Review Article Open Access
Neuropathology of Dementia Disorders
Kurt A Jellinger*
Institute of Clinical Neurobiology, Kenyongasse 18, A 1070, Vienna, Austria
- Corresponding Author:
- Kurt A Jellinger
Institute of Clinical Neurobiology
Kenyongasse 18, A 1070, Vienna, Austria
Tel: +43-1-526 65 34
Fax: +43-1-526 65 34
E-mail: kurt.jelllinger@univie.ac.at
Received date: November 28, 2013; Accepted date: January 15, 2014; Published date: January 25, 2014
Citation: Jellinger KA (2014) Neuropathology of Dementia Disorders. J Alzheimers Dis Parkinsonism 4:135. doi: 10.4172/2161-0460.1000135
Copyright: © 2014 Jellinger KA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Dementia, being not a specific disease but a syndrome characterized by deficits in several cognitive domains, is a major public health and socio-economic problem of our century. It is caused by dysfunction/loss of synapses and neurons inducing default neuronal networks. Despite updated concensus criteria for the clinical diagnosis of the major neurodegenerative disorders and new biomarkers, the diagnostic accuracy ranges from 65 to 96% (for Alzheimer’s disease /AD), with a sensitivity versus other dementias of around 85.4% and a specificity of up to 77.7%. Pathologic assessment, using genetic and molecular biological methods, based on homogenous definitions, harmonized interlaboratory and assessment standards, can achieve a classification in up to 95%, without, however, clarifying the etiology of most of these disorders. The new National Institute on Aging-Alzheimer Association guidelines (“ABC” score) for the neuropathologic diagnosis of AD combine β-amyloid plaque phases and Braak neurofibrillary scores, also considering other concomitant pathologies, but do not consider distinct clinico-pathologic subtypes of AD. Revised research criteria are available for dementia with Lewy bodies, Parkinson disease-dementia, frontotemporal lobe degeneration, vascular cognitive impairment, prion diseases, and other degenerative dementias. However, due to overlap between proteinopathies, frequent confounding lesions and co-occurence of multiple pathologies in aged brains, human postmortem studies entail biases that affect both their general applicability and validity. Although most degenerative dementias are incurable at present, prospective studies using validated protocols and data fusion may overcome the limitations of the current diagnostic framework as a basis for future personalized therapy options.
Keywords
Dementia, Diagnostic criteria; Neuropathology; Alzheimer’s disease; Lewy body disease; Neurodegenerative disorders; Proteinopathies; Prion diseases; Mixed pathologies
Introduction
Dementia, encompassing deficits in several cognitive domains that are severe enough to interfere with daily functioning [1], in the new DSM V classification is classified within the broad category of major neurocognitive disorders, proposing specific criteria for the various etiologies [2]. Previously defined as the manifestation of deteriorating brain functions over time due to cell deaths in the brain caused by neurodegeneration or any other disease [3], according to recent research dementia is not primarily caused by neuronal cell death/loss, but by dysfunction and loss of synapses [4] in AD [5] and in α-synucleinopathies [6]. Other causes include cholinergic neuronal and axonal abnormalities [7,8], as well as pre- and postsynaptic cortical cholinergic deficits also occurring in early AD [9]. These changes due to disconnection of major nervous circuitries causing default networks [10-13] have been demonstrated in vivo in early AD [14], suggesting that disease progress is transmitted by neuronal pathways [15]. A prionlike spread of misfolded protein aggregates in the pathogenesis and progression of neurodegeneration is a hot spot of discussion [16-21].
Both the prevalence and incidence of dementia increase exponentially with age. In 2010, 35.6 million people worldwide lived with dementia, with around 8 million new cases every year. Numbers are expected to double or triple every 5-10 years, to 135 millions in 2050, 16 millions in Europe. In 2010, 58% of all demented people lived in low-cost countries, with their proportion anticipated to rise to 71% in 2050 [22]. According to recent data from China the incidence of dementia was 9.87/1000 person years and the median standardised mortality ratio was 1.94:1 [23]. With the disproportional growth of the elderly population, dementia has become a major public health and socio-economic problem that threatens to become the scourge of our century. The total costs for dementia were US$ 604 billion in 2010 worldwide, up to 70% for social care alone [24], total payments for AD for 2013 were expected to be $ 203 billion in USA alone, not included contributions of unpaid caregivers [25].
Clinical Diagnostic Criteria-Diagnostic Challenges
Updated consensus criteria for the clinical diagnosis of the major dementing disorders include: revised NICDS-ADRDA and EFNS criteria for AD [1,26], the National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria for AD [27-30], criteria for Parkinson disease-dementia (PDD) [31,32], dementia with Lewy bodies (DLB) [33], frontotemporal lobe degeneration [34,35], vascular cognitive disorder/dementia NINDS-AIREN DSM-IV [36], and other degenerative dementia syndromes [37].
Assessment of clinical data with fusion of different biomarkers has improved the clinical diagnostic accuracy of AD up to 95%. A metaanalysis of 20 (among 1189) records on the accuracy in distingishing AD from other dementia types and healthy controls using autopsy as standard for truth calculated a sensitivity at 85.4% (95% CI 80.9-90.0%) and a specificity at 77.7% (95% C 70.2-85.1%), both values being slightly higher for imaging procedures than for cerebrospinal fluid (CSF) markers. The study also highlights the limited evidence on autopsyconfirmation and heterogeneity of study design [38]. Combination of the best CSF and magnetic resonance imaging (MRI) data using standardized operating measures allows a more precise diagnostic prediction [39,40], and will be further increased by using multimodal techniques and novel CNS biomarkers [41-47]. The validity of plasma biomarkers for the (preclinical) diagnosis of AD and the relation between CSF ApoE levels and cognitive decline have been reviewed recently [48,49]. Correlation between CSF biomarkers for AD with amyloid plaques and tau pathology was seen in cortical brain biopsies [50], although there are conflicting results with disease progression and biomarker changes [51-53]. Recent data suggest that both “amyloid first” and “neurodegeneration first” biomarker profile pathways to preclinical AD exist [54]. Although biopsy findings in dementia frequently are nonspecific [55], with an overall sensitivity of 65% [56], simulated cerebral biopsies from postmortem brains allowed accurate neuropathologic diagnosis in the majority of neurodegenerative diseases [57].
Identification of fibrillar Aβ in vivo by (11)CPIB PET is feasable for both research and clinical settings [58-61]. Recent evidence comparing amyloid PET studies with postmortem or biopsy results raises doubt about this method as representative of Aβ loads in the living human brain, which may be due to various reasons [62-64]. On the other hand, 55% prevalence of PiB positivity was observed in non-demented subjects aged >80 years, and 85% positivity in the ApoE ε4-positive non-demented elderly subjects [65]. Demonstration that SDS-soluble Aβ measured by immunoassay was better than post mortem PiB binding has important implications for imaging-based biomarkers [66]. In some PiB-negative cases, a combination of pre-existent non- AD pathology and tau-mediated neurodegeneration may be present prior to Aβ pathology [67].
Hippocampal atrophy in the elderly, demonstrated by modern imaging methods and confirmed by postmortem diagnosis of AD [44,68], has been shown to be an important and under-appreciated brain lesion of aging [69].
A review of 2,861 neurodegenerative disease cases of the National Alzheimer’s Coordinating Center Registry (NACCR) showed high diagnostic accuracy for AD (85% sensitivity, 51.1% specificity) and low sensitivity for DLB (32% for pure AD and 12% for AD+DLB) with a specificity over 58% [70]. Evaluation of the accuracy of current clinical diagnostic methods for AD in 919 autopsy cases from the database of the NACC (2005-2010) revealed a sensitivity from 70.9 to 87.3% and a specificity of 44.3 to 70.8%. When the clinical diagnosis was not confirmed by minimum levels of AD pathology, the most frequent primary diagnoses were neurofibrillary tangle-predominant dementia (NFTD), argyrophilic grain disease, frontotemporal lobe degeneration (FTLD), cerebrovascular disease (CVD), Lewy body disease, and hippocampal sclerosis. 39% of these cases met or exceeded minimum threshold levels of AD histopathology [71]. In a recent clinicopathologic restudy of 200 demented brain donors (mean age 78.7 ± 6.9 years, 26% AD, 15.5% mixed dementia, 28% combined diagnoses), the overall agreement between clinical and postmortem diagnoses was 44% (85% for prion disease, 49% for AD), with frequent co-occurrence of multiple pathologies [72]. However, histopathology is still considered to add to premortem diagnostic accuracy [71,73,74]. Clinico-pathologic correlations in the most common neurodegenerative dementias have been summarized recently [75].
This review will discuss the diagnostic validity and limitations of current neuropathologic criteria of neurodegenerative dementias, and give recommendations for future clinico-pathologic research.
Updated Pathologic Diagnostic Criteria
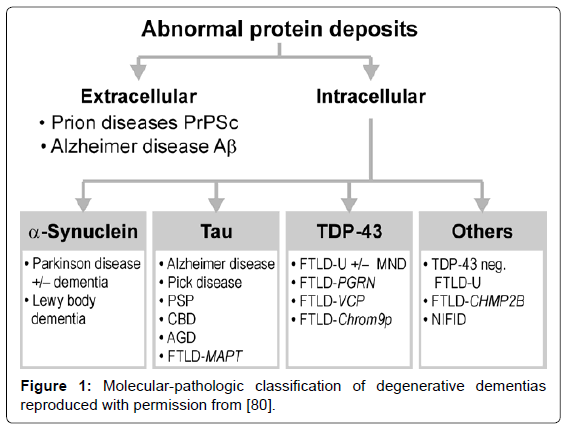
Several guidelines for the neuropathologic diagnosis of major dementing disorders are used, relying on qualitative, semiquantitative, and topographic assessment of morphologic and bio/histochemical markers, in particular specific protein inclusions in neurons, glia and other cells [76-79]. The classification of neurodegenerative disorders, previously based on the anatomical systems involved, has been replaced by molecular-pathologic classification (Figure 1), which may provide a basis for their neuropathologic diagnosis in the future [80].
Alzheimer disease
Neuropathologic criteria for Alzheimer disease (AD), include (1) cut-off quantitative values of senile plaques and tangles [81], (2) their semiquantitative assessment and age-adjustment in the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) protocol [82], (3) topographic staging of neuritic AD pathology [83], re-evaluated recently [84,85], and the progress and distribution of Aβ deposition being different from tau pathology [86].
Standard metrics for tangles and neuritic plaques are usually semiquantitative and, according to the Brain Net Europe consortium, good agreement can be reached in the diagnosis only when the lesions are substantial, having reached isocortical structures (Braak stage V-VI with absolute agreement 91%), while for mild lesions the agreement was poorer (Braak stage I-II, agreement 50%) [84], thereby limiting the ability to make accurate correlation of antemortem cognitive status and morphologic findings [87]. Furthermore, multiple concomitant brain damage contributes to the uncertainty of AD clinicopathologic correlations based only on plaques and tangles [88].
The combination of the CERAD and Braak scores in the National Institute of Aging - Reagan Institute (NIA-RI) criteria relates dementia to AD-typical lesions with high, intermediate and low likelihood [89]. These diagnostic categories apply only to demented individuals. Evaluation of the NIA-RI critera demonstrated their easy use in AD and nondemented subjects, but much less reliability for other dementing disorders. High Braak stages and CERAD criteria identified 54% and 97% of AD cases, respectively, and eliminated between 62 and 100% of nondemented ones with low Braak stages, whereas among non-AD neurodegenerative dementias only between 8 and 42% were identified [90].
Although the sensitivity and specificity of the NIA-RI criteria is suggested to be 90%, only 30 to 57% of the brains of patients with the clinical diagnosis of probable AD show “pure” AD pathology [80]. Thus, their predictive value may be reduced to 38-44% [91]. In a retrospective clinicopathological study of 1,700 demented elderly persons (66% female; MMSE score <20; mean age at death 84.3 ± 6.0 years; 90% over age 70 years), AD-related lesions were present in 83.2%, but pure AD (ABC 3/3/3) without other pathologies in only 41.0%, AD with concomitant pathologies including mixed dementia in 44.8%, vascular dementia in 10.7%, other disorders in 5.5%, and negative pathology in 0.9% [87,90]. Although cognitively normal elderlies may show variable neocortical AD-related pathology, in general, the number of isocortical tangles correlates best with the severity of cognitive impairment [92-94]. The pattern of gray matter loss associated with tangle pathology is an appropriate in vivo surrogate indicator of AD pathology [95]. The predictive value of widespread tau pathology (Braak stages V-VI) for dementia is high [93,94], while others found that both diffuse and neuritic plaques, rather than tangles in neocortical regions distinguish nondemented and AD subjects with high sensitivity and specificity [96]. A recent study found that the amyloid stage that has progressed to involve the striatum is highly predictive of dementia [97]. The cortical Aβ burden usually does not correlate with disease duration and the stage of tau pathology [98].
The recently revised guidelines for the neuropathologic evaluation of AD and other diseases consider levels of AD pathology in the brain regardless of the clinical status of a given individual [99,100]. They include (1) recognition that AD neuropathology may occur in the apparent absence of cognitive impairment, (2) an “ABC” score of AD neuropathologic changes that incorporates histological assessments of Aβ deposits (A), based on the phase assessment of amyloid [86], staging of neurofibrillary tangles/NFT) (B), based on the Braak staging system, and scoring of neuritic plaques, based on semiquantiative assessment in at least five neocortical regions (C), based on CERAD criteria [82] (Table 1), (3) more detailed approaches for assessing co-morbid conditions, such as LB disease, vascular brain injury, hippocampal sclerosis, and TAR DNA binding protein (TDP-43) immunoreactive lesions [100]. Correlations between neuropathologic AD changes with cognitive status were reviewed recently [94]. Testing the revised NIAAA guidelines for the assessment of AD in 390 autopsy cases (including 199 non-demented individuals) distinguished pure AD and non-AD dementia from non-demented cases with a sensitivity of 71% and a specificity of 99%. The sensitivity increased after neuropathologic exclusion of non-AD dementia cases, indicating that cognitive status and assessment according to NIA-AA guidelines appear ideal for distinguishing pure AD from non-AD dementia, preclinical AD and nondemented controls [101].
| Levels of AD neuropathologic changes | |||||
|---|---|---|---|---|---|
| "A" | Thal Phase for AÃÂ? plaques | "B" | Braak and Braak NFT stage | "C" | CERAD score |
| 0 | 0 | 0 | None | 0 | None (neg.) |
| 1 | 1 or 2 | 1 | IÂ Â Â Â Â Â Â Â Â Â Â Â Â or II | 1 | Sparse (A) |
| 2 | 3 | 2 | IIIÂ Â Â Â Â Â Â Â Â Â or IV | 2 | Moderate (B) |
| 3 | 4 or 5 | 3 | VÂ Â Â Â Â Â Â Â Â Â Â Â or VI | 3 | Frequent (C) |
| AD neuropathologic change | B | |||
|---|---|---|---|---|
| A | C | 0 or 1 | 2 | 3 |
| 0 | 0 | Nota | Nota | Nota |
| 1 | 0 or 1 | Low | Low | Lowb |
| 2 or 3f | Low | Intermediate | Intermediatee | |
| 2 | Any C | Lowc | Intermediate | Intermediatee |
| 3 | 0 or 1 | Lowc | Intermediate | Intermediatee |
| 2 or 3 | Lowc | Intermediate | High | |
a High levels of neuritic plaques and low Thal AÃÂ? phase are rare and should prompt reconsideration
b Widespread NFTs with some AÃÂ? plaques but limited neuritic plaques are infrequent
c High levels of AÃÂ? or neuritic plaques with low Braak stage should prompt consideration of co-morbidity, eg, CVLs, etc.
Table 1: "ABC" score for AD neuropathological changes. Modified with premission from [100].
Problems in the neuropathologic diagnosis of Alzheimer’s disease
Histopathologic examination of the brain establishes that ADrelated lesions are present in sufficient densities to distinguish AD from age-related lesions and other dementing disorders [99,100,102]. The current algorithms for the neuropathologic diagnosis of AD are based on (semiquantitative) assessment of plaques and tangles. Despite reasonable interrater agreement when using standardized criteria [84,103-105], no one set of histopathologic criteria for AD has been uniformely accepted by neuropathologists. These algorithms that only considered the classical “plaque and tangle” phenotype of AD did not recognize other subtypes [74,106,107]. Analysis of 1,677 cases with antemortem diagnosis of dementia from the NACCR showed that 82.4% fell into diagnostic “boxes” that are within the consensus recommendations, while the others were “atypical” cases [108]. Posterior cortical atrophy, a clinico-radiologic syndrome considered an atypical variant of AD [109] morphologically shows greater NFT burden in the occipital and parietal lobes and lower in hippocampus [110], while we recently published a 4-repeat tauopathy clinically presenting as posterior cortical atrophy of long duration [111].
The “plaque predominant” type with abundant amyloid plaques, no or very little neuritic pathology restricted to the hippocampus and abnormal phosphorylated tau in neocortical pyramidal cells but lacking overt tangle formation, accounts for 3.5-8% of demented subjects over age 85 [112,113]. Many of them are associated with cortical LBs representing a specific type of DLB or LB variant of AD (LBV/AD) [114]. The “neurofibrillary tangle predominant dementia” (NFTD) with tau pathology often restricted to the limbic system, absence of neuritic plaques, no or very little (diffuse) amyloid plaques and rare amyloid angiopathy accounts for 5-7% of oldest olds with low ApoE ε4 frequency. Recent molecular and genetic analysis confirmed an identical tau isoform composition in TPD and AD [115], and demonstrated absence of soluble Aβ but elevated soluble amyloid precursor protein α (APPα) in brain tissue, and association with the tau gene MAPT H1 haplotype, classifying it as a specific tauopathy independent of amyloid [116].
A recent study of autopsy-proven AD cases separated distinct subtypes: (a) hippocampal-sparing AD (HpSp-AD) with lower NFT counts in hippocampus but more frequent senile plaques compared to typical AD (11%), (b) limbic-predominant forms (LP-AD) with lower cortical NFT counts and tau burden (14%), and (c) typical AD (75%). AD subtypes havd pathologic, demographic, clinical, and genetic differences [117]. HpSP-AD cases were younger at death, while the disease duration in all three types was similar. Additional vascular pathology ranged from 16 to 36%, and Lewy pathology (11-26%) was lowest in HpSP-AD. While MAPT H1H1 genotype was high (~70 %) in NFTD and LP-AD, and similar to typical AD (59%), tau and Aβ burden in frontal cortex were very low in NFTD, differentiating it from AD subtypes, including LP-AD. Significant pathological differences between NFTD and LP-AD suggest that it may not merely be a variant of AD [106]. Volumetric MRI analysis could reliably track the distribution of NFT pathology and predict pathological subtypes of AD [118]. Hippocampal sclerosis of aging showing neuronal loss and gliosis out of proportion of AD-type pathology is a prevalent and highmorbidity brain disease [69,119].
Among 933 autopsy-confirmed AD cases, typical AD accounted for 82.5%, HsSP-AD and LP-AD 8.2 and 8.9%, respectively. Disease duration was lowest in LP-AD cases; age at death highest in typical AD. Additional cerebrovascular pathology was similar in all three types, Lewy pathology most frequent in HpSP-AD form [107] (Table 2). These two studies and one from the NACCR, separating “tangle intensive” and “plaque intensive” cases from classic AD [108] emphasize the need for prospective clinico-pathological studies for further elucidation of various phenotypes of AD.
In addition, considerable phenotypical and morphological differences exist between genetic/familial and sporadic AD [120-123]. In a recent autopsy study, upstream transcription factor 1 (USF1) carriers had lower tangle prevalence among 65+ year olds [124].
Another problem is the relationship between subcortical tau pathology and AD. The stepwise progression of tau pathology in aging and AD is generally assumed to begin in the entorhinal cortex, progressing via the hippocampus to neocortical regions [83]. However, recent studies indicate that tau pathology does not initially manifest in mediotemporal cortex but in selected subcortical nuclei, in particular the locus ceruleus that is suggested to have a distinctive role in the early development of sporadic AD [125,126]. Examination of 239 unselected elderly autopsy cases already in Braak stage 0 showed very sparse (pretangle) tau lesions in 53% in the olfactory bulb and in 44% in locus ceruleus, with increasing prevalence and severity of tau pathology in these nuclei with increasing Braak stages, suggesting that they are increasingly involved during AD progression rather than representing sites initially affected by AD-associated tau pathology [127,128]. Further studies are warranted to elucidate whether tau pathology in the locus ceruleus of young individuals is associated with AD or rather reflects non-specific neuronal damage.
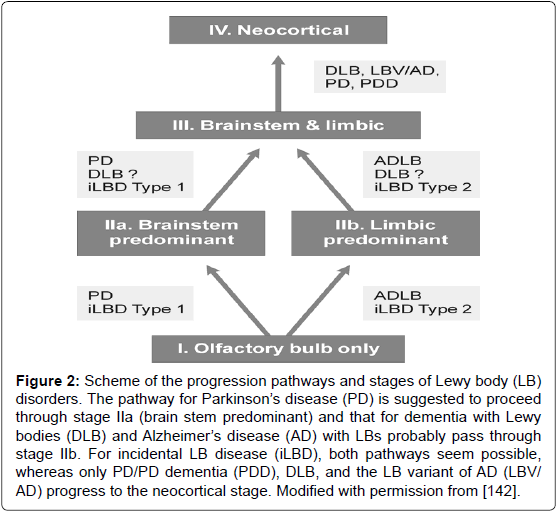
Figure 2: Scheme of the progression pathways and stages of Lewy body (LB) disorders. The pathway for ParkinsonâÂ?Â?s disease (PD) is suggested to proceed through stage IIa (brain stem predominant) and that for dementia with Lewy bodies (DLB) and AlzheimerâÂ?Â?s disease (AD) with LBs probably pass through stage IIb. For incidental LB disease (iLBD), both pathways seem possible, whereas only PD/PD dementia (PDD), DLB, and the LB variant of AD (LBV/ AD) progress to the neocortical stage. Modified with permission from [142].
Diagnostic criteria for other dementias
α-Synucleinopathies, in particular Lewy body disorders - Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) -, a frequent type of dementia in the aged, show a multiorgan distribution of phosporylated αSyn, with earliest involvement of the gastrointestinal tract and olfactory bulb [129-132]. Staging/classification systems based on the semiquantitative assessment of the progression pattern of αSyn pathology [33,133] are suggested to indicate a predictable sequence of lesions [130,131]. Clinico-pathologic studies, although partly confirming this grading system [134], revealed that between 6.3 and 47% of autopsy-proven PD cases did not follow the proposed caudorostral spread of Lewy pathology [132,135-138]. In 7-8.3% of PD cases, the dorsal motor nucleus of vagus was not involved despite definite αSyn inclusions in the higher brainstem or even in cortical regions [137-139]. On the other hand, 30-55% of elderly subjects with widespread/ cortical αSyn pathology had no neuropsychiatric symptoms or were not classifiable [139-141]. Therefore, the criteria for categorization of Lewy-related pathology have been modified (Figure 2). It should be emphasized that Lewy bodies (LB) occur early in the olfactory bulb and usually are present outside of the brain in the autonomic and peripheral system and in many organs [142,143].
PD-dementia (PDD) and DLB, sharing many clinical and morphologic features, are believed to form a continuum within the spectrum of LB diseases [33,144]. Clinically, an arbitrary cut-off is used: if dementia develops first or within one year of PD diagnosis, then DLB is diagnosed, while dementia developing more than one year after PD motor symptoms suggests PD-dementia. The pathologic hallmarks of both phenotypes are αSyn/LB pathology or a variable mixture of LB and AD pathologies, which may act synergistically [145]. The severity and extent of αSyn are scored semiquantitatively in specific brain regions [33,133]. DLB differs from PD-dementia by more severe diffuse amyloid load in the striatum [146,147], more frequent LB affection of the hippocampal CA 2-3 subareas [132], but lack of pedunculopontine cholinergic cell loss that was significant in PD with hallucinations, indicating different patterns of degenerations of cholinergic output structures in PD and DLB [148]. DLB cases had more severe Aβ load than PD-dementia; but no differences in neuritic and αSyn scores, while others reported higher Aβ load in cortical and subcortical areas [145,149], the Aβ load being similar to that in AD [150,151]. Between 10 and 50% of PD-dementia brains had enough AD-like lesions to attain the pathological diagnosis of definite AD [152], but cognitive impairment may also be related to higher Braak LB stages in the absence of significant AD pathology [153]. According to a recent clinico-pathologic study cortical LB/LN pathology is the most significant correlate of dementia in PD, while AD pathology, being abundant in a subset of patients, may modify the clinical phenotype [154]. On the other hand, up to 50% of AD cases exhibit additional LB pathology, which is associated with a more aggressive disease course and accelerated cognitive dysfunction [155].
| Characteristics | Hippocampal-sparing AD (n = 79, 8.2%) | Limbic-predom. AD (n = 85, 8.9%) | Typical AD (n = 769, 82.5%) | P value |
|---|---|---|---|---|
| Women | 59.5% | 64.7%* | 67.5%* | * versus HS P < 0.01 |
| Age at death | 76.3 ± 8.6 | 84.9 ± 3.8* | 81.3 ± 9.2* | * versus HS P < 0.01 |
| Age at onset | 68.0 ± 10.0 | 73.8 ± 6.4* | 79.7 ± 3.8* | P < 0.01; * versus HS <0.001 |
| Disease duration | 7.4 ± 3.6** | 4.8 ± 2.6*** | 9.1 ± 4.3* | ***P < 0.001; ** versus HS P < 0.001 |
| MMSE final (mean) | 10 (n = 20) | 11.5 (n = 16) | 4.6 (n = 78)* | * versus other forms P < 0.01 |
| Braak NFT stage (mean) | 4.5 (2âÂ?Â?5) | 4.5 (3âÂ?Â?5) | 5.6 (5âÂ?Â?6) | |
| Cerebrovascular pathology | 34.2% * | 41.1% | 36.4% * | * versus. LP P < 0.01 |
| Lewy body pathology | 24.9% * | 3.5% | 8.2% | * versus. other forms P < 0.001 |
Table 2: Major characteristics of AD subtypes. Reproduced with permission from [107].
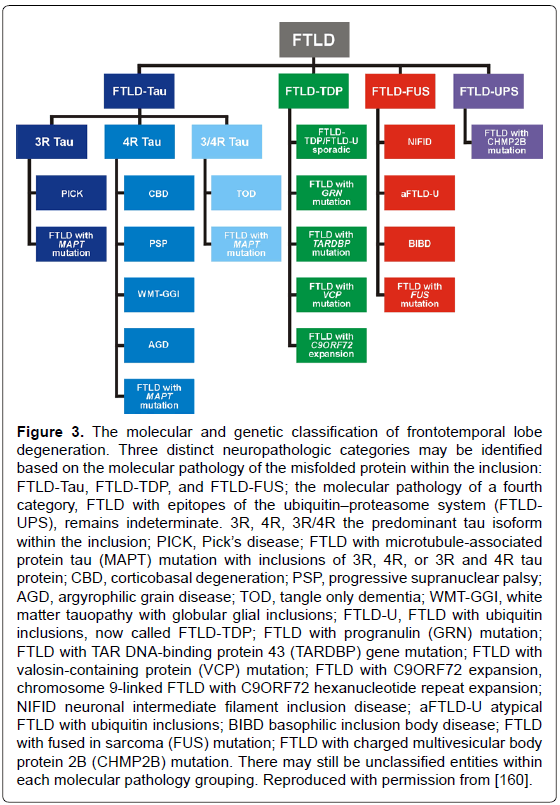
Frontotemporal lobe degeneration (FTLD), the third most frequent cause of dementias, with an estimated prevalence of 15-22/100.000 people aged 46 to 64 years [156] includes three major clinical subgroups: behavior variant (bvFTD), semantic dementia (SD), and progressive nonfluent aphasia (PNFA), showing distinct patterns of progressive brain atrophy [157]. The most common of their molecular correlates according to the predominant protein aggregates are: microtubulus-associated tau protein (FTLD-tau), TAR-DNA binding protein-43 (FTLD-TDP-43), and fusion sarcoma protein (FTLD-FUS), but there are cases with overlapping pathology [35,158-160]. Criteria for their neuropathologic diagnosis based on biochemical markers are summarized in Figure 3. Up to 40% of FTD patients have a familial background, and 30-50% of familial cases show autosomal-dominant traits; furthermore, various gene mutations have been described [159]. The three major genetic causes of FTLD are mutations in MAPT, progranulin (GRN) and C9orf72. The clinical and pathological phenotypes of FTLD with C9orf72 mutations have been reviewed recently [161]. An expanded GGGGCC repeat in a non-coding region of the C9orf72 gene is a common cause of FTLD and amyotrophic lateral sclerosis (ALS) [162]. FTLD in elderly patients presents features of several phenotypes and morphologic subgroups similar to that seen in the presenile group, though patients with MAPT (tau-gene), but not GRN mutations or FUS pathology are rare or even absent [163]. However, these authors found significantly more frequent hippocampal sclerosis but milder frontal atrophy in the elder group [164], which was confirmed in two cases of FTLD tau aged 85 and 88 years, respectively [165]. Inclusions in FTLD-TDP and ALS but not in FTLD-FUS have properties of amyoid [166]. TDP-43 positive inclusions are also observed in cases with AD and LB pathologies [167], and cognitively normal individuals [168], while FUS-immunoreactive intranuclear inclusions occur in various neurodegenerative diseases [169], but it is not clear whether these changes are a primary, secondary or coincidental event [170].
Figure 3: The molecular and genetic classification of frontotemporal lobe degeneration. Three distinct neuropathologic categories may be identified based on the molecular pathology of the misfolded protein within the inclusion: FTLD-Tau, FTLD-TDP, and FTLD-FUS; the molecular pathology of a fourth category, FTLD with epitopes of the ubiquitinâÂ?Â?proteasome system (FTLDUPS), remains indeterminate. 3R, 4R, 3R/4R the predominant tau isoform within the inclusion; PICK, PickâÂ?Â?s disease; FTLD with microtubule-associated protein tau (MAPT) mutation with inclusions of 3R, 4R, or 3R and 4R tau protein; CBD, corticobasal degeneration; PSP, progressive supranuclear palsy; AGD, argyrophilic grain disease; TOD, tangle only dementia; WMT-GGI, white matter tauopathy with globular glial inclusions; FTLD-U, FTLD with ubiquitin inclusions, now called FTLD-TDP; FTLD with progranulin (GRN) mutation; FTLD with TAR DNA-binding protein 43 (TARDBP) gene mutation; FTLD with valosin-containing protein (VCP) mutation; FTLD with C9ORF72 expansion, chromosome 9-linked FTLD with C9ORF72 hexanucleotide repeat expansion; NIFID neuronal intermediate filament inclusion disease; aFTLD-U atypical FTLD with ubiquitin inclusions; BIBD basophilic inclusion body disease; FTLD with fused in sarcoma (FUS) mutation; FTLD with charged multivesicular body protein 2B (CHMP2B) mutation. There may still be unclassified entities within each molecular pathology grouping. Reproduced with permission from [160].
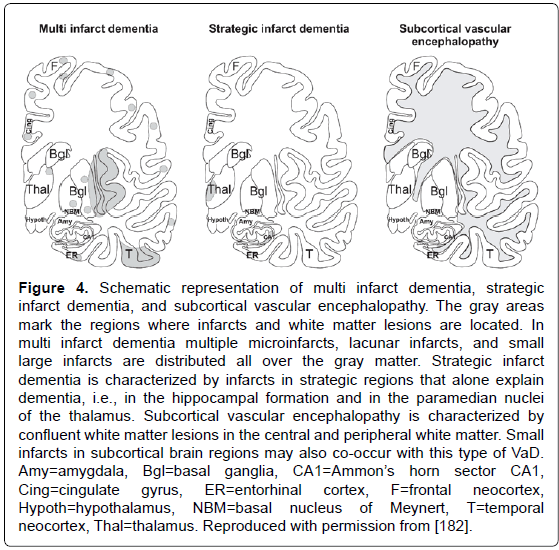
Figure 4: Schematic representation of multi infarct dementia, strategic infarct dementia, and subcortical vascular encephalopathy. The gray areas mark the regions where infarcts and white matter lesions are located. In multi infarct dementia multiple microinfarcts, lacunar infarcts, and small large infarcts are distributed all over the gray matter. Strategic infarct dementia is characterized by infarcts in strategic regions that alone explain dementia, i.e., in the hippocampal formation and in the paramedian nuclei of the thalamus. Subcortical vascular encephalopathy is characterized by confluent white matter lesions in the central and peripheral white matter. Small infarcts in subcortical brain regions may also co-occur with this type of VaD. Amy=amygdala, Bgl=basal ganglia, CA1=AmmonâÂ?Â?s horn sector CA1, Cing=cingulate gyrus, ER=entorhinal cortex, F=frontal neocortex, Hypoth=hypothalamus, NBM=basal nucleus of Meynert, T=temporal neocortex, Thal=thalamus. Reproduced with permission from [182].
Vascular dementias (VaD) or vascular cognitive impairment (VCI) (related to CVD, or more recently vascular cognitive disease), are suggested in 8-15% of demented patients; their prevalence in autopsy series varies from 0.03 to 58% (with means of 8 to 15%). Clinical diagnostic criteria show moderate sensitivity (around 50%) and variable specificity (range 64-98%) [171]. Despite several proposals for a categorization of major cerebrovascular lesions (CVLs) [172-176], a harmonization of the criteria and techniques for the assessment of cerebral lesions of presumable/possible vascular origin in cognitively impaired is necessary [177,178]. Due to the high variability of morphologic findings and multifactorial pathogenesis of vascular cognitive impairment, no generally accepted morphologic scheme for quantitating cerebrovascular lesions and no validated neuropathologic criteria for VaD have been established to date [172,175,179]. The revised NIA-AA guidelines recommend reporting all macroscopic and microscopic CVLs, multiple such lesions being associated with increased likelihood of cognitive impairment [100]. Major types of CVLs are multiple infarct encephalopathy, small vessel and strategic infarct type dementia (microinfarcts in functionally important brain areas interrupting major neuronal circuitries), subcortical lacunes, white matter lesions and microinfarcts (subcortical arteriosclerotic /leuko/ encephalopathy Binswanger) [172,180-182] (Figure 4). A recent staging strategy proposing semiquantitative assessment of CVLs in 4 brain areas with a score from I to IV/VI [183] (Table 3) needs further evaluation.
| Score | Staging |
|---|---|
| Frontal and temporal lobes | |
| 0 | Normal appearance of brain, vessels, white matter, and cortex |
| I | Mild modification of vessel walls, perivascular spaces, or white matter |
| II | Moderate to severe but isolated modification of the vessel walls (arteriolosclerosis or amyloid angiopathy), usually associated with hemosiderin deposits in the perivascular spaces |
| III | Moderate to severe perivascular space dilatations either in the deep or the juxtacortical white matter |
| IV | Moderate to severe myelin loss |
| V | Presence of cortical microinfarcts |
| VI | Presence of large infarcts |
| Hippocampus | |
| 0 | Normal appearance |
| I | Mild modification of vessel walls or perivascular spaces |
| II | Moderate to severe perivascular space dilatations |
| III | Presence of microinfarcts (usually in Ammon horn or the subiculum) |
| IV | Presence of large infarcts |
| Basal ganglia | |
| 0 | Normal appearance |
| I | Mild modification of vessel walls or perivascular spaces |
| II | Moderate to severe perivascular space dilatations |
| III | Presence of microinfarcts |
| IV | Presence of large infarcts |
| Total vascular score | Frontal + temporal lobe + hippocampus + basal ganglia (/20) |
Table 3: Staging of the cerebrovascular lesions. Reproduced with permission from [183].
Mixed type dementia, occurring in 25 to 80% of elderly demented persons, is diagnosed when a combination of several pathologies, e.g. AD with cerebrovascular lesions and/or Lewy pathology, is present [184-187]. CVD and vascular brain injury commonly are encountered in the brains of seniors with and without AD pathology [175,180,184,185], but uniform and reproducible criteria are currently not available. Subcortical CVLs were more frequent in AD patients than in nondemented controls and more frequent in severely demented patients with early onset AD [188].
Prion diseases (transmissible spongiform encephalopathies/TSE) are rare fatal neurodegenerative disorders, with tissue deposition of a misfolded isoform of the cellular prion protein (PrP), referred to as PrPSc [189]. They are classified by both clinicopathologic syndromes and etiology with subclassification according to molecular criteria [190,191]. Human prion diseases are grouped into three etiologic categories [192,193]: (1) Idiopathic forms: sporadic Creutzfeldt-Jakob disease (sCJD) - age at onset 50-78 (mean 60) years, mean duration 5 months, sporadic familial insomnia (sFI), and the recently described variably proteasesensitive prionopathy [194]; (2) inherited (genetic or familial) forms such as Gerstmann-Sträussler-Scheinker disease (GSS), fatal familial insomnia (FFI), octapeptide repeat insert [195,196], rare familial prion disease with AD-like tau pathology [197], genetic CJD associated with the E200K mutation [198], and (3) acquired forms, such as iatrogenic CJD (iCJD), Kuru, and variant CJD (vCJD) (transmission of bovine spongiform encephalopathy/BSE/ to humans) – age at onset 12-74 (mean 27) years, mean duration 14 months [199]. More than 80% of human prion diseases manifest as sCJD with an incidence of 1-2 cases/ million population/year across the world. Around 15% are associated with autosomal dominant pathogenic mutations of the PRNP gene located on the short arm of chromosome 20. To date, more than 37 pathogenic mutations and 29 non-coding polymorphisms have been described. Acquired human prion diseases are rare (only 5% of human prion diseases): transmission of sCJD prions occurred through treatment with pituitary hormones pooled from human cadavers, transplantation of dura mater or cornea, use of contaminated EEG electrodes, and by cannibalism among the Fore linguistic group in Papua New Guinea [200]. vCJD occurred mainly in the UK and in other countries due to human exposure to BSE prions, and more recently in a few cases by transmission of vCJD contaminated blood products [201].
Given the biological and phenotypic heterogeneity of prion diseases, the correct identification or “typing” of the prion strains associated with each case of human TSE has implications for diagnosis, epidemiologic surveillance, and future therapeutics. Either molecular or histopathologic data (or both) may, therefore, provided reliable surrogate markers for strains typing in humans. The current classification of sporadic human prion diseases recognized six major phenotypic subtypes with distinctive clinicopathologic features, which largely correlated at the molecular level with the genotype at the polymeric codon 129 (methionine, M, or valine, V) in the prion protein gene and with the size of the protease-resistent core of the PrPSc. Six different strains of prions according to the 129 PrP genotype have been distinguished showing differerent frequences, age at onset, duration, clinical and morphologic phenotypes. In addition, about 6% are mixed cases [192].
Based on this and other, slightly different definitions [194,202] and on the distribution and severity of histopathologic lesions, a general nomenclature for the diagnosis of sCJD in the absence of molecular data was proposed relating the histopathology lesion (type, location and distributon of PrP deposits and plaques) with molecular types (MM, MV, VV, etc.) [203]). According to a recent inter-rater study among surveillance centers in Europe and USA, the use of this consensus classification of human TSE histotypes allows reliable identification of molecular subtypes with full agreement for the two most common sCJD subtypes and vCJD, with high concordance for all pure phenotypes and the most common subtypes with mixed phenotypic features [203]. The current classification of human prion disease indicates that, besides molecular PrPSc typing, histopathologic analysis permits reliable disease classification with high inter-laboratory accuracy.
Rapidly Progressive and Early Onset Dementias
In contrast to most dementing conditions that typically develop over years, rapidly progressive dementia (RPD) being quickly fatal is an important challenging problem. The differential diagnosis shows a wide range, and in addition to frequent prion diseases includes rapidly progressing neurodegenerative tauopathies and synucleinopathies, autoimmune condition infections, toxic-metabolic and neoplastic diseases [204-206]. According to the US National Prion Disease Pathology Surveillance Center (NPDPSC) in patients with RPD, treatable disorders are frequently mistaken for CJD [207]. A rapidly progressive dementia with thalamic degeneration and cortical prion immunoreactivity but absence of resistent PrP has been reported recently [208].
Among 1,106 brain autopsies of RPD, 32% were negative for prion disease; most frequent were AD (50%) and VaD (12%), while 23% were potentially treatable diseases, eg., immune-mediated, infectious disorders or tumors [207]. In a rapidly progressing form of AD that clinically may mimic CJD, the genetic profile (absence of ApoE ε4 homozygenity and biomarkers) differs from classical AD, suggesting that it might represent a distinct subtype of AD [209].
The diagnosis of young-onset dementia, showing a highly variable epidemiology [210], presents challenges that differ from those of older patient frequently driven by the identification of genetic mutations causing early-onset familial disease [211]. However, despite absence of absolute concordance between clinical phenotype and underlying pathology, they can be distinguished with high accuracy. Among 228 cases with early-onset dementia, 46% were diagnosed with AD and the remaining cases DLB, CJD, VaD, or unclassified dementia. AD was identified with 97% sensitivity and 100% specificity, FTLD with 100% sensitivity and 97% specificity [212]. Non-degenerative non-vascular cases of dementia being more common than expected in patients with a younger onset (30% of younger-onset and 5% of older-onset group aged 70-99 years) include cancer, chronic alcoholism, chronic mental illness, and others [213]. The problem of young-onset dementia was reviewed recently [214].
Neuropathology in Nondemented Elderly Subjects and Pre-Alzheimer Disease
The presence of AD lesions in non-demented aged people may represent AD at a stage prior to clinical expression (presymptomatic, preclinical state) [215-219]. The mechanisms responsible for these changes in non-demented elderly appear similar if not identical to those found in AD [218,220]. The concept of “preclinical AD” pathology has been solidified by in vivo PET scanning, suggesting its high frequency in normal elderly similar to that seen in clinico-pathologic cohorts [42,61,221,222]. These data further suggest that preclinical stages are not static but progressive over time [58,223].
Preclinical AD, in clinical terms, includes all AD biomarkerpositive (CSF tau, Aβ, amyloid imaging) non-demented subjects [224-226] and is associated with future cognitive decline and mortality [227]. According to the neuropathologic definition it includes all nondemented cases with NIA-AA AD pathology. Dissociating clinical symptoms from pathologic findings better allows for investigation of preclinical AD. Although the severity of the pathology, particularly neurofibrillary tangles, has a large role in determining the extent of symptoms, other factors, including age, ApoE status, and comorbidities such as CVD also explain differences in clinical presentation [228].
In non-demented individuals, amyloid and neuritic plaques are usually accompanied by usually mild tangle scores (corresponding to Braak tau stages 0-IV, with a mean ranging from 1.2 to 2.3) and are considered to represent asymptomatic or preclinical AD [101,228,229]. Others reported considerable AD-related pathologies in cognitively intact seniors [216,219,230-232]. However, in the majority of older persons without cognitive impairment, AD-related lesions do not represent the single lesions but are frequently associated with CVD, cortical LBs, and other concomitant pathologies [229,233-237]. A recent comparison between AD, pre-AD and non-AD cases, classified according to current criteria, using neuropathology and detection of soluble, high-molecular-weight Aβ aggregates in a large autopsy series showed elevated Aβ in clinical AD compared to pre-AD and non-AD cases. This suggests that, in addition to more widespread AD-related pathologies, soluble Aβ aggregates in the neuropil play a role in the conversion of Pre-AD to clinical AD. In addition, detection of NFTs, cerebral amyloid angiopathy (CAA) and granulovacuolar degeneration in the absence of amyloid plaques suggests that these lesions may precede Aβ deposition and may represent a pre-amyloid stage of pre-AD not yet considered in current recommendations for the neuropathological diagnosis of AD [101].
Review of the data from National Disease Coordinating Center (NDCC) database and the NUN study emphasized that there may be no documented examples of truly endstage fibrillary pathology with intact cognition [238]. Although in the Adult Changes in Thought (ACT) and NUN studies, non-demented seniors with severe AD pathology (mean age 89 to 91 years) amounted to 8 and 12%, respectively, most of them showed neuritic Braak stage V and frontal NFT count was slightly lower than in a comparable dementia group [231]. However, most of the individuals classified as non-demented in those studies were indeed memory-impaired [231,239]. There is obviously no reported case of truly intact cognition despite severe AD pathology, ie., seniors with widespread neocortical NFTs [94]. Among non-demented elderly 62% demonstrated low and 28% high NFT levels [240], whereas AD cases showed much higher cortical neuritic and striatal amyloid plaque scores [97]. A 90+ study revealed significantly less severe Aβ, αSyn and TP’D-43 pathologies and hippocampal lesions in non-demented subjects, while Aβ distribution showed no essential differences; nondemented individuals had limited hippocampal tau and neocortical Aβ pathology [241]. On the other hand, NFTs in the occipital cortex of 24% of non-demented subjects aged 42-87 years were reported [242].
Thus, mounting evidence supports the view that AD is a continuous spectrum between asymptomatic lesions in cognitively unimpaired seniors and dementia, with mild cognitive impairment (MCI) as a transition between them [243]. Although correlations between cognitive deficits and the severity and extension of plaques and tangles have been found [94], at least in those brains without other pathologies, the distinction between “physiological” (in non-demented subjects) and “pathological” aging (PA) may be difficult. Recent biochemical studies found excessive overlap with only subtle quantitative differences between amyloid levels, peptide profiles, solubility, and oligomeric assemblies in PA and AD brains [244], suggesting that PA represents an initial Aβ prodromal stage of AD. They further suggest that these individuals would develop clinical symptoms, if they live long enough, or have an inherent individual resistence to the toxic effect of β-amyloid [245]. Larger brain and hippocampal values were associated with preserved cognitive function during life despite a high burden of AD pathology [246-248], but the mechanisms that protect from AD are unknown [249-251].
A default hypothesis for AD is that it is part of a “normal aging process”, such that plaques and tangles are secondary to aging or that the primary effect is on synapses and neurons independent of these morphological AD markers. AD is indeed a disease that accompanies human aging, but it is not an inevitable consequence of it [252,253]. However, the suggestion that plaques and NFTs, the morphologic markers of AD, may “cause” this disorder is oversimplified or even wrong, since accumulating evidence indicates that AD pathology represents effect rather than cause or at least a host response to injury, equaling adaptative or neuroprotective reaction [254-256]. A growing body of evidence supports the idea that plaques and NFTs actually define (but not fully represent) the disease process, which involves oxidative stress, neuroinflammation, and many other molecular processes, leading to progressive synaptic, neuronal, and axonal dysfunction and loss [257]. This cascade of pathogenic events is suggested to begin 10 to 20 years before the onset of cognitive decline and other clinical symptoms, but their sequence is still not fully understood [126,258,259].
The AD resiliant group (pathological AD without cognitive impairment) showed preserved densities of synaptophysin-positive presynaptic terminals and dendritic spines compared with the ADdementia group and increased densities of GFAP-positive astrocytes compared with the AD-dementia group and normal controls [246]. Hence, greater amounts of presynaptic proteins and distinct proteinprotein interactions may be components of cognitive reserve reducing the risk of dementia with aging [260]. A recent targeted proteomics study identified a number of factors related to resilience against AD pathology [246]. In conclusion, the aging process that results in loss of synapses and neurons may be far more detrimental for those with little cognitive/brain reserve as compared to those with a high one [261].
Dementia in the Oldest-Old
Neuropathology of AD in the very old demented subjects differs considerably in both intensity and distribution from that in younger age groups [262]. Increased densities of neuritic plaques and NFTs are absent in non-demented patients over age 85-90 [229,263-267], and there is considerable overlap in the pathologies found in demented and non-demented patients [268]. On the other hand, by age 80-85 years, many cognitively unimpaired subjects may have substantial cortical AD pathology [215], while others found significant positive correlation between the extent of dementia and senile plaque density (p=0.011), but not for the NFT density score (p=0.076) [269]. Recent studies suggest that dementia in the oldest-old group (90+ years) is only modestly related to AD, while both cardiovascular and cerebrovascular pathology may cause cognitive impairment in those with low AD pathology scores [175,270,271], and CVLs may contribute to the clinical expression of dementia [272]. However, several clinico-pathologic studies clearly showed that Braak NFT staging remains a significant predictor of cognitive status even in oldest-olds [273,274]. There may be no evidence for some elderly subjects having dementia without an apparent causative morphologic background [270,273], although dementia lacking a known pathologic substrate is extremely rare [275].
In a retrospective clinicopathologic study of 1,100 demented seniors (66% females, MMSE <20; mean age at death 83.3 ± 5.4 SD years) AD pathology increased with highest incidence in the 8th and 9th decade, and slightly decreased after age 90, while the relative prevalence of both AD + minor CVLs and mixed dementia significantly increased with age (7.8 to 32.9% and 0 to 7.%, respectively, p<0.001). VaD showed a continuing age-related decrease from 15.6 to 9.4% (p<0.05), whereas AD + Lewy pathology remained fairly stable (10.3 and 11%). In a prospective study of 180 demented patients (mean age 85 ± 3.4 years), autopsy showed AD in 48%, AD with vascular pathology in 19%, VaD in 11%, DLB in 9%, and dementia of unknown etiology in 13% [270], confirming the notion that a high percentage of demented persons aged 80+ do not meet the pathological criteria of AD or were classified as “dementia of unknown etiology” [97,276,277]. A 90+ study revealed significantly less severe Aβ, αSyn and TP’D-43 pathologies and hippocampal lesions in non-demented subjects, while Aβ distribution showed no esssential differences; non-demented individuals had limited hippocampal tau and neocortical Aβ pathology [241]. Non-AD pathology significantly improved precise differentiation between oldest-old and younger age groups [263].
The Importance of Multiple Pathologies
A major problem is the frequent presence of multiple pathologies in the aged brain that coexist with AD, as CVD, LB pathology, argyrophilic grain disease, hippocampal sclerosis, and others. About two-thirds of aged human brains show non-AD-type neuropathology [229,278-280], which, however, frequently has been missed clinically and could not be identified without neuropathologic examination [72]. Since 50 to 85% of the brains of persons who die aged 80-90+ show appreciable CVLs [281], a specific problem is the impact of CVLs in relation to AD pathology [175,185,237,282-284]. In several autopsy series of very old people, the frequency of AD ranged from 12 to 66%, that of VaD from 9 to 46.8%, that of DLB between 9 and 24%, and that of mixed pathologies between 2 and 86% (!), and was over 40% in a large autopsy series of patients over age 80 [270] (Table 4).
The burden of vascular and AD type pathologies are considered to be independent of each other, and are consistent with an additive or synergistic effect of both types of lesions on cognitive impairment [147,175,185,272,286-291]. The thresholds for vascular and degenerative lesions in distinguishing “pure” VaD or AD from mixed cases have been discussed [274,292,293]. AD pathology alone more frequently accounts for dementia than both microscopic and macroscopic infarcts [294], and in full-blown stages of AD concomitant small vascular lesions do not significantly influence the overall state and progression of cognitive decline, the severity and extent of AD pathology overwhelming the effects of CVD [175,185,295,296]. In a recent autopsy study, global AD pathology significantly correlated to global cognition, whereas infarcts and Lewy bodies did not [234].
| Pathologies [%] | ||||||
|---|---|---|---|---|---|---|
| Author | n | AD lesions | AD alone | AD+ CVL | AD+ LBD | VaD |
| Nolan et al. '98 [285] | 87 | 87 | 50 | 34 | âÂ?Â? | âÂ?Â? |
| Lim et al. '99 [286] | ? | AD cases | 36 | 45 | 22 | âÂ?Â? |
| NUN study - Riley et al. '02 [287] | AD cases | 57 | 73/93 | âÂ?Â? | âÂ?Â? | |
| HAAS study - Petrovitch '05 [281] | 333 | < 60 | 36 | 24 | âÂ?Â? | 24 |
| MRC-CFAS (UK) (Fernando-Ince '04) [268] | 209 (48% dem.) | 70 | 21 | âÂ?Â? | âÂ?Â? | 78 |
| Andin et al. '05 [288] | 175 (clin. VaD) | âÂ?Â? | 72 | âÂ?Â? | 28 | |
| Schneider et al. '07 [237] | 141 | 82.7 | 30 | 38 | 12 | 12 |
| Jellinger '08Â (retrospective) [172] | 1700 (dem.) | 82.9 | 48.0 | 19.0 | 9.1 | 10.7 |
| Jellinger (prospective, unpubl.) | 180 | 82.7 | 48.8 | 23.9 | 10.0 | 7.8 |
| Kovacs et al. '13 [289] (other pathologies 23.2%) | 233 | 100 | 12 | 48.9 | 24 | ? |
Table 4: Mixed pathologies frequent in demented elderly.
The contribution of CVD in neurodegenerative diseased was recently studied in 5715 autopsy cases of the NACC database. For comparison, 210 “unremarkable” cases without cognitive impairment and 280 cases with pure CVD were included. Cases with CVC were older than those without in all groups except for those with hippocampal sclerosis. α-Synucleinopathies, FTLD and prion diseases showed a lower prevalence of coincident CVD than AD patients and those with AD and synucleinopathies revealed a relatively lower burden of their relative lesions then those without CVD in the context of comparable severity of dementia. In conclusion, CVD as a common finding in aged subjects with dementia, is more common in AD than in other neurodegenerative disorders and lowers the threshold for dementia due to AD or synuclein pathologies [282], confirming previous findings [172,175,272,291,293]. Another recent study of 2083 autopsy cases from the NACC database correlating the clinical dementia rate within 2 years before death in 835 subjects showed that the cause of mild to moderate dementia remained uncertain in 14% of the patients. Plaques and tangles independently correlated with cognitive dysfunction and severe small vessel disease, CAA and hippocampal sclerosis were also independently associated with the degree of cognitive impairment, while concomitent CVD strongly correlated with cognitive impairment in the sample selected to represent the AD pathologic continuum, confirming the uncertainty of AD clinicopathologic correlations based only on tangles and plaques [88].
Many studies emphasize multiple confounding pathologies in non-demented elders, in particular CVLs, e.g., small or large cerebral infarctions, lacunes, and white matter lesions in up to almost 10% [230,236,297,298]. Evaluation of 336 cognitively normal (CN) seniors from four studies revealed moderately to frequent neuritic plaque density in 47%, of these 6% also had Braak stages I to VI, medullary, nigral, and cortical Lewy bodies in 15, 8 and 4%, respectively, cerebral microinfarcts in 33%, and high-level microinfarcts in 10%. The burden of brain lesions and comorbidities varied widely within each study but was similar across studies [299].
Among 418 non-demented partizipants of the Religious Order study (mean age 88.5 ± 5.3 years), 35% showed macroscopic infarcts, 8% microinfarcts, 14.8% arteriosclerosis, 5.7% both, only 37.5% being free of CVLs [300]. Up to 75% of CN seniors had various degrees of CAA [298], argyrophilic grains in up to 23% [298], Lewy pathologies in up to 18% [229,230,236,280,301], occasional hippocampal sclerosis [298,301], and mixed pathologies in 7 to 15% [230,280]. Among 100 non-demented elderly, mild, moderate and severe intracranial atherosclerosis was present in 31,17 and 6%, respectively, lacunar state in basal ganglia and/or white matter in 73%, hippocampal sclerosis in 3%, LBs in 5%, tau pathology in brainstem in 60%, and mixed cerebral pathologies in 6%, whereas only 9% were free of CVLs [229]. A recent British non-demented sample (n=53; mean age 81.5 ± 7.4 years) showed maximum score neuritic plaques in 32-49%, NFTs in hippocampus and neocortex in 81 and 30.8%, respectively, white matter lesions in 55-83%, small vessel disease in 45%, infarcts in 13.7%, lacunes in 6%, and cerebral hemorrhages in 10% [302]. A community-based autopsy series from the Viennese VITA study [303] of 233 individuals over 75 years of age (age at death 77-87), in addition to some degree of NFT in 100%. showed Aβ deposits (68.7%), CVLs (48.9%), non-Alzheimer tauopathies (23.2%), TDP proteinopathy (13.3%), and others (inflammation, tumors, etc, 15.1%). Most of these lesions did not increase the probability of the co-occurrence of others, while the number of observed pathologies correlated significantly with AD-neuropathologic changes [289]. A recent cross-sectional study in a community-based sample of 72 cognitively normal older individuals (mean age 74.9 ± 5.7 years) confirmed that a substantial number harbor neurodegeneration without Aβ burden, but association of neurodegenerative lesions with CVD can emerge through non-Aβ pathways within regions most affected by AD [222].
The synergistic interaction between Aβ, tau, and αSyn, accelerating neuropathology and cognitive decline, has been summarized recently [76,304].
Conclusions and Outlook
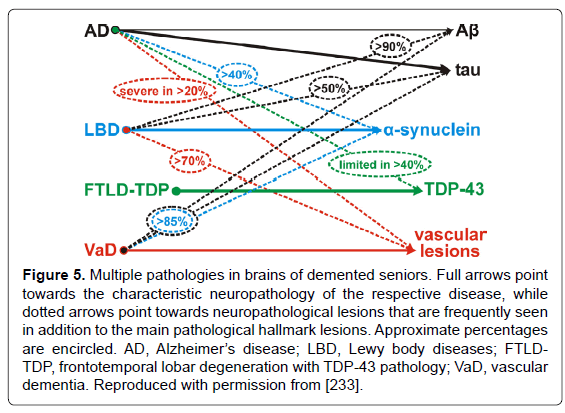
Increases of biochemical, genetic, and experimental approaches are used for refinement of diagnosis and analysis of the relevant contribution of different disease processes to neurodegeneration in AD and other dementias [305]. Since the majority of degenerative dementing disorders are associated with intracellular and/or extracellular deposition of misfolded proteins, most of them can be diagnosed by morphologic and/or molecular identification of these deposits representing characteristic markers or signposts of particular disorders. Algorithms for the molecular-pathologic classification of sporadic (nongenetic/nonhereditary) forms of neurodegenerative dementias have been proposed [77,78,306]. However, due to variable overlap, these changes may fail to distinguish between cognitively intact aged subjects from those with MCI or preclinical or mild AD [4,8,92,307,308]. These latter groups show a wide variety in intensity and pattern of AD-related lesions and other (vascular) pathologies [230,302]. Although they often differ from “normal” aging, only a small proportion of cognitively intact aged subjects are free of AD pathology, while up to 50% may show AD-related changes or even definite AD pathology [215,232,236,297,309]. Additional challenges arise from frequent coexistence with other pathologies that may have an additive or synergistic effects (Figure 5), although their mutual impact often remains unclear. Cohorts with comprehensive neuropathological assessment and multimodal biomarkers are needed to characterize independent predictors for the different neuropathological substrates of cognitive impairment [310].
Figure 5: Multiple pathologies in brains of demented seniors. Full arrows point towards the characteristic neuropathology of the respective disease, while dotted arrows point towards neuropathological lesions that are frequently seen in addition to the main pathological hallmark lesions. Approximate percentages are encircled. AD, AlzheimerâÂ?Â?s disease; LBD, Lewy body diseases; FTLDTDP, frontotemporal lobar degeneration with TDP-43 pathology; VaD, vascular dementia. Reproduced with permission from [233].
Neuropathology using immunohistochemistry, molecular biological and genetic methods can achieve a diagnosis or classification in up to 95%, using homogenous and harmonized definitions and standardized inter-laboratory methods, standards for the assessment of nervous system lesions, and considering exact clinical data. Interdisciplinary projects/ initiatives for the standardized assessment of clinical, neuroimaging, biomarker, and neuropathological data are currently under way [39,42,47,311-316]. In the majority of cases except those with known genetic or metabolic backgrounds, however, pathologic examination may not be able to clarify the causes or etiology of most dementing disorders [74], while some conservative authors emphasized that autopsy examination of well-studied cases of AD and other dementias still has a critical role to play [317]. Therefore, the reliabilty and clinical relevance of the current criteria for the neuropathologic diagnosis of neurodegenerative disorders need better qualification and validation in order to find a way out of the “chaos” regarding histological diagnosis of dementia and their clinical implications [1,87]. Molecular genetics, biochemistry and animal models, at least in part reproducing the morphology of human AD and related disorders, have produced a large body of data on the pathogenesis and pathophysiology of these diseases, showing a complex cascade of events leading from preclinical to fully developed neurodegeneration [318-323]. However, both their molecular backgrounds, basic etiologic factors, pathogenic interrelations of various concomitant pathologies, and their impact on the manifestation of AD need further validation. Harmonized techniques are required to increase the accuracy and reproducibility of neuropathological diagnosis as a basis for further personalized treatment and neuroprotection; an enormous challenge for modern neurosciences.
Acknowledgements
The study was supported in part by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria. The author thanks Mr. E. Mitter-Ferstl, PhD, for secretarial and computer work.
References
- Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, et al. (2010) Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol 9: 1118-1127.
- American Psychiatric Association (2012) DSM-5 Development. American Psychiatric Association accessed 2013-11-26.
- Peng FC (2003) Is dementia a disease? Gerontology 49: 384-391.
- Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ (2011) Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 24: 547-557.
- Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, et al. (2005) Differential loss of synaptic proteins in Alzheimer's disease: implications for synaptic dysfunction. J Alzheimers Dis 7: 103-117.
- Schulz-Schaeffer WJ (2010) The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia. Acta Neuropathol 120: 131-143.
- Geula C, Nagykery N, Nicholas A, Wu CK (2008) Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol 67: 309-318.
- Mufson EJ, Binder L, Counts SE, DeKosky ST, de Toledo-Morrell L, et al. (2012) Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol 123: 13-30.
- Potter PE, Rauschkolb PK, Pandya Y, Sue LI, Sabbagh MN, et al. (2011) Pre- and post-synaptic cortical cholinergic deficits are proportional to amyloid plaque presence and density at preclinical stages of Alzheimer's disease. Acta Neuropathol 122: 49-60.
- McCaffrey P, Fagan T, Landhuis E (2010) Alzheimer research series on the default network. J Alzheimers Dis 19: 747-758.
- Palop JJ, Mucke L (2010) Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci 13: 812-818.
- Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB (2011) Functional network disruption in the degenerative dementias. Lancet Neurol 10: 829-843.
- Schroeter ML, Vogt B, Frisch S, Becker G, Barthel H, et al. (2012) Executive deficits are related to the inferior frontal junction in early dementia. Brain 135: 201-215.
- Wang K, Liang M, Wang L, Tian L, Zhang X, et al. (2007) Altered functional connectivity in early Alzheimer's disease: a resting-state fMRI study. Hum Brain Mapp 28: 967-978.
- Raj A, Kuceyeski A, Weiner M (2012) A network diffusion model of disease progression in dementia. Neuron 73: 1204-1215.
- Costanzo M, Zurzolo C (2013) The cell biology of prion-like spread of protein aggregates: mechanisms and implication in neurodegeneration. Biochem J 452: 1-17.
- Duyckaerts C (2013) Neurodegenerative lesions: seeding and spreading. Rev Neurol (Paris) 169: 825-833.
- Jellinger KA (2013) Commentary on "shining a light on posterior cortical atrophy". Alzheimers Dement 9: 475-476.
- Morales R, Moreno-Gonzalez I, Soto C (2013) Cross-seeding of misfolded proteins: implications for etiology and pathogenesis of protein misfolding diseases. PLoS Pathog 9: e1003537.
- Piccardo P, King D, Telling G, Manson JC, Barron RM (2013) Dissociation of prion protein amyloid seeding from transmission of a spongiform encephalopathy. J Virol 87: 12349-12356.
- Soto C (2012) Transmissible proteins: expanding the prion heresy. Cell 149: 968-977.
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, et al. (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9: 63-75.
- Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, et al. (2013) Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet 381: 2016-2023.
- Wimo A, JÃÂ?¶nsson L, Bond J, Prince M, Winblad B; Alzheimer Disease International (2013) The worldwide economic impact of dementia 2010. Alzheimers Dement 9: 1-11.
- Thies W, Bleiler L; Alzheimer's Association (2013) 2013 Alzheimer's disease facts and figures. Alzheimers Dement 9: 208-245.
- Sorbi S, Hort J, Erkinjuntti T, Fladby T, Gainotti G, et al. (2012) EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol 19: 1159-1179.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, et al. (2011) The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 263-269.
- Reiman EM, McKhann GM, Albert MS, Sperling RA, Petersen RC, et al. (2011) Clinical impact of updated diagnostic and research criteria for Alzheimer's disease. J Clin Psychiatry 72: e37.
- Sarazin M, de Souza LC, LehÃÂ?©ricy S, Dubois B (2012) Clinical and research diagnostic criteria for Alzheimer's disease. Neuroimaging Clin N Am 22: 23-32,viii.
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, et al. (2011) Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 280-292.
- Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, et al. (2007) Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord 22: 2314-2324.
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, et al. (2007) Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 22: 1689-1707.
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, et al. (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65: 1863-1872.
- Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, et al. (2011) Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 122: 137-153.
- Seltman RE, Matthews BR (2012) Frontotemporal lobar degeneration: epidemiology, pathology, diagnosis and management. CNS Drugs 26: 841-870.
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, et al. (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42: 2672-2713.
- Lopez OL, McDade E, Riverol M, Becker JT (2011) Evolution of the diagnostic criteria for degenerative and cognitive disorders. Curr Opin Neurol 24: 532-541.
- Cure S, Abrams K, Belger M, Dell'Agnello G, Happich M (2013) Systematic literature review and meta-analysis of diagnostic test accuracy in Alzheimer's disease and other dementia using autopsy as standard of truth. J Alzheimer's Dis in print.
- Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, et al. (2012) Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement 8: 65-73.
- Zabel M, Schrag M, Mueller C, Zhou W, Crofton A, et al. (2012) Assessing candidate serum biomarkers for Alzheimer's disease: a longitudinal study. J Alzheimers Dis 30: 311-321.
- Engelborghs S, Le Bastard N (2012) The impact of cerebrospinal fluid biomarkers on the diagnosis of Alzheimer's disease. Mol Diagn Ther 16: 135-141.
- Johnson KA, Fox NC, Sperling RA, Klunk WE (2012) Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med 2: a006213.
- Price JC (2012) Molecular brain imaging in the multimodality era. J Cereb Blood Flow Metab 32: 1377-1392.
- Teipel SJ, Wegrzyn M, Meindl T, Frisoni G, Bokde AL, et al. (2012) Anatomical MRI and DTI in the diagnosis of Alzheimer's disease: a European multicenter study. J Alzheimers Dis 31 Suppl 3: S33-47.
- Fagan AM, Perrin RJ (2012) Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer's disease. Biomark Med 6: 455-476.
- Blennow K, Zetterberg H, Fagan AM (2012) Fluid biomarkers in Alzheimer disease. Cold Spring Harb Perspect Med 2: a006221.
- Toledo JB, Korff A, Shaw LM, Trojanowski JQ, Zhang J (2013) CSF ÃÂ?ŽÃÂ?±-synuclein improves diagnostic and prognostic performance of CSF tau and AÃÂ?ŽÃÂ?² in Alzheimer's disease. Acta Neuropathol 126: 683-697.
- Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, et al. (2012) Plasma amyloid-ÃÂ?ŽÃÂ?² as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol 69: 824-831.
- Toledo JB, Da X, Weiner MW, Wolk DA, Xie SX, et al. (2014) CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol .
- SeppÃÂ?¤lÃÂ?¤ TT, Nerg O, Koivisto AM, Rummukainen J, Puli L, et al. (2012) CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology 78: 1568-1575.
- Foster JK, Albrecht MA, Savage G, Lautenschlager NT, Ellis KA, et al. (2013) Lack of reliable evidence for a distinctive epsilon4-related cognitive phenotype that is independent from clinical diagnostic status: findings from the Australian Imaging, Biomarkers and Lifestyle Study. Brain 136: 2201-2216.
- Toledo JB, Xie SX, Trojanowski JQ, Shaw LM (2013) Longitudinal change in CSF Tau and AÃÂ?ŽÃÂ?² biomarkers for up to 48 months in ADNI. Acta Neuropathol 126: 659-670.
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, et al. (2013) Amyloid ÃÂ?ŽÃÂ?² deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 12: 357-367.
- Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, et al. (2013) Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12: 207-216.
- Schott JM, Reiniger L, Thom M, Holton JL, Grieve J, et al. (2010) Brain biopsy in dementia: clinical indications and diagnostic approach. Acta Neuropathol 120: 327-341.
- Josephson SA, Papanastassiou AM, Berger MS, Barbaro NM, McDermott MW, et al. (2007) The diagnostic utility of brain biopsy procedures in patients with rapidly deteriorating neurological conditions or dementia. J Neurosurg 106: 72-75.
- King A, Maekawa S, Bodi I, Troakes C, Curran O, et al. (2013) Simulated surgical-type cerebral biopsies from post-mortem brains allows accurate neuropathological diagnoses in the majority of neurodegenerative disease groups. Acta Neuropathol Commun 1: 53.
- Beckett TL, Webb RL, Niedowicz DM, Holler CJ, Matveev S, et al. (2012) Postmortem Pittsburgh Compound B (PiB) binding increases with Alzheimer's disease progression. J Alzheimers Dis 32: 127-138.
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, et al. (2012) Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-ÃÂ?ŽÃÂ?² plaques: a prospective cohort study. Lancet Neurol 11: 669-678.
- Jack CR Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, et al. (2012) An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol 71: 765-775.
- Villain N, ChÃÂ?©telat G, Grassiot B, Bourgeat P, Jones G, et al. (2012) Regional dynamics of amyloid-ÃÂ?ŽÃÂ?² deposition in healthy elderly, mild cognitive impairment and Alzheimer's disease: a voxelwise PiB-PET longitudinal study. Brain 135: 2126-2139.
- Jack CR Jr, Barrio JR, Kepe V (2013) Cerebral amyloid PET imaging in Alzheimer's disease. Acta Neuropathol 126: 643-657.
- Ikonomovic MD, Abrahamson EE, Price JC, Hamilton RL, Mathis CA, et al. (2012) Early AD pathology in a [C-11]PiB-negative case: a PiB-amyloid imaging, biochemical, and immunohistochemical study. Acta Neuropathol 123: 433-447.
- Kepe V, Moghbel MC, LÃÂ?Â¥ngstrÃÂ?¶m B, Zaidi H, Vinters HV, et al. (2013) Amyloid-ÃÂ?ŽÃÂ?² positron emission tomography imaging probes: a critical review. J Alzheimers Dis 36: 613-631.
- Mathis CA, Kuller LH, Klunk WE, Snitz BE, Price JC, et al. (2013) In vivo assessment of amyloid-ÃÂ?ŽÃÂ?² deposition in nondemented very elderly subjects. Ann Neurol 73: 751-761.
- Niedowicz DM, Beckett TL, Matveev S, Weidner AM, Baig I, et al. (2012) Pittsburgh compound B and the postmortem diagnosis of Alzheimer disease. Ann Neurol 72: 564-570.
- Jack CR Jr, Wiste HJ, Weigand SD, Knopman DS, Lowe V, et al. (2013) Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 81: 1732-1740.
- Dawe RJ, Bennett DA, Schneider JA, Arfanakis K (2011) Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One 6: e26286.
- Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang WX, et al. (2013) Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol 126: 161-177.
- Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, et al. (2010) Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol 257: 359-366.
- Beach TG, Monsell SE, Phillips LE, Kukull W (2012) Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol 71: 266-273.
- EchÃÂ?¡varri C, Burgmans S, Caballero MC, GarcÃÂ?Âa-Bragado F, Verhey FR, et al. (2012) Co-occurrence of different pathologies in dementia: implications for dementia diagnosis. J Alzheimers Dis 30: 909-917.
- Durand-Martel P, Tremblay D, Brodeur C, Paquet N (2010) Autopsy as gold standard in FDG-PET studies in dementia. Can J Neurol Sci 37: 336-342.
- Jellinger KA (2010) Con: Can neuropathology really confirm the exact diagnosis? Alzheimers Res Ther 2: 11.
- Taipa R, Pinho J, Melo-Pires M (2012) Clinico-pathological correlations of the most common neurodegenerative dementias. Front Neurol 3: 68.
- Jellinger KA (2012) Interaction between pathogenic proteins in neurodegenerative disorders. J Cell Mol Med 16: 1166-1183.
- Jellinger KA (2010) Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med 14: 457-487.
- Kovacs GG, Botond G, Budka H (2010) Protein coding of neurodegenerative dementias: the neuropathological basis of biomarker diagnostics. Acta Neuropathol 119: 389-408.
- Arnold SE, Toledo JB, Appleby DH, Xie SX, Wang LS, et al. (2013) Comparative survey of the topographical distribution of signature molecular lesions in major neurodegenerative diseases. J Comp Neurol 521: 4339-4355.
- Jellinger KA (2013) Challenges in the neuropathological diagnosis of dementias. Int J Neuropathol 1: 8-25.
- Duyckaerts C, Dickson DW (2011) Neuropathology of Alzheimer's disease and its variants. In: Dickson DW, Weller RO (eds) Neurodegeneration. The Molecular Pathology of Dementia and Movement Disorders, 2nd Edition. Wiley-Blackwell, pp 62-91.
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, et al. (1991) The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 41: 479-486.
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: 239-259.
- Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, et al. (2008) Staging of neurofibrillary pathology in Alzheimer's disease: a study of the BrainNet Europe Consortium. Brain Pathol 18: 484-496.
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112: 389-404.
- Thal DR, RÃÂ?¼b U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58: 1791-1800.
- Jellinger KA (2011) Criteria for the neuropathological diagnosis of dementing disorders: Routes out of the swamp? In: Braissant O, Wakamatsu H, Liu IKK, Allegaert K, Lenbury Y, Wachholtz A (eds) Recent Researches in Modern Medicine. Proceedings of the WSEAS Intl. Conferences in Cambridge, UK, Feb. 23-25, 2011. WSEAS Press Cambridge, UK, pp 71-97.
- Serrano-Pozo A, Qian J, Monsell SE, Frosch MP, Betensky RA, et al. (2013) Examination of the clinicopathologic continuum of Alzheimer disease in the autopsy cohort of the National Alzheimer Coordinating Center. J Neuropathol Exp Neurol 72: 1182-1192.
- Hyman BT, Trojanowski JQ (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 56: 1095-1097.
- Jellinger KA (2009) Criteria for the neuropathological diagnosis of dementing disorders: routes out of the swamp? Acta Neuropathol 117: 101-110.
- Bowler JV, Munoz DG, Merskey H, Hachinski V (1998) Fallacies in the pathological confirmation of the diagnosis of Alzheimer's disease. J Neurol Neurosurg Psychiatry 64: 18-24.
- Markesbery WR (2010) Neuropathologic alterations in mild cognitive impairment: a review. J Alzheimers Dis 19: 221-228.
- Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, et al. (2007) Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles "do count" when staging disease severity. J Neuropathol Exp Neurol 66: 1136-1146.
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, et al. (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71: 362-381.
- Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, et al. (2008) MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 71: 743-749.
- McKeel DW Jr, Price JL, Miller JP, Grant EA, Xiong C, et al. (2004) Neuropathologic criteria for diagnosing Alzheimer disease in persons with pure dementia of Alzheimer type. J Neuropathol Exp Neurol 63: 1028-1037.
- Beach TG, Sue LI, Walker DG, Sabbagh MN, Serrano G, et al. (2011) Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinicopathological Alzheimer's disease: implications for amyloid imaging. J Alzheimers Dis 28: 869-876.
- Cupidi C, Capobianco R, Goffredo D, Marcon G, Ghetti B, et al. (2010) Neocortical variation of Abeta load in fully expressed, pure Alzheimer's disease. J Alzheimers Dis 19: 57-68.
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 8: 1-13.
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 123: 1-11.
- Thal DR, von Arnim C, Griffin WS, Yamaguchi H, Mrak RE, et al. (2013) Pathology of clinical and preclinical Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci 263 Suppl 2: S137-145.
- Duyckaerts C, Delatour B, Potier MC (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118: 5-36.
- Bancher C, Paulus W, Paukner K, Jellinger K (1997) Neuropathologic diagnosis of Alzheimer disease: consensus between practicing neuropathologists? Alzheimer Dis Assoc Disord 11: 207-219.
- Mirra SS, Gearing M, McKeel DW Jr, Crain BJ, Hughes JP, et al. (1994) Interlaboratory comparison of neuropathology assessments in Alzheimer's disease: a study of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) J Neuropathol Exp Neurol 53: 303-315.
- Nagy Z, Vatter-Bittner B, Braak H, Braak E, Yilmazer DM, et al. (1997) Staging of Alzheimer-type pathology: an interrater-intrarater study. Dement Geriatr Cogn Disord 8: 248-251.
- Janocko NJ, Brodersen KA, Soto-Ortolaza AI, Ross OA, Liesinger AM, et al. (2012) Neuropathologically defined subtypes of Alzheimer's disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol 124: 681-692.
- Jellinger KA (2012) Neuropathological subtypes of Alzheimer's disease. Acta Neuropathol 123: 153-154.
- Nelson PT, Kukull WA, Frosch MP (2010) Thinking outside the box: Alzheimer-type neuropathology that does not map directly onto current consensus recommendations. J Neuropathol Exp Neurol 69: 449-454.
- Crutch SJ, Schott JM, Rabinovici GD, Boeve BF, Cappa SF, et al. (2013) Shining a light on posterior cortical atrophy. Alzheimers Dement 9: 463-465.
- Levine DN, Lee JM, Fisher CM (1993) The visual variant of Alzheimer's disease: a clinicopathologic case study. Neurology 43: 305-313.
- Jellinger KA, Grazer A, Petrovic K, Ropele S, Alpi G, et al. (2011) Four-repeat tauopathy clinically presenting as posterior cortical atrophy: atypical corticobasal degeneration? Acta Neuropathol 121: 267-277.
- Terry RD, Hansen LA, DeTeresa R, Davies P, Tobias H, et al. (1987) Senile dementia of the Alzheimer type without neocortical neurofibrillary tangles. J Neuropathol Exp Neurol 46: 262-268.
- Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J (2004) The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 62: 1984-1989.
- Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, et al. (1990) The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology 40: 1-8.
- Jellinger KA, Attems J (2007) Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol 113: 107-117.
- Santa-Maria I, Haggiagi A, Liu X, Wasserscheid J, Nelson PT, et al. (2012) The MAPT H1 haplotype is associated with tangle-predominant dementia. Acta Neuropathol 124: 693-704.
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, et al. (2011) Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10: 785-796.
- Whitwell JL, Dickson DW, Murray ME, Weigand SD, Tosakulwong N, et al. (2012) Neuroimaging correlates of pathologically defined subtypes of Alzheimer's disease: a case-control study. Lancet Neurol 11: 868-877.
- Brenowitz WD, Monsell SE, Schmitt FA, Kukull WA, Nelson PT (2013) Hippocampal Sclerosis of Aging is a Key Alzheimer's Disease Mimic: Clinical-Pathologic Correlations and Comparisons with both Alzheimer's Disease and Non-Tauopathic Frontotemporal Lobar Degeneration. J Alzheimers Dis.
- Woodhouse A, Shepherd CE, Sokolova A, Carroll VL, King AE, et al. (2009) Cytoskeletal alterations differentiate presenilin-1 and sporadic Alzheimer's disease. Acta Neuropathol 117: 19-29.
- Shepherd C, McCann H, Halliday GM (2009) Variations in the neuropathology of familial Alzheimer's disease. Acta Neuropathol 118: 37-52.
- Maarouf CL, Daugs ID, Spina S, Vidal R, Kokjohn TA, et al. (2008) Histopathological and molecular heterogeneity among individuals with dementia associated with Presenilin mutations. Mol Neurodegener 3: 20.
- Martikainen P, Pikkarainen M, PÃÂ?¶ntynen K, Hiltunen M, Lehtovirta M, et al. (2010) Brain pathology in three subjects from the same pedigree with presenilin-1 (PSEN1) P264L mutation. Neuropathol Appl Neurobiol 36: 41-54.
- Isotalo K, Kok EH, Luoto TM, Haikonen S, Haapasalo H, et al. (2012) Upstream transcription factor 1 (USF1) polymorphisms associate with Alzheimer's disease-related neuropathological lesions: Tampere autopsy study. Brain Pathol 22: 765-775.
- Braak H, Del Tredici K (2011) The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol 121: 171-181.
- Braak H, Del Tredici K (2013) Reply: the early pathological process in sporadic Alzheimer's disease. Acta Neuropathol 126: 615-618.
- Attems J, Thomas A, Jellinger K (2012) Correlations between cortical and subcortical tau pathology. Neuropathol Appl Neurobiol 38: 582-590.
- Attems J, Thal DR, Jellinger KA (2012) The relationship between subcortical tau pathology and Alzheimer's disease. Biochem Soc Trans 40: 711-715.
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, et al. (2010) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119: 689-702.
- Braak H, Del Tredici K, RÃÂ?¼b U, de Vos RA, Jansen Steur EN, et al. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197-211.
- Braak H, Bohl JR, MÃÂ?¼ller CM, RÃÂ?¼b U, de Vos RA, et al. (2006) Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov Disord 21: 2042-2051.
- Jellinger KA (2012) Neuropathology of sporadic Parkinson's disease: evaluation and changes of concepts. Mov Disord 27: 8-30.
- Alafuzoff I, Ince PG, Arzberger T, Al-Sarraj S, Bell J, et al. (2009) Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium. Acta Neuropathol 117: 635-652.
- Kingsbury AE, Bandopadhyay R, Silveira-Moriyama L, Ayling H, Kallis C, et al. (2010) Brain stem pathology in Parkinson's disease: an evaluation of the Braak staging model. Mov Disord 25: 2508-2515.
- Burke RE, Dauer WT, Vonsattel JP (2008) A critical evaluation of the Braak staging scheme for Parkinson's disease. Ann Neurol 64: 485-491.
- Jellinger KA (2008) A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116: 1-16.
- Jellinger KA (2009) A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta 1792: 730-740.
- Parkkinen L, PirttilÃÂ?¤ T, Alafuzoff I (2008) Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 115: 399-407.
- Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG; MRC Cognitive Function, et al. (2008) Patterns and stages of alpha-synucleinopathy: Relevance in a population-based cohort. Neurology 70: 1042-1048.
- Fujishiro H, Ferman TJ, Boeve BF, Smith GE, Graff-Radford NR, et al. (2008) Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J Neuropathol Exp Neurol 67: 649-656.
- Leverenz JB, Hamilton R, Tsuang DW, Schantz A, Vavrek D, et al. (2008) Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol 18: 220-224.
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, et al. (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117: 613-634.
- Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, et al. (2013) The Lewy body in Parkinson's disease and related neurodegenerative disorders. Mol Neurobiol 47: 495-508.
- Goldmann Gross R, Siderowf A, Hurtig HI (2008) Cognitive impairment in Parkinson's disease and dementia with lewy bodies: a spectrum of disease. Neurosignals 16: 24-34.
- Compta Y, Parkkinen L, O'Sullivan SS, Vandrovcova J, Holton JL, et al. (2011) Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain 134: 1493-1505.
- Halliday GM, Song YJ, Harding AJ (2011) Striatal ÃÂ?ŽÃÂ?²-amyloid in dementia with Lewy bodies but not Parkinson's disease. J Neural Transm 118: 713-719.
- Jellinger KA, Attems J (2006) Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol 112: 253-260.
- Hepp DH, Ruiter AM, Galis Y, Voorn P, Rozemuller AJ, et al. (2013) Pedunculopontine cholinergic cell loss in hallucinating Parkinson disease patients but not in dementia with Lewy bodies patients. J Neuropathol Exp Neurol 72: 1162-1170.
- Kalaitzakis ME, Walls AJ, Pearce RK, Gentleman SM (2011) Striatal AÃÂ?ŽÃÂ?² peptide deposition mirrors dementia and differentiates DLB and PDD from other parkinsonian syndromes. Neurobiol Dis 41: 377-384.
- Dugger BN, Serrano GE, Sue LI, Walker DG, Adler CH, et al. (2012) Presence of Striatal Amyloid Plaques in Parkinson's Disease Dementia Predicts Concomitant Alzheimer's Disease: Usefulness for Amyloid Imaging. J Parkinsons Dis 2: 57-65.
- Gomperts SN, Locascio JJ, Marquie M, Santarlasci AL, Rentz DM, et al. (2012) Brain amyloid and cognition in Lewy body diseases. Mov Disord 27: 965-973.
- Sabbagh MN, Adler CH, Lahti TJ, Connor DJ, Vedders L, et al. (2009) Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord 23: 295-297.
- Braak H, RÃÂ?¼b U, Jansen Steur EN, Del Tredici K, de Vos RA (2005) Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64: 1404-1410.
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, et al. (2012) Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 72: 587-598.
- Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM (2010) Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci 30: 7281-7289.
- Deramecourt V, Lebert F, Pasquier F (2013) Frontotemporal dementia. In: Dening T, Thomas A (eds) Oxford Textbook of Old Age Psychiatry (2 ed.). Oxford Univ. Press Oxford, UK, pp 479-490, DOI: 410.1093/med/9780199644957.9780199644003.9780199640036
- Lu PH, Mendez MF, Lee GJ, Leow AD, Lee HW, et al. (2013) Patterns of brain atrophy in clinical variants of frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 35: 34-50.
- Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, et al. (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122: 111-113.
- Goedert M, Ghetti B, Spillantini MG (2012) Frontotemporal dementia: implications for understanding Alzheimer disease. Cold Spring Harb Perspect Med 2: a006254.
- Halliday G, Bigio EH, Cairns NJ, Neumann M, Mackenzie IR, et al. (2012) Mechanisms of disease in frontotemporal lobar degeneration: gain of function versus loss of function effects. Acta Neuropathol 124: 373-382.
- Liu Y, Yu JT, Sun FR, Ou JR, Qu SB, et al. (2013) The clinical and pathological phenotypes of frontotemporal dementia with C9ORF72 mutations. J Neurol Sci 335: 26-35.
- Mizielinska S, Lashley T, Norona FE, Clayton EL, Ridler CE, et al. (2013) C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci. Acta Neuropathol 126: 845-857.
- Baborie A, Griffiths TD, Jaros E, McKeith IG, Burn DJ, et al. (2011) Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol 121: 365-371.
- Baborie A, Griffiths TD, Jaros E, Momeni P, McKeith IG, et al. (2012) Frontotemporal dementia in elderly individuals. Arch Neurol 69: 1052-1060.
- Jellinger KA (2013) Elderly individuals with FTLD. JAMA Neurol 70: 412-413.
- Bigio EH, Wu JY, Deng HX, Bit-Ivan EN, Mao Q, et al. (2013) Inclusions in frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) and amyotrophic lateral sclerosis (ALS), but not FTLD with FUS proteinopathy (FTLD-FUS), have properties of amyloid. Acta Neuropathol 125: 463-465.
- Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, et al. (2009) Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol 117: 125-136.
- Arnold SJ, Dugger BN, Beach TG (2013) TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol 126: 51-57.
- Woulfe J, Gray DA, Mackenzie IR (2010) FUS-immunoreactive intranuclear inclusions in neurodegenerative disease. Brain Pathol 20: 589-597.
- Wilson AC, Dugger BN, Dickson DW, Wang DS (2011) TDP-43 in aging and Alzheimer's disease - a review. Int J Clin Exp Pathol 4: 147-155.
- Jellinger KA (2013) Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci 5: 17.
- Jellinger KA (2008) The pathology of "vascular dementia": a critical update. J Alzheimers Dis 14: 107-123.
- RomÃÂ?¡n GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, et al. (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43: 250-260.
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, et al. (1992) Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology 42: 473-480.
- Jellinger KA (2007) The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol 113: 349-388.
- Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, et al. (2004) Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci 226: 75-80.
- Alafuzoff I, Gelpi E, Al-Sarraj S, Arzberger T, Attems J, et al. (2012) The need to unify neuropathological assessments of vascular alterations in the ageing brain: multicentre survey by the BrainNet Europe consortium. Exp Gerontol 47: 825-833.
- Pantoni L, Sarti C, Alafuzoff I, Jellinger K, Munoz DG, et al. (2006) Postmortem examination of vascular lesions in cognitive impairment: a survey among neuropathological services. Stroke 37: 1005-1009.
- RomÃÂ?¡n GC (2008) The epidemiology of vascular dementia. In: Duyckaerts C, Litvan I (eds) Dementias - Handbook of Clinical Neurology, vol 89 (3rd series). Elsevier Edinburgh, pp 639-658.
- Grinberg LT, Thal DR (2010) Vascular pathology in the aged human brain. Acta Neuropathol 119: 277-290.
- Richardson K, Stephan BC, Ince PG, Brayne C, Matthews FE, et al. (2012) The neuropathology of vascular disease in the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Curr Alzheimer Res 9: 687-696.
- Thal DR, Grinberg LT, Attems J (2012) Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol 47: 816-824.
- Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, et al. (2012) Staging and natural history of cerebrovascular pathology in dementia. Neurology 78: 1043-1050.
- Ferrer I (2010) Cognitive impairment of vascular origin: neuropathology of cognitive impairment of vascular origin. J Neurol Sci 299: 139-149.
- Jellinger KA (2007) The enigma of mixed dementia. Alzheimers Dement 3: 40-53.
- Kovacs GG, Alafuzoff I, Al-Sarraj S, Arzberger T, Bogdanovic N, et al. (2008) Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord 26: 343-350.
- Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, et al. (2012) The natural history of cognitive decline in Alzheimer's disease. Psychol Aging 27: 1008-1017.
- Geppert AM, Wroblewska KA, Przedpelska-Ober EM (2007) Greater frequency of subcortical lesions in severely demented patients with early onset Alzheimer's disease. Alzheimers Dement 3: 54-57.
- Prusiner SB (1998) Prions. Proc Natl Acad Sci U S A 95: 13363-13383.
- Safar JG (2012) Molecular pathogenesis of sporadic prion diseases in man. Prion 6: 108-115.
- Wadsworth JD, Collinge J (2011) Molecular pathology of human prion disease. Acta Neuropathol 121: 69-77.
- Parchi P, Strammiello R, Giese A, Kretzschmar H (2011) Phenotypic variability of sporadic human prion disease and its molecular basis: past, present, and future. Acta Neuropathol 121: 91-112.
- Parchi P, Saverioni D (2012) Molecular pathology, classification, and diagnosis of sporadic human prion disease variants. Folia Neuropathol 50: 20-45.
- Gambetti P, Puoti G, Zou WQ (2011) Variably protease-sensitive prionopathy: a novel disease of the prion protein. J Mol Neurosci 45: 422-424.
- Cochran EJ, Bennett DA, CervenÃÂ?¡kovÃÂ?¡ L, Kenney K, Bernard B, et al. (1996) Familial Creutzfeldt-Jakob disease with a five-repeat octapeptide insert mutation. Neurology 47: 727-733.
- Jansen C, Voet W, Head MW, Parchi P, Yull H, et al. (2011) A novel seven-octapeptide repeat insertion in the prion protein gene (PRNP) in a Dutch pedigree with Gerstmann-Straussler-Scheinker disease phenotype: comparison with similar cases from the literature. Acta Neuropathol 121: 59-68.
- Jayadev S, Nochlin D, Poorkaj P, Steinbart EJ, Mastrianni JA, et al. (2011) Familial prion disease with Alzheimer disease-like tau pathology and clinical phenotype. Ann Neurol 69: 712-720.
- Kovacs GG, Seguin J, Quadrio I, HÃÂ?¶ftberger R, KapÃÂ?¡s I, et al. (2011) Genetic Creutzfeldt-Jakob disease associated with the E200K mutation: characterization of a complex proteinopathy. Acta Neuropathol 121: 39-57.
- Heath CA, Cooper SA, Murray K, Lowman A, Henry C, et al. (2011) Diagnosing variant Creutzfeldt-Jakob disease: a retrospective analysis of the first 150 cases in the UK. J Neurol Neurosurg Psychiatry 82: 646-651.
- Collinge J, Whitfield J, McKintosh E, Beck J, Mead S, et al. (2006) Kuru in the 21st century--an acquired human prion disease with very long incubation periods. Lancet 367: 2068-2074.
- Brandner S (2011) Diversity of prion diseases: (no) strains attached? Acta Neuropathol 121: 1-4.
- Gambetti P, Cali I, Notari S, Kong Q, Zou WQ, et al. (2011) Molecular biology and pathology of prion strains in sporadic human prion diseases. Acta Neuropathol 121: 79-90.
- Parchi P, de Boni L, Saverioni D, Cohen ML, Ferrer I, et al. (2012) Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: an inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol 124: 517-529.
- Geschwind MD, Shu H, Haman A, Sejvar JJ, Miller BL (2008) Rapidly progressive dementia. Ann Neurol 64: 97-108.
- Josephs KA, Ahlskog JE, Parisi JE, Boeve BF, Crum BA, et al. (2009) Rapidly progressive neurodegenerative dementias. Arch Neurol 66: 201-207.
- Capellari S, Strammiello R, Saverioni D, Kretzschmar H, Parchi P (2011) Genetic Creutzfeldt-Jakob disease and fatal familial insomnia: insights into phenotypic variability and disease pathogenesis. Acta Neuropathol 121: 21-37.
- Chitravas N, Jung RS, Kofskey DM, Blevins JE, Gambetti P, et al. (2011) Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann Neurol 70: 437-444.
- Kovacs GG, Peden A, Weis S, HÃÂ?¶ftberger R, Berghoff AS, et al. (2013) Rapidly progressive dementia with thalamic degeneration and peculiar cortical prion protein immunoreactivity, but absence of proteinase K resistant PrP: a new disease entity? Acta Neuropathol Commun 1: 72.
- Schmidt C, HaÃÂ?¯k S, Satoh K, RÃÂ?¡bano A, Martinez-Martin P, et al. (2012) Rapidly progressive Alzheimer's disease: a multicenter update. J Alzheimers Dis 30: 751-756.
- Vieira RT, Caixeta L, Machado S, Silva AC, Nardi AE, et al. (2013) Epidemiology of early-onset dementia: a review of the literature. Clin Pract Epidemiol Ment Health 9: 88-95.
- Rossor MN, Fox NC, Mummery CJ, Schott JM, Warren JD (2010) The diagnosis of young-onset dementia. Lancet Neurol 9: 793-806.
- Snowden JS, Thompson JC, Stopford CL, Richardson AM, Gerhard A, et al. (2011) The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain 134: 2478-2492.
- Knopman DS, Petersen RC, Cha RH, Edland SD, Rocca WA (2006) Incidence and causes of nondegenerative nonvascular dementia: a population-based study. Arch Neurol 63: 218-221.
- Kuruppu DK, Matthews BR (2013) Young-onset dementia. Semin Neurol 33: 365-385.
- Price JL, McKeel DW Jr, Buckles VD, Roe CM, Xiong C, et al. (2009) Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 30: 1026-1036.
- Morris JC, Storandt M, McKeel DW Jr, Rubin EH, Price JL, et al. (1996) Cerebral amyloid deposition and diffuse plaques in "normal" aging: Evidence for presymptomatic and very mild Alzheimer's disease. Neurology 46: 707-719.
- Galvin JE, Powlishta KK, Wilkins K, McKeel DW Jr, Xiong C, et al. (2005) Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol 62: 758-765.
- Troncoso JC, Cataldo AM, Nixon RA, Barnett JL, Lee MK, et al. (1998) Neuropathology of preclinical and clinical late-onset Alzheimer's disease. Ann Neurol 43: 673-676.
- Price JL, Morris JC (1999) Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol 45: 358-368.
- Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, et al. (1998) Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol 55: 1185-1191.
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, et al. (2006) [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67: 446-452.
- Wirth M, Villeneuve S, Haase CM, Madison CM, Oh H, et al. (2013) Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol 70: 1512-1519.
- Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, et al. (2010) Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch Neurol 67: 217-223.
- Driscoll I, Troncoso J (2011) Asymptomatic Alzheimer's disease: a prodrome or a state of resilience? Curr Alzheimer Res 8: 330-335.
- Ewers M, Insel PS, Stern Y, Weiner MW; Alzheimer's Disease Neuroimaging Initiative (ADNI) (2013) Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology 80: 1194-1201.
- Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, et al. (2009) Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol 66: 632-637.
- Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, et al. (2013) Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol 12: 957-965.
- Monsell SE, Mock C, Roe CM, Ghoshal N, Morris JC, et al. (2013) Comparison of symptomatic and asymptomatic persons with Alzheimer disease neuropathology. Neurology 80: 2121-2129.
- Jellinger KA, Attems J (2012) Neuropathology and general autopsy findings in nondemented aged subjects. Clin Neuropathol 31: 87-98.
- Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA (2009) The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 18: 691-701.
- SantaCruz KS, Sonnen JA, Pezhouh MK, Desrosiers MF, Nelson PT, et al. (2011) Alzheimer disease pathology in subjects without dementia in 2 studies of aging: the Nun Study and the Adult Changes in Thought Study. J Neuropathol Exp Neurol 70: 832-840.
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, et al. (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66: 1837-1844.
- Attems J, Jellinger KA (2013) Neuropathology. In: Dening T, Thomas A (eds) Oxford Textbook of Old Age Psychiatry (2 ed.). Oxford Univ. Press Oxford, UK, pp 87-106, DOI: 110.1093/med/9780199644957.9780199644003.9780199640006
- Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA (2012) Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol 72: 599-609.
- Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, et al. (2013) Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol 74: 478-489.
- Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, et al. (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62: 1087-1095.
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69: 2197-2204.
- Abner EL, Kryscio RJ, Schmitt FA, Santacruz KS, Jicha GA, et al. (2011) "End-stage" neurofibrillary tangle pathology in preclinical Alzheimer's disease: fact or fiction? J Alzheimers Dis 25: 445-453.
- Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA (2006) Memory complaints are related to Alzheimer disease pathology in older persons. Neurology 67: 1581-1585.
- Kramer PL, Xu H, Woltjer RL, Westaway SK, Clark D, et al. (2011) Alzheimer disease pathology in cognitively healthy elderly: a genome-wide study. Neurobiol Aging 32: 2113-2122.
- Robinson JL, Geser F, Corrada MM, Berlau DJ, Arnold SE, et al. (2011) Neocortical and hippocampal amyloid-ÃÂ?ŽÃÂ?² and tau measures associate with dementia in the oldest-old. Brain 134: 3708-3715.
- Pikkarainen M, Kauppinen T, Alafuzoff I (2009) Hyperphosphorylated tau in the occipital cortex in aged nondemented subjects. J Neuropathol Exp Neurol 68: 653-660.
- Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, et al. (2009) Mild cognitive impairment: ten years later. Arch Neurol 66: 1447-1455.
- Maarouf CL, Daugs ID, Kokjohn TA, Walker DG, Hunter JM, et al. (2011) Alzheimer's disease and non-demented high pathology control nonagenarians: comparing and contrasting the biochemistry of cognitively successful aging. PLoS One 6: e27291.
- Moore BD, Chakrabarty P, Levites Y, Kukar TL, Baine AM, et al. (2012) Overlapping profiles of AÃÂ?ŽÃÂ?² peptides in the Alzheimer's disease and pathological aging brains. Alzheimers Res Ther 4: 18.
- Arnold SE, Louneva N, Cao K, Wang LS, Han LY, et al. (2013) Cellular, synaptic, and biochemical features of resilient cognition in Alzheimer's disease. Neurobiol Aging 34: 157-168.
- Esiri MM, Chance SA (2012) Cognitive reserve, cortical plasticity and resistance to Alzheimer's disease. Alzheimers Res Ther 4: 7.
- Negash S, Xie S, Davatzikos C, Clark CM, Trojanowski JQ, et al. (2013) Cognitive and functional resilience despite molecular evidence of Alzheimer's disease pathology. Alzheimers Dement 9: e89-95.
- Jellinger KA, Attems J (2013) Neuropathological approaches to cerebral aging and neuroplasticity. Dialogues Clin Neurosci 15: 29-43.
- Erten-Lyons D, Woltjer RL, Dodge H, Nixon R, Vorobik R, et al. (2009) Factors associated with resistance to dementia despite high Alzheimer disease pathology. Neurology 72: 354-360.
- Goh JO, Park DC (2009) Neuroplasticity and cognitive aging: the scaffolding theory of aging and cognition. Restor Neurol Neurosci 27: 391-403.
- Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, et al. (2011) Alzheimer's disease is not "brain aging": neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 121: 571-587.
- Swerdlow RH (2007) Is aging part of Alzheimer's disease, or is Alzheimer's disease part of aging? Neurobiol Aging 28: 1465-1480.
- Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA (2008) Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol 67: 523-531.
- Castellani RJ, Zhu X, Lee HG, Smith MA, Perry G (2009) Molecular pathogenesis of Alzheimer's disease: reductionist versus expansionist approaches. Int J Mol Sci 10: 1386-1406.
- Castellani RJ, Lee HG, Siedlak SL, Nunomura A, Hayashi T, et al. (2009) Reexamining Alzheimer's disease: evidence for a protective role for amyloid-beta protein precursor and amyloid-beta. J Alzheimers Dis 18: 447-452.
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1: a006189.
- Braak H, Zetterberg H, Del Tredici K, Blennow K (2013) Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-ÃÂ?ŽÃÂ?² changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol 126: 631-641.
- Mann DM, Hardy J (2013) Amyloid or tau: the chicken or the egg? Acta Neuropathol 126: 609-613.
- Honer WG, Barr AM, Sawada K, Thornton AE, Morris MC, et al. (2012) Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry 2: e114.
- Stern Y (2012) Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 11: 1006-1012.
- von Gunten A, KÃÂ?¶vari E, Rivara CB, Bouras C, Hof PR, et al. (2005) Stereologic analysis of hippocampal Alzheimer's disease pathology in the oldest-old: evidence for sparing of the entorhinal cortex and CA1 field. Exp Neurol 193: 198-206.
- Middleton LE, Grinberg LT, Miller B, Kawas C, Yaffe K (2011) Neuropathologic features associated with Alzheimer disease diagnosis: age matters. Neurology 77: 1737-1744.
- Haroutunian V, Schnaider-Beeri M, Schmeidler J, Wysocki M, Purohit DP, et al. (2008) Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol 65: 1211-1217.
- Nelson PT, Braak H, Markesbery WR (2009) Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol 68: 1-14.
- Prohovnik I, Perl DP, Davis KL, Libow L, Lesser G, et al. (2006) Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology 66: 49-55.
- Polvikoski T, Sulkava R, Rastas S, Sutela A, NiinistÃÂ?¶ L, et al. (2006) Incidence of dementia in very elderly individuals: a clinical, neuropathological and molecular genetic study. Neuroepidemiology 26: 76-82.
- Fernando MS, Ince PG; MRC Cognitive Function and Ageing Neuropathology Study Group (2004) Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci 226: 13-17.
- Purohit D, Batheja N, Haroutunian V, Sano M, Grossman H, et al. (2008) A clinicopathological correlation study of cognitive status and senile plaques and neurofibrillary tangles in clinically well-characterized individuals of extreme old age (abstract). J Neuropath Exp Neurol 67: 494
- Jellinger KA, Attems J (2010) Prevalence and pathology of vascular dementia in the oldest-old. J Alzheimers Dis 21: 1283-1293.
- Laukka EJ, Fratiglioni L, BÃÂ?¤ckman L (2010) The influence of vascular disease on cognitive performance in the preclinical and early phases of Alzheimer's disease. Dement Geriatr Cogn Disord 29: 498-503.
- Strozyk D, Dickson DW, Lipton RB, Katz M, Derby CA, et al. (2010) Contribution of vascular pathology to the clinical expression of dementia. Neurobiol Aging 31: 1710-1720.
- Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, et al. (2010) Age, Alzheimer's disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain 133: 2225-2231.
- Sinka L, KÃÂ?¶vari E, Gold G, Hof PR, Herrmann FR, et al. (2010) Small vascular and Alzheimer disease-related pathologic determinants of dementia in the oldest-old. J Neuropathol Exp Neurol 69: 1247-1255.
- Jicha GA, Saligram U, Abner EL, Van Eldik L, Nelson PT (2010) Dementia lacking a known pathologic substrate: results from the University of Kentucky Alzheimer's Disease Center Brain Bank (abstr.). Ann Neurol 68, Suppl 14: S48
- Crystal HA, Dickson D, Davies P, Masur D, Grober E, et al. (2000) The relative frequency of "dementia of unknown etiology" increases with age and is nearly 50% in nonagenarians. Arch Neurol 57: 713-719.
- Jellinger K (2001) Frequency of "dementia of unknown etiology" increases with age. Arch Neurol 58: 1498-1499.
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, et al. (2010) Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol 20: 66-79.
- Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, et al. (1997) The effects of additional pathology on the cognitive deficit in Alzheimer disease. J Neuropathol Exp Neurol 56: 165-170.
- White L (2009) Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis 18: 713-725.
- Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, et al. (2005) AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol 57: 98-103.
- Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, et al. (2013) Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain 136: 2697-2706.
- Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, et al. (2006) Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol 60: 677-687.
- Giannakopoulos P, Gold G, KÃÂ?¶vari E, von Gunten A, Imhof A, et al. (2007) Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol 113: 1-12.
- Nolan KA, Lino MM, Seligmann AW, Blass JP (1998) Absence of vascular dementia in an autopsy series from a dementia clinic. J Am Geriatr Soc 46: 597-604.
- Lim A, Tsuang D, Kukull W, Nochlin D, Leverenz J, et al. (1999) Clinico-neuropathological correlation of Alzheimer's disease in a community-based case series. J Am Geriatr Soc 47: 564-569.
- Riley KP, Snowdon DA, Markesbery WR (2002) Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol 51: 567-577.
- Andin U, Gustafson L, Passant U, Brun A (2005) A clinico-pathological study of heart and brain lesions in vascular dementia. Dement Geriatr Cogn Disord 19: 222-228.
- Kovacs GG, Milenkovic I, WÃÂ?¶hrer A, HÃÂ?¶ftberger R, Gelpi E, et al. (2013) Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol 126: 365-384.
- Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR (2008) AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging 29: 1587-1590.
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA (2004) Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 62: 1148-1155.
- Gold G, Giannakopoulos P, Herrmann FR, Bouras C, KÃÂ?¶vari E (2007) Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain 130: 2830-2836.
- Zekry D, Duyckaerts C, Belmin J, Geoffre C, Herrmann F, et al. (2003) The vascular lesions in vascular and mixed dementia: the weight of functional neuroanatomy. Neurobiol Aging 24: 213-219.
- Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, et al. (2008) Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol 64: 168-176.
- Lee JH, Olichney JM, Hansen LA, Hofstetter CR, Thal LJ (2000) Small concomitant vascular lesions do not influence rates of cognitive decline in patients with Alzheimer disease. Arch Neurol 57: 1474-1479.
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS (2005) Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 64: 834-841.
- Davis DG, Schmitt FA, Wekstein DR, Markesbery WR (1999) Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol 58: 376-388.
- Jentoft M, Parisi J, Dickson D, Johnson K, Boeve B, et al. (2011) Neuropathologic findings in 32 nondemented elderly subjects (abs.). J Neuropathol Exp Neurol 70: 531
- Sonnen JA, Santa Cruz K, Hemmy LS, Woltjer R, Leverenz JB, et al. (2011) Ecology of the aging human brain. Arch Neurol 68: 1049-1056.
- Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA (2011) Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke 42: 3183-3189.
- Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, et al. (2000) "Preclinical" AD revisited: neuropathology of cognitively normal older adults. Neurology 55: 370-376.
- Stephan BC, Matthews FE, Ma B, Muniz G, Hunter S, et al. (2012) Alzheimer and vascular neuropathological changes associated with different cognitive States in a non-demented sample. J Alzheimers Dis 29: 309-318.
- Fischer P, Jungwirth S, Krampla W, Weissgram S, Kirchmeyr W, et al. (2002) Vienna Transdanube Aging "VITA": study design, recruitment strategies and level of participation. J Neural Transm Suppl : 105-116.
- Colom-Cadena M, Gelpi E, Charif S, Belbin O, Blesa R, et al. (2013) Confluence of ÃÂ?ŽÃÂ?±-synuclein, tau, and ÃÂ?ŽÃÂ?²-amyloid pathologies in dementia with Lewy bodies. J Neuropathol Exp Neurol 72: 1203-1212.
- Marchesi VT (2012) Alzheimer's disease 2012: the great amyloid gamble. Am J Pathol 180: 1762-1767.
- Dickson DW (2009) Neuropathology of non-Alzheimer degenerative disorders. Int J Clin Exp Pathol 3: 1-23.
- Jicha GA, Abner EL, Schmitt FA, Kryscio RJ, Riley KP, et al. (2012) Preclinical AD Workgroup staging: pathological correlates and potential challenges. Neurobiol Aging 33: 622.
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, et al. (2006) Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 63: 665-672.
- Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology 42: 1681-1688.
- Toledo JB, Cairns NJ, Da X, Chen K, Carter D, et al. (2013) Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun 1: 65.
- Cairns NJ, Taylor-Reinwald L, Morris JC; Alzheimer's Disease Neuroimaging Initiative (2010) Autopsy consent, brain collection, and standardized neuropathologic assessment of ADNI participants: the essential role of the neuropathology core. Alzheimers Dement 6: 274-279.
- Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, et al. (2010) The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement 6: 221-229.
- Trojanowski JQ, Vandeerstichele H, Korecka M, Clark CM, Aisen PS, et al. (2010) Update on the biomarker core of the Alzheimer's Disease Neuroimaging Initiative subjects. Alzheimers Dement 6: 230-238.
- Frisoni GB (2010) Alzheimer's disease neuroimaging initiative in Europe. Alzheimers Dement 6: 280-285.
- Weiner MW, Aisen PS, Jack CR Jr, Jagust WJ, Trojanowski JQ, et al. (2010) The Alzheimer's disease neuroimaging initiative: progress report and future plans. Alzheimers Dement 6: 202-211.
- Jack CR Jr (2012) Alzheimer disease: new concepts on its neurobiology and the clinical role imaging will play. Radiology 263: 344-361.
- Esiri MM (2010) Pro: Can neuropathology really confirm the exact diagnosis? Alzheimers Res Ther 2: 10.
- Balducci C, Forloni G (2011) APP transgenic mice: their use and limitations. Neuromolecular Med 13: 117-137.
- de Calignon A, Polydoro M, SuÃÂ?¡rez-Calvet M, William C, Adamowicz DH, et al. (2012) Propagation of tau pathology in a model of early Alzheimer's disease. Neuron 73: 685-697.
- GÃÂ?¶tz J, GÃÂ?¶tz NN (2009) Animal models for Alzheimer's disease and frontotemporal dementia: a perspective. ASN Neuro 1.
- Hunter JM, Bowers WJ, Maarouf CL, Mastrangelo MA, Daugs ID, et al. (2011) Biochemical and morphological characterization of the AÃÂ?ŽÃÂ?²PP/PS/tau triple transgenic mouse model and its relevance to sporadic Alzheimer's disease. J Alzheimers Dis 27: 361-376.
- Leon WC, Canneva F, Partridge V, Allard S, Ferretti MT, et al. (2010) A novel transgenic rat model with a full Alzheimer's-like amyloid pathology displays pre-plaque intracellular amyloid-beta-associated cognitive impairment. J Alzheimers Dis 20: 113-126.
- Petiet A, Delatour B, Dhenain M (2011) Models of neurodegenerative disease - Alzheimer's anatomical and amyloid plaque imaging. Methods Mol Biol 771: 293-308.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 21352
- [From(publication date):
February-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 16335
- PDF downloads : 5017