tone of the vaginal smooth muscle, showing that phosphodiesterase type-5 inhibitors can relax the clitoral and vaginal muscles and possibly increase arousal in ladies [22]. Only sildenafil has been clinically studied to date for its potential effects on female sexual function; nevertheless, the findings have been underwhelming [22]. Nonetheless, the impact of neuropeptides on vaginal blood flow has only been somewhat studied, with a particular focus on vasoactive intestinal peptide [23]. Similarly, non-vascular vaginal smooth muscle strips have been used to explore the functional effects of neuropeptides on sexual arousal, although the available data is similar. Once the nerves of the vagina were identified by histochemical means as containing VIP, PACAP, peptide histidine methonine, peptide histidine valine, and helospectin [24] showed that each of these neuropeptides could directly relax the rabbit vagina when bath applied to pre-contracted vaginal tissue slits. There haven’t been any in vitro research done to look into how neuropeptides affect the vaginal arteries that supply vaginal blood flow. Thus, the objectives of the research were to: (a) examine the impact of pro-and anti-sexual hypothalamic neuropeptides in the rabbit vagina in vitro; (b) assess the potency and effectiveness of neuropeptides in vaginal strips and arteries; and (c) determine the receptor subtypes responsible for neuropeptide expressions.
Techniques
Preparing the vagina in rabbits
Every experiment was conducted in accordance with ACP and was subject to a regional ethics assessment. By injecting an excessive amount of pentobarbitone (Pentoject, Animalcare, York, UK) into the marginal ear vein, female rabbits weighing B3 kg were slaughtered. Removed, the vagina was submerged in Krebs solution (mM) (NaCl 119, KCl 4.7, At 37°C, CaCl2, 2.5, KH2PO4, 1.2, MgSO4, 1.2, NaHCO3, and 11 glucose and 95% O2/5% CO2 gassed.
The dissection and installation of vaginal strips
The lowest 3 cm of the vagina were removed, and the vagina was then divided into four longitudinal strips, four upper and four lower by cutting it in half horizontally. The Krebs solution, which was aerated with 95% O2/5% CO2, was used to mount the strips in 5 mL organ baths. The strips were then left to equilibrate for one hour at a resting tension of 19.6 mN.
Mounting and dissection of the vaginal artery
After the vagina was removed, the arteries were separated and cut into rings that measured 2 mm. The term “extra-vaginal artery” refers to the artery that enters the vagina, whereas the term “lower 2 cm of the the vagina is called the “Intra Vaginal Artery” (IVA). Using isometric wire myography, the vascular rings (i.e. the extra-vaginal artery: 263 ± 15 mM; IVA: 173 ± 3.6 mM) were recorded after 30 minutes of equilibration at a resting tension of 2.94 mN. Every experiment was run in Krebs solution at 37°C with 95% CO2/5% O2 gassed. After the system had settled, artery rings were pre constricted three times with 10 mM phenylephrine then had a 30-minute washout interval before applying neuropeptide.
Curves for concentration response
Agonist concentration response curves were created by cumulatively applying half-log concentration increments in order to examine the contractile action of neuropeptides in arteries and vaginal strips. After a one-hour washout period, either the vehicle or the antagonist was incubated for a further twenty minutes prior to carry out another repeatable concentration response curve. Pre-contraction of arteries and vaginal strips with phenylephrine (10-20 mM) was done for relaxation tests. By using cumulative half-log additions, neuropeptide agonist concentration response curves were produced. After a onehour washout period, a repeatable concentration response curve was repeated after either a vehicle or antagonist incubation for 20 minutes.
Analysis of data
Data are presented as mean ± s.e. of the mean (s.e. mean), with n denoting the quantity of artery rings or vaginal strips. EC50, slope, and pKB were calculated with Labstats (Pfizer Excel add-in). Lew and Angus (1995) [25] introduced the global nonlinear regression method that was used to analyze experimental data with three antagonist concentrations in order to determine the pKB values. Benefits of this approach above the conventional Schild method have been explored. In brief, the approach does not require the within-tissue control concentration response curves needed for conventional Schild regression approaches, as previously described [25]. In experiments where the antagonist was known to be competitive, the Gaddum equation was applied with a single antagonist concentration to oppose the agonist concentration response curve. Student’s t-test or ANOVA were used to establish statistical significance.
Mixtures
The compounds were acquired from Sigma-Aldrich (Gillingham, Dorset, UK), which provided phenylephrine, AVP, oxytocin, BIBP 3226, and MCH. PACAP 6-38, GR231118, NPY (13-36), cPP1-7, NPY (human, rat), NPY19-23, Gln, Ala, and AibLeu31, Pro34, and NPY, the pancreatic polypeptide PYY (3-36), were provided. As well as VIP, VIP6-28, PACAP 1-27, PACAP 1-38, and aMSH from Tocris (Avonmouth, Bristol, UK) at St. Helens, Merseyside, UK. According to reports in the literature [26-28] SR 49059, L-368899, and bremelanotide were synthesized.
Outcomes
Neuropeptide-induced contractions in rabbit smooth muscle, both vascular and non-vascular. Neuropeptides were added to the organ bath in order to study their effects on the rabbit vagina. Oxytocin (0.1 nM-3 mM) and AVP (0.01-100 nM) produced consciousness maximal contractions of 5.7 ± 0.7 mN (n=28) and 12.4 ± 2.2 mN (n=13) in upper vaginal strips, respectively, that are dependent contraction (Figures 1a and 1b). Both peptides did not cause the lower 1.5 cm vaginal strips to contract. Compared to oxytocin, the contraction caused by AVP was 20 times stronger (Table 1).
Figure 1: Effect of Oxytocin (OT) and Vasopressin (AVP) on rabbit vaginal strips and arteries in vitro. 1a) shows an experimental trace of increasing concentrations of AVP on (i) vaginal strips and (ii) vaginal arteries. (iii) This is the average concentration response curve to AVP in both tissues. 1b is the same as 1a, but in the presence of oxytocin.
Peptide |
EC50 (nM) |
Slope |
Emax (mN) |
n |
|---|---|---|---|---|
| AVP | 9.5 ± 1.8 | 1.5 ± 0.2 | 5.7 ± 0.7 | 28 |
| Oxytocin | 206 ± 83.2 | 0.8 ± 0.1 | 12.4 ± 2.2 | 13 |
| NPY | NE | NE | NE | 6 |
| MCH | NE | NE | NE | 8 |
Table 1: Neuropeptide-induced contraction in rabbit vaginal strip.
The rabbit vaginal artery was demonstrated to contract in all locations in response to AVP (0.01 nM-100 nM) and oxytocin (0.1 nM- 10 mM), with maximum contractile responses of 6.3 ± 0.5 mN (n¼ 53) and 5.7 ± 0.5 mN (n¼ 49), respectively. AVP was more effective than oxytocin, much like in the vaginal strips (Table 2). When compared to strips of vaginal smooth muscle, both neuropeptides were 15-fold and 3-fold more powerful in the vaginal arteries NPY did not cause a concentration-dependent contraction in vaginal strips (n¼ 6; P40.05), in contrast to AVP and oxytocin. But NPY effectively produced a strong vaginal artery constriction that is concentration-dependent. In comparison to the extra-vaginal artery (0.7 ± 0.2 mN, n¼ 9; P o0.05), the IVA experienced larger NPY-induced contractions (4.3 ± 0.7 mN, n¼ 25). The endogenous MCH receptor agonist MCH, like NPY, did not cause any contraction in arterial rings at high concentrations (1 mM, Emax¼ 3.7 ± 0.6 mN, n¼ 13), however it did have an impact in vaginal strips (n¼ 8). Rabbit vascular and non-vascular smooth muscle relaxations induced by neuropeptides Pre-contracted vaginal strips were relaxed by VIP (0.1 nM-3 mM) and PACAP 1-27 (0.1 nM-3 mM), as previously demonstrated by Ziessen et al. (2002) (Figure 2).
| Peptide | EC50 (nM) | Slope | Emax (mN) | n |
|---|---|---|---|---|
| AVP | 0.6 ± 0.1 | 1.1 ± 0.1 | 6.3 ± 0.5 | 53 |
| Oxytocin | 76.9 ± 6.4 | 1.4 ± 0.1 | 5.7 ± 0.5 | 49 |
| NPY | 26.9 ± 4 | 0.9 ± 0.1 | 4.3 ± 0.7 | 23 |
| MCH | 41000 | - | 3.7 ± 0.6 | 13 |
Table 2: Neuropeptide-induced contraction in rabbit vaginal artery.
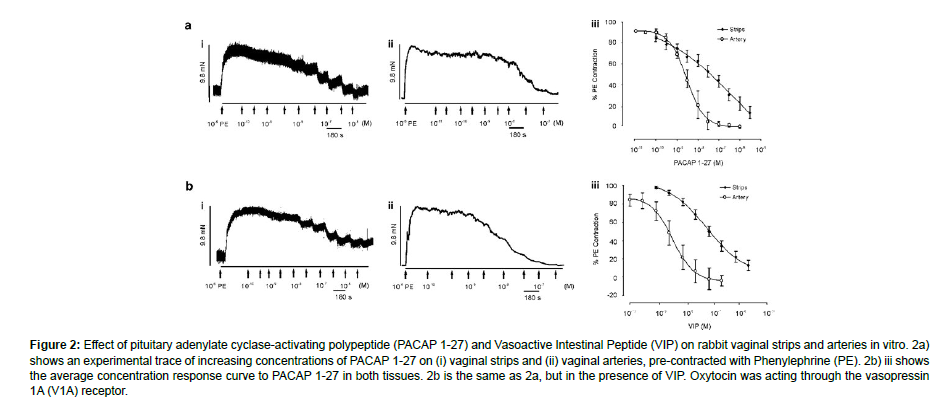
Figure 2: Effect of pituitary adenylate cyclase-activating polypeptide (PACAP 1-27) and Vasoactive Intestinal Peptide (VIP) on rabbit vaginal strips and arteries in vitro. 2a) shows an experimental trace of increasing concentrations of PACAP 1-27 on (i) vaginal strips and (ii) vaginal arteries, pre-contracted with Phenylephrine (PE). 2b) iii shows the average concentration response curve to PACAP 1-27 in both tissues. 2b is the same as 2a, but in the presence of VIP. Oxytocin was acting through the vasopressin 1A (V1A) receptor.
Maximum relaxation was 87 ± 5.3% (n¼ 18) and 95.9 ± 10.5% (PACAP 1-27) respectively.
It’s noticed that tissue strips are not vascular. PACAP 1-38 was unable to relax smooth muscle that is vascular or non-vascular both (n¼ 7; P40.05). Both vaginal strips and arteries relaxed when aMSH, a non-selective melanocortin agonist, was applied; this effect did not depend on concentration and was only observed at 1 mM (36 ± 6.7% (n¼ 12) in vaginal strips and 43.6 ± 10.2% (n¼ 12) in arteries). However, Bremelanotide (1 mM, n¼ 4-5; P40.05), another nonselective melanocortin agonist, showed no impact in determination of the receptor subtype(s) responsible for the contractions in vascular and non-vascular smooth muscle caused by oxytocin and AVP. In order to ascertain if oxytocin was affecting vascular and non-vascular smooth muscle via oxytocin receptors, the strong oxytocin antagonist L-368899 (Ki¼ 3.6 nM); (Thompson et al., 1997) was employed. L-368899 (10- 100 nM) was unable to change the level of oxytocin response curve in small quantities. The effects on the vaginal arteries (oxytocin, EC50) were only observed at high, non-selective, micromolar concentrations. In strips oxytocin EC50 206.4 ± 83.2 nM; oxytocin and 3 mM L-368899 EC50 1.8 ± 0.8 mM, n¼ 10; Po0.05 and 75 ± 3.9 nM; oxytocin and 1 mM L-368899 EC50 300 ± 43 nM, n¼ 10; Po0.01 (Figure 3a). AVP concentration response curves in vaginal arteries (AVP EC50 1.2 ± 0.07 nM; AVP and 1 mM L-368899 EC50 6.2 ± 0.5 nM, n¼ 4, Po0.05) were only marginally affected by 1 mM L-368899, as was the case with oxytocin. However, AVP was unaffected by concentrations up to 3 mM in vaginal strips (n¼ 4) (Figure 3b).
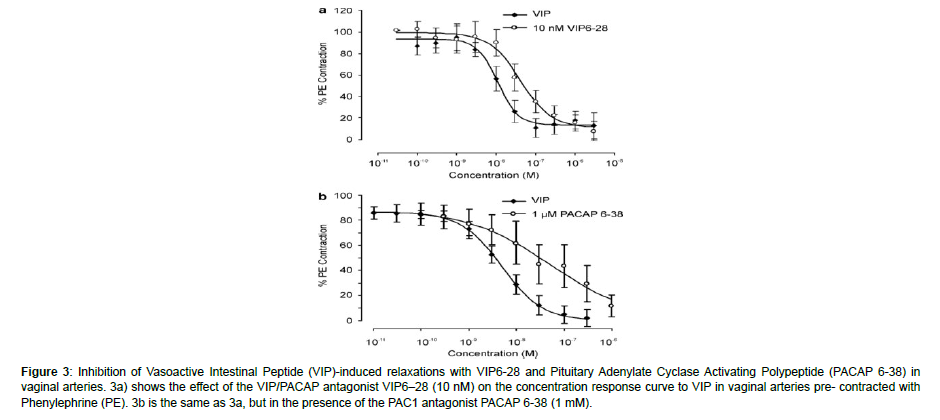
Figure 3: Inhibition of Vasoactive Intestinal Peptide (VIP)-induced relaxations with VIP6-28 and Pituitary Adenylate Cyclase Activating Polypeptide (PACAP 6-38) in vaginal arteries. 3a) shows the effect of the VIP/PACAP antagonist VIP6–28 (10 nM) on the concentration response curve to VIP in vaginal arteries pre- contracted with Phenylephrine (PE). 3b is the same as 3a, but in the presence of the PAC1 antagonist PACAP 6-38 (1 mM).
Finding the receptor subtype that underlies the constriction of the rabbit vaginal arteries caused by NPY
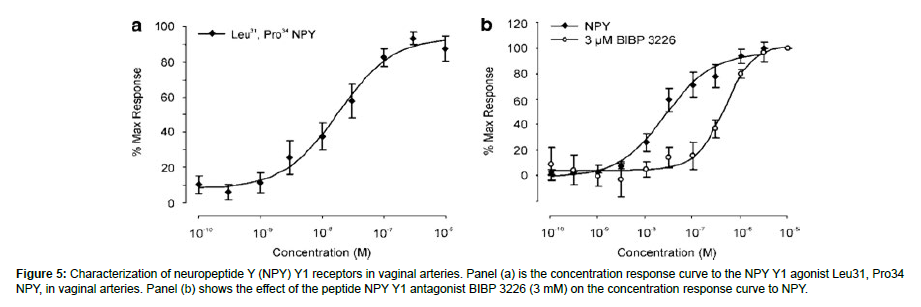
On the rabbit vaginal artery, a variety of NPY selective agonists were evaluated, including the non-selective agonist PYY (3-36), NPY (13-36) (Y2 agonist), GR231118 (Y4 agonist), and [cPP1-7, NPY19-23, Ala31, Aib32, Gln34]-h pancreatic polypeptide (Y5 agonist). None of them were able to cause a contraction (1 mM; n¼ 3-4; P40.05). On the other hand, a concentration-dependent contraction was elicited by the Y1 agonist Leu31, Pro34 NPY. comparable to NPY’s (EC50 38.9 ± 14.1 nM, slope) amplitude Emax 4.5 ± 1.6 mN, n¼ 11, 1.1 ± 0.2) (Figure 4a). To thoroughly look into the existence of NPY Y1 receptors, the IVA-only test for NPY was conducted using the selective peptide NPY Y1 antagonist BIBP 3226 (rabbit pKB¼ 6.98) [29]. The NPY contractile response was competitively reduced by BIBP 3226 (apparent pKB¼ 7, slope 0.7, n¼ 3-9) (Figure 4b). Identification of the receptor subtypes in non-vascular and vascular smooth muscle that underlie the relaxations brought on by VIP and PACAP. In rabbit vaginal strips (n=6; P40.05) and extra-vaginal arteries (n=8; P40.05), the selective peptide PAC1 receptor antagonist PACAP 6-38 (1 mM) and VIP/PACAP antagonist VIP6-28 (10 nM) were unable to prevent either PACAP 1-27 or VIPinduced relaxations. Conversely, in the IVA, the VIP EC50 (n¼ 5-8) (Figure 5) was approximately four times more to the right after the administration of both VIP6-28 (10 nM) and PACAP 6-38 (1 mM).
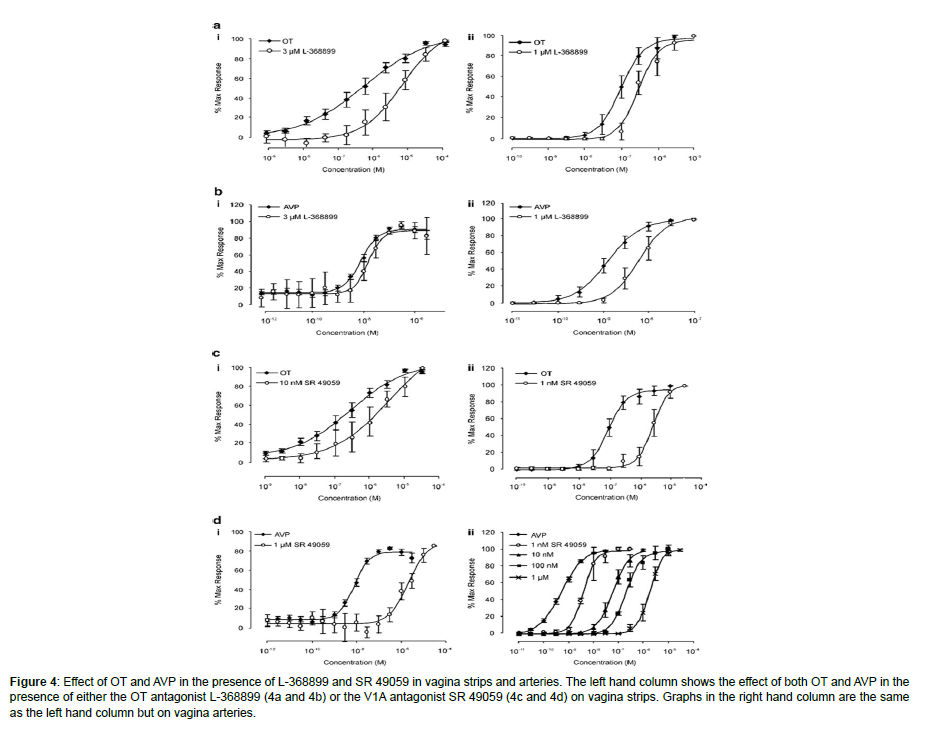
Figure 4: Effect of OT and AVP in the presence of L-368899 and SR 49059 in vagina strips and arteries. The left hand column shows the effect of both OT and AVP in the presence of either the OT antagonist L-368899 (4a and 4b) or the V1A antagonist SR 49059 (4c and 4d) on vagina strips. Graphs in the right hand column are the same as the left hand column but on vagina arteries.
Figure 5: Characterization of neuropeptide Y (NPY) Y1 receptors in vaginal arteries. Panel (a) is the concentration response curve to the NPY Y1 agonist Leu31, Pro34 NPY, in vaginal arteries. Panel (b) shows the effect of the peptide NPY Y1 antagonist BIBP 3226 (3 mM) on the concentration response curve to NPY.
Comments and argument
The research yielded several novel findings, including: (a) AVP and oxytocin contract rabbit vaginal strips and arteries via V1A receptors; (b) AVP and oxytocin have a higher affinity for vaginal artery receptors than non-vascular smooth muscle; and (c) V1A antagonists are equipotent in vascular and non-vascular vaginal wall, (f) via distinct receptor subtypes, PACAP 1-27 and VIP relax pre-contracted vaginal strips and arteries, (g) Vaginal strips are less effective at relaxing both vascular and non-vascular vaginal smooth muscle than PACAP and VIP. (h) aMSH, but not bremelanotide, has a stronger affinity for arteries. Many studies have demonstrated that oxytocin can enhance female receptivity by acting through the oxytocin receptor [30,31]. Furthermore, studies have shown that women’s plasma oxytocin levels vary during their menstrual cycles and are strongly correlated with vaginal lubrication, suggesting that oxytocin plays a peripheral role in the activation of sexual function [32]. On the other hand, oxytocin in this study constricts both arteries and vaginal strips, suggesting that peripheral oxytocin might reduce blood flow. The ineffectiveness of a selective oxytocin antagonist and the low potency of oxytocin suggest that oxytocin does not cause contractions through the oxytocin receptor [33]. Utilizing oestrogenized rabbit vaginal strips, demonstrated that oxytocin only constricted the tissue at micromolar quantities, corroborating these findings. Furthermore, binding tests showed that the rabbit vagina lacks oxytocin receptors. The strong contractile effect of AVP in this investigation and the strong antagonistic action of SR 49059 on both oxytocin and AVP suggest the existence of an anti-arousal V1A receptor. It has recently been demonstrated that prosexual V1A antagonistism exists in the hypothalamus [3]. All of these facts confirm the theorized that V1A antagonists may increase arousal in the sexual center as well as the peripheral.
The anti-sexual properties of NPY, which are released from the forebrain into the hypothalamus, are also widely known. It is still unclear which receptor subtype NPY’s primary activities are caused by. According to research using immunohistochemistry, NPY is abundant in the human vagina and has been demonstrated to alter uterine blood flow peripherally [34,35]. NPY, however, was unable to contract vaginal strips in this investigation. It’s interesting to note that the NPY Y1 receptor allowed NPY to contract vaginal arteries. The IVA showed larger NPY-induced contractions, which might be the result of a rise in NPY Y1 density as the vessel’s size decreases. These findings imply that NPY Y1 antagonists could potentially increase vaginal blood flow.
MCH was found to exhibit unique pharmacology for vascular and non-vascular smooth muscle, since it alone contracted the vaginal arteries. Since rabbits and other non-primate species lack functioning MCH2 receptors, the receptor subtype responsible for the response to MCH is most likely the MCH1 receptor [36,37]. The development of a commercially available selective MCH1 antagonist is awaited for further assessment. The current information runs counter to the idea that central MCH promotes female sexual behavior [12]. Application of exogenous VIP and PACAP 1-27 relaxed the vaginal wall, as previously reported [24]. However, PACAP 1-38 was unable to possess an impact. Additionally, VIP and PACAP 1-27 had much greater power in the IVA in contrast to the vaginal wall, which could be explained by a variation in the receptor subtypes, a higher receptor density, or a different coupling efficiency. A clinical case study demonstrating that VIP improves vaginal blood flow and lubrication in healthy women is supported by the blood vessel data [23]. Although these antagonists were effective in the IVA, the lack of blocking of both agonists with traditional peptide PAC1 and VIP/PACAP antagonists within the vaginal strips may indicate distinct receptor subtypes. New studies in this unique class B receptor family have revealed the existence of several splice variants with distinct signal-transduction pathways [38]. Apart from PAC1/VIP Recent research has demonstrated that PACAP, acting through PAC1 receptors within the hypothalamus, can enhance peripheral sexual arousal through receptor agonism. Both pre-clinically in rats and in ongoing clinical trials including women with female sexual arousal disorder, activation of central melanocortin receptors has been suggested as a means of restoring female sexual arousal. In rat solicitative proceptive copulatory behaviors (hops and darts), the nonselective peptide melanocortin agonist bremelanotide boosted desire, arousal, and satisfaction in proof-of-concept tests (http://www.palatin. com/pdfs/bremelanotide.pdf) [14]. The ability of aMSH to directly relax vascular and non-vascular smooth muscle has never been documented before; however, bremelanotide was unable to replicate this relaxation effect. These contradicting results could be caused by Variations in the effectiveness and potency of melanocortin agents among species. On the other hand, it’s possible that a novel, non-selective mode of action for aMSH has been discovered, which calls for more research.
Conclusion
Gaining a deeper comprehension of the pharmacology and physiology of female sexual arousal is essential to comprehending the pathophysiology behind female sexual dysfunction. Currently, there are two categories of female sexual arousal: physical (genital) and subjective (mental). As with male erectile dysfunction, the pathogenesis of female sexual dysfunction is currently unknown, however it could stem from a psychogenic or centrally mediated deficit like anxiety or depression, or it could be due to a genital deficit. The two possible therapeutic approaches are (a) increasing genital arousal, or increased lubrication and blood flow; and (b) increasing central subjective arousal, or subjective arousal, or enhanced desire. and increased contentment or (c) raise the subjective arousal levels in the genitalia and centrally. Combining the results of this investigation with behavioral data may lead to the suggestion that NPY Y1 and MCH1 antagonists could be useful in reestablishing genital arousal. While melanocortin, VIP/PACAP agonists, V1A and NPY1 antagonists, and MCH and oxytocin agonists may be effective in restoring subjective arousal, V1A antagonists, as well as MCH and oxytocin agonists, may be helpful in restoring both genital and subjective arousal. Due to the lack of knowledge regarding female sexual dysfunction, it might be necessary to test several of these theories on female patients in order to develop a therapy that effectively treats female sexual dysfunction and restores normal sexual function.
References
- Clark JT (1992) Benextramine, a putative neuropeptide Y receptor antagonist, attenuates the termination of receptivity. Physiol Behav 52: 965-969.
- Marin Bivens CL, Kalra SP, Olster DH (1998) Intraventricular injection of neuropeptide Y antisera curbs weight gain and feeding, and increases the display of sexual behaviors in obess Zucker female rats. Regul Pept 76: 327-334.
- Pedersen CA, Boccia ML (2006) Vasopressin interactions with oxytocin in the control of female sexual behavior. Neuroscience 139: 843-851.
- Arletti R, Bertolini A (1985) Oxytocin stimulates lordosis behavior in female rats. Neuropeptides 6: 247-253.
- Caldwell JD, Prange AJ, Pedersen CA (1986) Oxytocin facilitates the sexual receptivity of estrogen-treated female rats. Neuropeptides 7: 175-189.
- Caldwell JD, Jirikowski GF, Greer ER, Pedersen CA (1989) Medial preoptic area oxytocin and female sexual receptivity. Behav Neurosci 103: 655-662.
- Caldwell JD (1992) Central oxytocin and female sexual behavior. Ann N Y Acad Sci 652: 166-179.
- Benelli A, Poggioli R, Luppi P, Ruini L, Bertolini A, et al. (1994) Oxytocin enhances, and oxytocin antagonism decreases, sexual receptivity in intact female rats. Neuropeptides 27: 245-250.
- Pedersen CA, Boccia ML (2002) Oxytocin maintains as well as initiates female sexual behavior: effects of a highly selective oxytocin antagonist. Horm Behav 41: 170-177.
- Gonzalez MI, Vaziri S, Wilson CA (1996) Behavioral effects of alpha- MSH and MCH after central administration in the female rat. Peptides 17: 171-177.
- Cragnolini A, Scimonelli T, Celis ME, Schioth HB (2000) The role of melanocortin receptors in sexual behavior in female rats. Neuropeptides 34: 211-215.
- Gonzalez MI, Baker BI, Hole DR, Wilson CA (1998) Behavioral effects of neuropeptide E-I (NEI) in the female rat: interactions with alpha-MSH, MCH and dopamine. Peptides 19: 1007-1016.
- Nocetto C, Cragnolini AB, Schioth HB, Scimonelli TN (2004) Evidence that the effect of melanocortins on female sexual behavior in preoptic area is mediated by the MC3 receptor; Participation of nitric oxide. Behav Brain Res 153: 537-541.
- Pfaus JG, Shadiack A, Van Soest T, Tse M, Molinoff P (2004) Selective facilitation of sexual solicitation in the female rat by a melano-cortin receptor agonist. Proc Natl Acad Sci USA 101: 10201-10204.
- Park K, Ahn K, Lee S, Ryu S, Park Y, et al. (2001) Decreased circulating levels of estrogen alter vaginal and clitoral blood flow and structure in the rabbit. Int J Impot Res 13: 116-124.
- Park K, Goldstein I, Andry C, Siroky MB, Krane RJ, et al. (1997) Vasculogenic female sexual dysfunction: the hemodynamic basis for vaginal engorgement insufficiency and clitoral erectile insufficiency. Int J Impot Res 9: 27-37.
- Min K, Munarriz R, Berman J, Kim NN, Goldstein I, et al. (2001) Hemodynamic evaluation of the female sexual arousal response in an animal model. J Sex Marital Ther 27: 557-565.
- Kim NN, Min K, Huang YH, Goldstein I, Traish AM (2002) Biochemical and functional characterization of alpha-adrenergic receptors in the rabbit vagina. Life Sci 71: 2909-2920.
- Kim SW, Jeong SJ, Munarriz R, Kim NN, Goldstein I, Traish AM (2003) Role of the nitric oxide-cyclic GMP pathway in regulation of vaginal blood flow. Int J Impot Res 15: 355-361.
- Min K, Munarriz R, Kim NN, Goldstein I, Traish A (2002) Effects of ovariectomy and estrogen and androgen treatment on sildenafil- mediated changes in female genital blood flow and vaginal lubrication in the animal model. Am J Obstet Gynecol 187: 1370-1376.
- Min K, Munarriz R, Yerxa BR, Goldstein I, Shaver SR, et al. (2003) Selective P2Y2 receptor agonists stimulate vaginal moisture in ovariectomized rabbits. Fertil Steril 79: 393-398.
- Sandner P, Hu¨tter J, Tinel H, Ziegelbauer K, Bischoff E (2007) PDE5 inhibitors beyond erectile dysfunction. Int J Impot Res 19: 533-543.
- Levin R (1991) VIP, vagina, clitoral and periurethral glans–an update on human female genital arousal. Exp Clin Endocrinol 98: 61-69.
- Ziessen T, Moncada S, Cellek S (2002) Characterisation of the nonnitregic NANC relaxation responses in the rabbit vaginal wall. Br J Pharmacol 135: 546-554.
- Lew MJ, Angus JA (1995) Analysis of competitive agonist-antagonist interactions by nonlinear regression. Trends Pharmacol Sci 16: 328-337.
- Serradeil-Le Gal C, Wagnon J, Garcia C, Lacour C, Guiraudou P, et al. (1993) Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. J Clin Invest 92: 224-231.
- Thompson KL, Vincent SH, Miller RR, Colletti AE, Alvaro RF, et al. (1997) Pharmacokinetics and disposition of the oxytocin receptor antagonist L-368,899 in rats and dogs. Drug Metab Dispos 25: 1113-1118.
- Blood CH, Shadiack AM, Bernstein JK, Herbert GW (2001) Compositions and methods for treatment of sexual dysfunction WO 2001/000224.
- Doods HN, Weinen W, Eentzeroth M, Rudolf K, Eberlein W, E, et al. (1995) Pharmacological characterization of the selective nonpeptide neuropeptide Y1 receptor antagonist BIBP 3226. JPET 275: 136-142.
- Witt DM, Insel TR (1991) A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinol 128: 3269-3276.
- Caldwell JD, Johns JM, Faggin BM, Senger MA, Pedersen CA (1994) Infusion of an oxytocin antagonist into the medial preoptic area prior to progesterone inhibits sexual receptivity and increases rejection in female rats. Horm Behav 28: 288-302.
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, et al. (2005) Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav 47: 164-169.
- Maggi M, Genazzani AD, Giannini S, Torrisi C, Baldi E, et al. (1988) Vasopressin and oxytocin receptors in vagina, myometrium, and oviduct of rabbits. Endocrinol 122: 2970-2980.
- Tenmoku S, Otteson B, O’Hare MM, Shiekh S, Bardrum B, et al. (1988) Interaction of NPY and VIP in regulation of myometrial blood flow and mechanical activity. Peptides 9: 269-275.
- Jorgensen JC, Sheikh SP, Forman A, Norgard M, Schwartz TW, et al. (1989). Neuropeptide Y in the human female genital tract: localization and biological action. Am J Physiol 257: E220-E227.
- Hoyle CH, Stones RW, Robson T, Whitley K, Burnstock G (1996) Innervation of vasculature and microvasculature of the human vagina by NOS and neuropeptide-containing nerves. J Anat 188: 633-644.
- Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, et al. (2002) Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics 79: 785-792.
- Spengler D, Waeber C, Pantaloni C, Holshoer F, Bockaert J, et al. (1993) Differential signal transduction by five splice variants of the PACAP receptor. Nature 365: 170-175.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Sonwani HP, Kumar N, Verma V, Sen A, Sahu R, et al. (2023)Neuropeptide Pharmacological Profiling on the Rabbit Vaginal Wall and SmoothMuscle of the Vaginal Arteries In Vitro. World J Pharmacol Toxicol 6: 218.
Copyright: © 2023 Sonwani HP, et al. This is an open-access article distributedunder the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided theoriginal author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 818
- [From(publication date): 0-2023 - Feb 23, 2025]
- Breakdown by view type
- HTML page views: 740
- PDF downloads: 78