Review Article Open Access
Neurological Manifestations of Bartonellosis in Immunocompetent Patients: A Composite of Reports from 2005��?2012
E. B. Breitschwerdt1,* S. Sontakke1,2 and S. Hopkins3
1Intracellular Pathogens Research Laboratory, Center for Comparative Medicine and Translational Research, North Carolina State University, Raleigh, NC 27607, USA
2Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607, USA
3Department of Neurology, Nemours/Alfred I. duPont Hospital for Children, Wilmington, DE 19803, USA
- *Corresponding Author:
- E. B. Breitschwerd
Intracellular Pathogens Research Laboratory
Center for Comparative Medicine and Translational
Research, North Carolina State University
Raleigh, NC 27607, USA
E-mail: ed breitschwerdt@ncsu.edu
Received Date: 8 October 2012 Revised Date: November 2012 Accepted Date: 4 November 2012
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
In recent years, an increasing number of Bartonella species have been identified as zoonotic pathogens, transmitted by animal bites, scratches or by arthropods. Although historically the term bartonellosis was attributed to infections with Bartonella bacilliformis, transmitted by sandflies in the Peruvian Andes, a more inclusive medical use of this term now includes infections caused by any Bartonella sp., anywhere in the world. Potentially, because Bartonella spp. can infect erythrocytes, endothelial cells, and various macrophage-type cells, including brain-derived dendritic cells in vitro, the clinical and pathological manifestations of bartonellosis appear to be very diverse. The purpose of this review is to focus attention on neurological bartonellosis cases reported in immunocompetent patients since 2005. Among these patients, disease course has varied substantially in length and severity, including one fatal case of encephalitis in a child. Based upon the evolving literature, a high clinical index of suspicion is warranted.
Keywords
Bartonella; zoonosis; encephalopathy
Introduction
The genus Bartonella is comprised of a rapidly increasing number of important pathogenic species that induce a spectrum of animal and human diseases, including seemingly diverse neurological abnormalities. These fastidious, hemotropic, Gram-negative bacteria are mainly transmitted by arthropod or insect vectors [25,26]. Most Bartonella spp. have evolved to infect one or more mammalian reservoir hosts, within which the bacteria often cause a long lasting intra-erythrocytic bacteremia [11]. During the past decade, there has been a rapid escalation in knowledge relative to those animals that harbor Bartonella, the vectors that transmit these bacteria among animals and people, and their pathogenic potential, particularly in pets and people. Infection with one or more Bartonella sp. has been associated with a wide range of disease manifestations; including granulomatous lymphadenopathy, fever of unknown origin, endocarditis, neurological and ophthalmological syndromes, Carrion’s disease, trench fever, cat scratch disease (CSD), osteomyelitis, myalgia, peliosis hepatis, splenomegaly and splenic rupture [26] (Table 1), and epithelioid hemangioendothelioma [18,51].
| Bartonella | Primary reservoir | Vector | Accidental host(s) and(human disease) |
|---|---|---|---|
| Bartonella alsatica | (Rabbit Oryctolagus cuniculus) | (Rabbit flea? Spilopsyllus cuniculi) | Human (endocarditis [59]) |
| Bartonella bacilliformis | Human | (Sandfly Lutzomia verrucarum) | None (Carrion’s disease,Oroya fever, verruga peruna) |
| Bartonella clarridgeiae | (Cat Felis catus) | (Cat flea Ctenocephalides felis) | Human, dog (cat scratch disease) |
| Bartonella elizabethae | (Rat Rattus norvegicus) | (Oriental rat flea Xenopsylla cheopis) | Human, dog (endocarditis,neuroretinitis) |
| Bartonella grahamii | (Wild mice agrestris,Apodemus Clethrionomysglareolus, Microtus flavicollis) | Rodent fleas | Human (neuroretinitis) |
| Bartonella henselae | (Cat Felis catus) | (Cat flea Ctenocephalides felis) | Human, dog, horse, marine animals(cat scratch disease, bacillaryangiomatosis, endocarditis,neuroretinitis, bacteraemia, epitheliodhemangioendothelioma) [51] |
| Bartonella koehlerae | Cat | Cat flea | Human, dog (endocarditis) [2] |
| Bartonella melophagi | (Sheep Ovis aries) | (Sheep ked Melophagus ovinus) | Human [46] |
| Bartonella quintana | Human | (Body louse Pediculus humanis) | Cat, dog (endocarditis, trench fever,bacillary angiomatosis) |
| Bartonella rochalimae | Canids | (Fleas? Pulex irritans, Pulex simulans) | Human, dog (bacteraemia, fever) |
| Bartonella tamiae | (Rat?) unknown | Mites? Ticks? | Humans (bacteraemia, fever [41]) |
| Bartonella vinsonii arupensis | (White-footed mousePeromyscus leucopus) | (Fleas? ticks?) unknown | Human (bacteraemia, fever,endocarditis) |
| Bartonella vinsonii berkhoffii | (Coyote Canis latrans)dog (Canis familiaris) | (Ticks?) unknown | Human, cat (endocarditis, epitheliodhemangioendothelioma) [18] |
| Bartonella washoensis | (Californian ground squirrelSpermophilus beecheyii) | (Fleas Oropsylla montana) | Human, dog (myocarditis,endocarditis) |
Table 1: Species and subspecies of Bartonella that are confirmed or potential human pathogens, primary hosts and vectors, known accidental hosts, and human diseases (adapted from Chomel and Kasten, 2010 [26] and Kaiser [37]).
Some of the animals that harbor Bartonella include cats, coyotes, cows, dogs, foxes, horses, kangaroos, sheep, numerous rodent species, [35] and bats [3]. There is ample evidence that cats are the primary, but not the sole, reservoirs for transmission of Bartonella henselae, Bartonella clarridgiae, and Bartonella koehlerae to human beings. Direct cat flea transmission to humans has been suspected [53], whereas epidemiological and experimental transmission studies clearly support cat fleas as the primary vector for transmission of Bartonella spp. among cats and likely dogs throughout the world. There are also reports that support bite transmission of bartonellosis from dogs to people [22]. Needle stick transmission has been recently reported in two veterinarians, both of whom developed neurological complications [43,56]. Transmission potentially can occur through cat bites, if infectious flea excrement or cat blood contaminates the bite wound. Other Bartonellae reported to cause human infections, their vectors, and primary reservoirs are summarized in Table 1. With the exception of Bartonella bacilliformis, for which human beings are the only known reservoir host, all Bartonella spp. listed in Table 1 are considered to be zoonotic pathogens. Historically, Bartonella quintana was considered a nonzoonotic species because the human body louse was thought to be the sole vector and human beings the sole reservoir host. However, in recent years, B. quintana DNA has been PCR amplified and sequenced from cat fleas [63,66], rodent fleas [66], ticks, [66], dog saliva [31], and from dogs with B. quintana endocarditis [39]. In addition, B. quintana bacteremia was confirmed in a woman after being bitten by a feral cat, that months later was shown to be B. quintana bacteremic [17]. Thus, there is evolving evidence to support B. quintana as a zoonotic pathogen, potentially transmitted among cat and rodent fleas to pets, and thus posing a risk for human infection. Clearly, the zoonotic risk of acquiring bartonellosis is of substantial importance to veterinary professionals, groomers, animal rescue workers, and pet owners, particularly for those individuals who come into frequent contact with feral animals or with privately owned pets for which flea and tick prevention strategies are not used to avoid infestations [47]. Other recent publications have emphasized the ecological complexity of Bartonella spp. transmission in nature [23,66]. Tick transmission of a Bartonella sp. has been a controversial subject for some years [27,65]. Recently, vector competence for tick (Ixodes ricinus) transmission of a Bartonella sp. was demonstrated experimentally, thus supporting the possibility that Ixodes sp. ticks are transmitting B. henselae throughout the northern hemisphere [60]. Previous studies from Europe and North America have documented the presence of B. henselae DNA in Ixodes ricinus [29], Ixodes scapularis [66] and Ixodes pacificus [36].
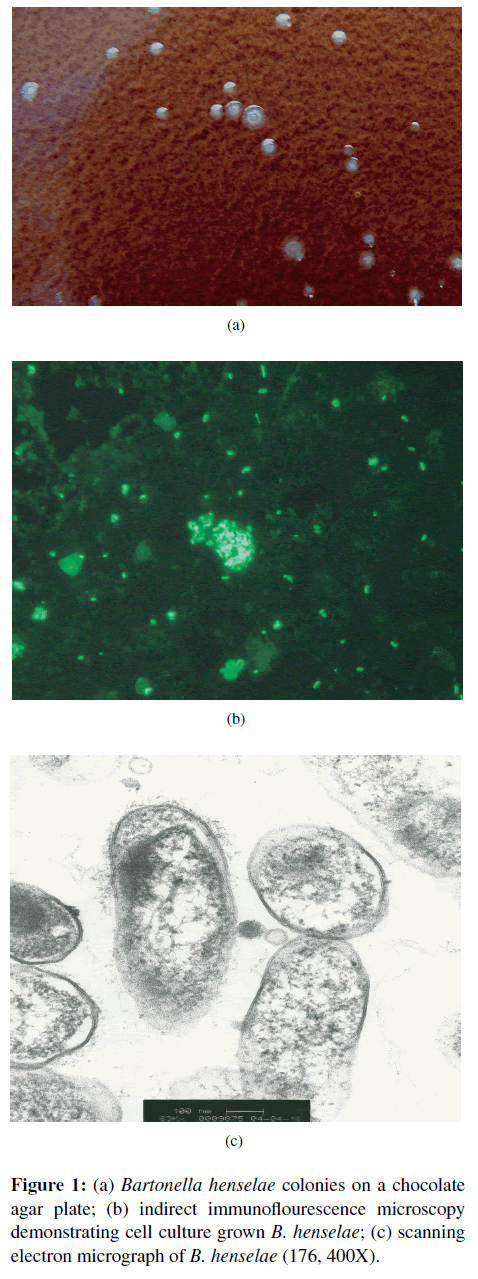
Historically, CSD has represented a prototypical disease process in immunocompetent people, characterized by the triad of a cat scratch or bite, fever, and regional lymphadenopathy [9]. Generally, within 7–12 days following transmission of B. henselae, the primary if not the sole cause of classical CSD, a papule and then a pustule develop at the inoculation site. One to 3 weeks later, regional lymphadenopathy (usually involving a single lymph node) develops with the persistence of lymphadenomegaly for a few weeks to several months [24,26]. Low-grade fever, malaise, and muscle pain are often reported by CSD patients. Some patients develop severe headaches, anorexia, and splenomegaly. Abscessation of lymph nodes is reported occasionally. Approximately 10% of CSD patients develop what have historically been considered atypical manifestations, including Parinaud’s oculo-glandular syndrome, encephalitis, endocarditis, haemolytic anaemia, hepatosplenomegaly, glomerulonephritis, pneumonia, pleural and pericardial effusions, relapsing bacteremia, and osteomyelitis [9,26]. Bartonella henselae appears to be the primary, if not the sole, cause of CSD. As discussed in the diagnosis section, isolation, serology, and PCR testing combined with enrichment bacterial culture have facilitated the diagnosis of CSD and neurobartonellosis (Figure 1). With improvements in diagnostic testing modalities, these “atypical” manifestations may be much more prevalent (more recent estimates have been revised to 25% of “atypical” cases) in human patient populations than was previously suspected [54]. In the past decade, numerous cases of neuroretinitis or neurobartonellosis optical neuritis have been reported and as these cases have recently been reviewed by Kalogeropoulos et al. [38]; these cases will not be included in this review.
The purpose of this review is to summarize the historical and neurological manifestations reported between 2005 and 2012 in immunocompetent people with bartonellosis. While there are rare exceptions, encephalopathy is a unifying factor in these cases. The additional manifestations are varied; including seizures, cranial neuropathies, aphasia, white matter abnormalities, and peripheral nervous system diseases. Testing limitations, as well as progress in the development of more sensitive and specific diagnostic modalities, will be briefly reviewed. Cerebrospinal fluid findings are often normal or minimally abnormal [49]. Also, because there appears to be a spectrum of disease outcomes ranging from death to self-limiting illness, challenges associated with the selection of an effective treatment regimen and the need for additional research to define the neuropathogenesis of bartonellosis will be discussed.
Overview of neurological case reports in immunocompetent patients (2005–2011)
Encephalitis
Based upon the spectrum of neurological disease manifestations, researchers have hypothesized that Bartonella may cause nervous system damage either by producing neural toxins, by direct injury to cells within the CNS, or by triggering an aberrant autoimmune or immune-mediated response. To a great extent none of these hypotheses have been rigorously tested and the remarkable variability among clinical presentations in patients with neurological bartonellosis supports to a varying degree involvement of multiple or individual mechanisms of CNS injury among patients. For example, in 2005, Dyachenko et al. described a 22-year-old patient with fever (38 °C), an epitrochlear mass, and a history of cat scratches, who after a detailed evaluation was treated with a doxycycline for a presumptive diagnosis of CSD [32]. Despite continued administration of oral doxycycline, six days following discharge from the hospital the patient developed generalized tonic-clonic convulsions, followed by a loss of consciousness. CSF analysis revealed only a few leukocytes (3/μL), and normal protein (39 mg/dL), and glucose (80 mg/dL) concentrations. CSF bacterial culture did not result in growth and a herpes simplex virus PCR was negative. Computed tomography of the patient’s brain was normal, but an EEG revealed epileptiform discharges in the temporo-parietal regions. B. henselae DNA was amplified from the CSF and following surgical exploration and excision of an encapsulated abscess in the right epitrochlear lymph node, B. henselae DNA was PCR amplified and infection was documented by restriction fragment length polymorphism (RFLP) analysis. Treatment with oral rifampin and intravenous minocycline elicited rapid improvement in consciousness. One year after developing encephalopathy, the patient remained seizurefree without residual neurological deficits. In this patient, PCR amplification of B. henselae DNA from the CSF provided a circumstantial support for direct CNS invasion by Bartonella. This case also illustrates a potential need to select antibiotics that both penetrate the central nervous system and acquire high intracellular and extracellular antimicrobial concentrations, so as to more rapidly achieve resolution of the neurological symptoms.
In 2008, Nishio et al. described the development of encephalopathy in a 9-year-old girl following admission to the hospital for fever, right cervical lymphadenopathy, and a history of a cat scratch (classical CSD) [55]. Routine laboratory tests were normal, with the exception of slight elevations in erythrocyte sedimentation rate and C-reactive protein (CRP). Initially intravenous ceftriaxone and oral minocycline were administered, with defervescence in the first 24-hours. Due to a minimal decrease in serum CRP over the next 4 days, panipenem/betamiprone was substituted for the initial antibiotics. Once CRP decreased to 1.5mg/dL, oral clarithromycin was substituted for minocycline. On the 14th day of hospitalization, CRP increased in conjunction with a rise in temperature. On the 16th day of hospitalization, the patient developed generalized tonic convulsions for 5 minutes followed by a comatose state. A CT scan did not reveal any abnormalities, whereas an EEG indicated a generalized slow wave activity, a common but non-specific finding of encephalopathy. CSF analysis was normal with the exception of a slightly increased white blood cell count (12 cells/mm3). The patient was reported to be B. henselae seroreactive. After intravenous administration of ceftriaxone and erythromycin, the patient had a rapid neurological recovery. One month after admission, a right cervical lymph node biopsy revealed granulomas with central necrosis. B. henselae DNA was amplified from the lymph node, but retrospective attempts to amplify Bartonella DNA from CSF were not successful. The patient subsequently remained free of seizures during a six year follow-up period.
In 2007, Fouch and Coventry described a 6-yearold previously healthy Hispanic boy, who was treated with cephalexin after being diagnosed with left axillary lymphadenopathy associated with CSD. During the subsequent 48 hours, the patient developed fever, persistent vomiting, and abdominal pain, indicative of systemic bartonellosis [67] and was hospitalized. During the next two days, the child complained of a worsening headache. By day 4 of hospitalization, mental status deteriorated and the boy developed seizures. No abnormalities were observed on a brain CT scan. Thirty six hours after the scan he had another seizure, and lumbar puncture revealed 51 WBC/μL (94% lymphocytes, 5 % monocytes, 1% segmented neutrophils), glucose (114 mg/dL), and protein (31 mg/dL). Gram stain was negative. Twenty minutes following the lumbar puncture, the boy developed respiratory arrest requiring cardiopulmonary resuscitation. Pupils became fixed and despite medical therapy the boy died. Following autopsy, B. henselae DNA (htr gene) was amplified from the granulomatous lymph node at the Centers for Disease Control and Prevention; however, B. henselae serology results were equivocal (IgM < 16, IgG 1:128) and a Warthin-Starry stain of the lymph node was negative. The brain contained extensive, diffuse perivascular lymphocytic infiltrates with microglial nodules, involving the frontal, parietal, and occipital lobes and pons. B. henselae DNA was not amplified from the brain and organisms were not identified by special stains. Tests for other infectious causes of encephalitis were negative (Table 2).
| Case | Microbiological blood and CSF examination andCT and MRI examinations of brain | Biochemical assays | |
|---|---|---|---|
| Recurrent expressiveaphasia in a 15-year-oldmale | Brain MRI suggested an acute disseminatedencephalomyelitis. B. henselae titers were positive:IgM > 1.32 and IgG > 1:128. CSF PCR was negative. | Negative for metabolic disorders,hypercoagulability, thromboembolism,toxic ingestions, toxic exposures,autoimmune disorders, encephalitis,and evidence of traumatic injury. | [34] |
| Encephalitis lethargicain a 16-year-old girl | Increased B. henselae antibody titers. Blood and CSFPCR performed after antibiotic therapy were B. henselaenegative. Tests for HHV6, VZV, EBV, HIV, Measles,Enteroviruses, L. monocytogenes, and Rubella werenegative. Negative for antibodies against basal ganglia. | Normal WBC count, C reactive protein,antistreptolysin O titer, and all otherroutine blood analyses. CSF identifiedlymphocytosis and intrathecal synthesisof oligoclonal bands. | [20] |
| Encephalopathy in a9-year-old girl duringadministration ofantibiotics | Elevated erythrocyte sedimentation rate. Sera were negativefor EBV, CMV, and T. gondii. B. henselae IgM titer was1:80 and IgG titer was 1:1024. Lymph node was PCRpositive for B. henselae. CSF PCR was negative. | Elevation of C reactive protein.Histology of lymph node biopsyshowed granulomatous mass with nomalignant cells. | [55] |
| Fatal encephalopathyin a 6-year-old male | WBC count 9600/mL, CSF contained 51 WBC/mL, CSFwas negative for HSV, Eneovirus, WNV, flavivius, andEastern equine encephalitis virus. CT was negative. B.henselaeIgM titer was 1:16 and IgG titer was 1:128.PCR of lymph nodes was positive. | Toxicology screen was negative. | [33] |
| Transverse myelitis ina 46-year-old man | Cranial CT was negative. Culture and stain of CSF forbacteria, fungi, and acid fast bacilli were negative. CSFcryptococcal antigens test was negative. Serological testingfor coccidioidomycosis, HIV, Lyme disease, and syphilis wasnegative. B. henselae IgM titer was 1:64 and IgG antibodytiter was 1:512. | Chest radiograph, urinalysis, bloodchemistry, and ANA were negative;except lactate dehydrogenase whichwas 266 mU/mL | [4] |
| Transverse myelitis ina 13-year-old boy | B. henselaeand B. quintanaIgG antibody titers were 1:512.Warthin-Starry stain was positive. | [4] | |
| Expressive Aphasia ina 59-year-old male | VDRL and CSF culture were negative; PCR for HSV, VZV,and enterovirus was negative and WBCs were absent.Bartonella henselaetiters were reported as 1: 1024. | CSF glucose level was 60 mg/dL andprotein was 64 mg/dL | [50] |
| MS-like disease in a27-year-old-man | Brain and spinal cord MRI were normal. No oligoclonalbands. Borrelia burdorferi, syphilis, HIV, Epstein Barrvirus, cytomegalovirus, HSV 1 and 2, leptospirosis,leishmaniasis, listeriosis, Q fever, tularemia, and M.pneumoniae testing was negative. | ANA, ENA, ANCA, antiphospho lipidantibodies were negative; normalbiochemistry profile. | [21] |
| Psychiatric illness in a41-year-old male | Bartonella henselaeIgM titer was 1:256 andBartonellahenselaePCR was positive. | [64] | |
| Psychiatric illness in amale | Bartonella henselaeIg G titer was 1:128.Bartonella PCRwas negative. | [64] | |
| Psychiatric illness in afemale | Unusual rash on thigh. No diagnostically supportive data. | [64] | |
| Meningitis in a47-year-old woman | Cranial and abdominal CT were normal. CSF culture wasnegative, CSF WBC count was 6 cells/mL. Blood cultureafter five days of incubation was identified as Bartonellawashoensis by FAME analysis and glt A PCR and DNAsequencing. | Coagulation, serum chemistries, andliver function tests were normal. | [57] |
| Neurological diseasein a 50-year-old male | Positive Romberg sign and difficulty with heel-toe walking.Cranial nerves, muscle strength, sensation, and deep tendonreflexes were normal. CSF analyses were normal. By IFAtesting, the patient was B. vinsonii berkhoffii genotypes IIand III and B. henselae seroreactive. B. vinsonii subspberkhoffi DNA was amplified and sequenced from blood andCSF using BAPGM enrichment and culture. Periodontalswab sample was B. vinsonii subsp. berkhoffii PCR positive. | [14] | |
| Headaches and insomniain a 7-year-old female | Seroreactive to B.vinsonii subsp. berkhoffii genotypes II andIII and B. henselae, B. vinsonii subsp. berkhoffii DNA wasamplified and sequenced from two BAPGM enrichmentblood cultures performed 3 weeks apart | [14] | |
| Hallucinations andvisual defects in an18-year-old female | Over a 1-year period, the patient was consistently B.koehleraeseroreactive and B. koehleraewas repeatedly PCRamplified and sequenced from blood or BAPGM enrichmentcultures. There were no abnormalities on a noncontrast brainMRI or an electrocephalogram. | [19] |
Table 2: A summary of the diagnostic features of cases of neurobartonellosis.
Aphasia/encephalopathy
In 2007, Fox et al. described a 15-year-old male patient who was sent home from school due to nausea, vomiting, and “an inability to speak” [34]. Expressive aphasia, mild right facial droop, right sided weakness, and an inability to ambulate were found when examined at a local hospital. Laboratory tests and a CT of the head were unrevealing. Subsequently, a diffuse white matter periventricular signal abnormality was interpreted from an MRI of his brain. Aphasia resolved during a one-day hospitalization and as a cerebrovascular insult was suspected, the patient was discharged with instructions for aspirin therapy. Within hours of discharge the boy could not speak and was readmitted the next day due to agitation. A mildly tender right axillary lymph node was palpated and healing papules and scratches were identified on his chest, which was attributed to being scratched on several occasions by the family kitten. Following review of the previous MRI by a pediatric neuroradiologist, intravenous steroids were administered for acute disseminated encephalomyelitis. The only abnormality identified during an extensive inpatient evaluation was “strongly positive” IgM and IgG B. henselae antibody titers. The boy was treated with a 5-day course of azithromycin, after which he remained normal during the 5-month follow-up period (Table 2).
In 2010, Marienfeld et al. reported sudden-onset expressive aphasia, word substitution errors, and impaired repetition in a 59-year-old male with a history of a previous stroke. The patient had recently adopted several stray kittens. Ten days prior to presentation the patient reported a skin infection that progressed to right axillary lymphadenopathy. CSD was diagnosed and treated with a 5-day course of doxycycline. A head CT identified a low-density lesion in the region of the left insula, with no mass effect. An MRI showed his prior stroke, but no changes consistent with an acute or subacute infarction. EEG demonstrated generalized slowing, but no epileptiform activity. Eighteen hours later, the patient had a generalized seizure and a waning level of consciousness. A lumbar puncture was normal with the exception of a slight increase in protein (64 mg/dL). Viral and bacterial antibody and PCR testing were negative and CSF culture did not result in bacterial growth. Overnight, the patient responded rapidly to intravenous azithromycin, after which he was discharged with instructions to take oral doxycycline and rifampin for 14 days. B. henselae antibody titers were positive (IgM 1:20 and IgG 1:1024).
Encephalitis lethargica (EL)
Encephalitis lethargica is characterized by psychiatric disturbances, lethargy, sleep abnormalities, parkinsonism, and dyskinesia. In March 2005, a 16-year-old girl was referred for evaluation of lethargy, disorientation, and behavioral changes that began 14 days prior to presentation [20]. Six weeks earlier, the girl had complained of swollen lymph nodes in her neck. Physical examination findings were consistent with classical encephalitis lethargica, including lethargy, oral dyskinesias, and dystonia. The girl did not recall exposure to animals and there was no prior history of psychiatric disorders or drug abuse. After hospitalization, seizures progressed from focal to generalized tonic-clonic, accompanied by respiratory arrest requiring mechanical ventilation for over four days. Interictal EEG demonstrated focal slowing over the left temporal lobe. Lumbar puncture revealed a lymphocytosis with intrathecal synthesis of oligoclonal bands. Repeated serological testing identified B. henselae antibody titers of 1:64 (day 8 of hospitalization) and 1:256 three weeks later. B. henselae PCR from serum and cerebrospinal fluid was negative; however, the samples were obtained after three weeks of antibiotic therapy. Initial and two follow-up cerebral MRIs showed no pathological findings. However, [123]IBZMSPECT revealed a marked decrease of striatal dopamine D2 receptor availability which was more pronounced on the left side corresponding to the predominantly right-sided rigor. Antibodies reactive against basal ganglia were not found. Symptomatic treatment of parkinsonism with both levodopa (300 mg/tid) and amantadine (200 mg daily IV) did not improve motor disturbances. Treatment with erythromycin (1 g q8h IV) over 14 days in combination with methylprednisolone (initially 1000 mg daily with slow tapering off) also had no effect on lethargy and parkinsonism. Consequently, antibiotic therapy was changed to rifampicin (600 mg daily IV) and doxycycline (100 mg q12h PO) resulting in a significant improvement of her neurological symptoms within one week’s time and total resolution of motor, cognitive, and behavioral disturbances within two months. Encephalitis lethargica is thought to be an immune-mediated disorder due to the presence of intrathecal oligoclonal bands, antibodies reactive against basal ganglia. Steroids are often beneficial. This patient lacked antibodies to basal ganglia and had no discernible beneficial effect from steroids, whereas there was rapid and progressive improvement in neurological status after administration of rifampicin and doxycycline. The authors speculated that B. henselae caused encephalitis with the phenomenology of encephalitis lethargica. There is overlap between encephalitis lethargica anti-N-methyl-D-aspartate (NMDA) receptor encephalitis, but this case occurred prior to wide recognition of the latter [28].
Transverse myelitis, multiple sclerosis (MS) like disease, and Guillain-Barr´e syndrome (GBS)
Transverse myelitis is associated with spinal inflammation induced by viral infections, syphilis, vaccinations, demyelinating disease, or idiopathic immune reactions that result in extensive damage to nerve fibers of the spinal cord. In 2007, Baylor et al. described two patients with transverse myelitis, both of whom were exposed to young cats and had enlarged lymph nodes (classical CSD). Both patients had positive Bartonella serological test results (Table 2). Patient 1 was a 46-year-old man who had a 4-week history of intermittent fever, night sweats, a 5.5 kg weight loss and development of a painful swelling in the right groin. Surgical exploration identified a large lymph node, which was excised. The following day, the patient developed numbness in the right foot and difficulty voiding, and subsequently an inability to ambulate due to flaccid areflexic paraplegia with an incomplete sensory level at T5. Routine laboratory findings were unrevealing, chest radiographs and a cranial CT scan were normal. An MRI identified focal cord changes at C5–C7, consistent with an inflammatory process. Lumbar puncture yielded 47 WBC cells/mm3 (70% lymphocytes, 26% monocytes, and 4% neutrophils) and a protein level of 58 mg/dL. CSF stains and culture were negative, as were a battery of serological tests. The lymph node contained necrotizing granulomatous inflammation. Acid fast and methanamine silver stains were negative, whereas Warthin- Starry staining at the Armed Forces Institute of Pathology identified a myriad of branched bacilli consistent with CSD. Serum B. henselae IgM and IgG antibody titers were 1:32 and 1:128 respectively, whereas no antibodies were detected in CSF. Serology, repeated three weeks later, identified a B. henselae IgG titer of 1:512, supporting seroconversion. The patient was initially treated with methylprednisone and intravenous immunoglobulin. After diagnosis of bartonellosis, the patient was treated with oral doxycycline and rifampin for two months. Patient 2 was a 13-year-old boy who developed right axillary lymphadenopathy, one week following a cat scratch. Subsequently fever, anorexia and headaches developed. By day 17 post-scratch, the boy developed dysthesias, abdominal pain, difficulty in walking followed by paralysis, and urinary retention. CSF contained increased WBC (300 cells/mm3 with 70% lymphocytes and 30% neutrophils) protein level of 90mg/dL. Antibiotic treatment with ceftazidime and netilimicin for 10 days and dexamethasone intravenously for 5 days resulted in defervesence, and resolution of neurological symptoms beginning on post-treatment day 7, with complete resolution reported 6 weeks later. A serological diagnosis of myelitis secondary to bartonellosis was made based upon B. henselae antibody titers of 1:128 at admission, 1:512 at day 15, and 1:32 at 6 months post-discharge.
In 2010, Brinar and Habek described a patient with multiple sclerosis-like symptoms [21]. The patient had developed encephalopathy, fever, non-tender-pustulous skin lesions, lymphadenopathy, splenomegaly, and relapsing neurological symptoms: slight motor weakness of the left extremities, diminished visual acuity and double vision, paresthesia in the left extremities with demyelinating brain MRI changes. Some symptoms, such as non-tender papules on legs, remittent febrile status, regional lymphadenopathy and splenomegaly pointed to a systemic illness, while the neurological symptoms characterized by remittent encephalopathy, loss of visual acuity, and minor left-sided hemiparesis in conjunction with the MRI findings were consistent with multiple sclerosis. Findings supportive of an infectious process included signs of systemic illness, negative CSF oligoclonal bands, encephalopathy, and an MRI that was more typical of a non-inflammatory/vascular demyelinating disorder. Serological testing for infectious agents was negative (Table 2). Historically, the patient transported wild animal meat across Europe and had exposure to cats. Because of the non-tender pustules, regional lymphadenopathy, splenomegaly, and oscillations in neurological status, neurobartonellosis became a diagnostic consideration. B. henselae antibodies were found in serum and CSF. One month after antibiotic therapy, the patient’s clinical status normalized and he made a full recovery.
In 2006, Massei et al. described a 10-year-old girl who was hospitalized due to leg weakness [52]. Seven days earlier, the girl had a self-limiting episode of fever and vomiting of 1 day duration. Four days later, she had difficulty walking, became irritable, and complained of severe myalgia in the lower limbs. Laboratory findings were not remarkable. Nerve conduction studies identified decreases in motor conduction velocity and amplitude, consistent with axonal damage. An exhaustive search for known causes of Guillian-Barre syndrome was negative. The patient was treated with intravenous immunoglobulins for 5 days, antibiotics were not administered, and within two weeks her neurological status had normalized. There was no history of cat scratches, no palpable lymphadenopathy, and no hepatic or splenic lesions on an abdominal ultrasound. Because she lived in a rural area and played with kittens, B. henselae serology was requested. She had an IgG titer of 1:1024 and a specific IgM titer (value not reported). CSF PCR was negative. Convalescent serology did not identify IgM antibodies and IgG antibodies were decreased (titer not provided).
In 2007, Mantadakis et al. described a 16-month-old girl who presented to an emergency room for respiratory distress, cough, vomiting, diarrhea, and a decreased level of consciousness during the preceding week. The child lived in a rural area with cat, dog, rabbit, and bird contact. A CT scan of the brain was unremarkable. Lumbar CSF contained 3 cells/mm3 (all lymphocytes), normal glucose and protein, and negative Gram stain and culture. She was empirically treated with cefotaxime and ampicillin intravenously and was admitted to the pediatric intensive care unit. Thoracic radiographs revealed a right upper lobe infiltrate, requiring intubation and mechanical ventilation. Ampicillin was discontinued and clindamycin and acyclovir were administered intravenously. On the third day of hospitalization, an electroencephalogram showed diffuse slowing and a repeat lumbar puncture identified a marked increase in CSF protein (322 mg/dL) with 5 nucleated cells (80% lymphocytes, 20% neutrophils). While the child remained on mechanical ventilation, there were recurrent episodes of “unexplained” tachycardia and hypertension. During weaning from sedatives, deep tendon reflexes were absent. An MRI scan of the brain and spinal cord with gadolinium showed homogeneous uptake throughout the lower spinal roots. Repeat lumbar puncture identified a protein of 379 mg/dL. GBS was diagnosed and the child was treated with intravenous gamma globulin and a 2-week course of intravenous clarithromycin. Within 3 days, the patient could move her legs. Reduced motor conduction velocity and amplitude supported peripheral axonal damage. The patient was extubated 1 week after receiving immunoglobulin when a repeat MRI identified acute hydrocephalus, necessitating a ventriculperitoneal shunt. Eight months following discharge, the child had a normal neurological examination. Subsequent efforts to identify an infectious etiology for GBS in this patient resulted in documentation of greater than a four-fold decrease in IgM titer for B. quintana. In addition, B. quintana DNA was amplified and sequenced from 2 CSF samples. There was no historical evidence of louse infestations or flea bites to the patient’s body.
Meningitis
In 2009, Probert et al. described a 47-year-old previously healthy woman who developed fever, chills, headache, nausea, vomiting, epigastric and lower abdominal pain and joint pain that had progressed over 24 hours. Examination findings included fever (39.3 °C), photophobia, neck stiffness with mild nuchal rigidity, consistent with meningitis. The patient reported recent handling of a dead mole and dead ground squirrel, but denied flea, tick or other insect bites. Lumbar CSF contained 6 WBC/mm3 (51% neutrophils, 49% monocytes), protein 40 mg/dL and glucose 64mg/dL. CSF cultures and PCR for enterovirus were negative. When discharged three days later, the platelet count had decreased from 168 to 112 × 103 cells/μL). The patient was treated empirically with vancomycin and chloramphenicol, followed by replacement of chloramphenicol with aztreonam, and oral moxifloxacin prescribed at discharge. At follow-up 6 days later, urinalysis revealed WBCs, RBCs, and nitrates, and the patient was switched to levofloxacin for 10 days. Eight weeks after hospitalization the patient was clinically improved, but reported persistent body ache and bone pain. Additional patient follow-up was not provided. A blood culture obtained at admission yielded aerobic growth after 5 days of incubation and a subculture isolate was eventually identified as Bartonella washoensis. Ground squirrels have been suggested as the major reservoir for B. washoensis in California and Nevada. Oropsylla montana is the most common flea species to parasitize California ground squirrels. The gltA sequence from O. montana fleas collected from the patient’s putative site of exposure matched the gltA sequence from bacteria isolated from the patient’s blood. Although the patient did not recall recent arthropod bites, it seems likely that she was bitten by an infected flea while handling the squirrel carcass or while walking near ground squirrel burrows on her property.
Neurological diseases with psychological illness
In 2007, Schaller et al. described three patients with acute psychiatric disorders (personality changes, agitation, panic attacks, and depression) associated with “Bartonella-like” symptoms. Tick transmission was suspected in two patients, and the third was exposed to cat fleas. Symptoms consistent with Bartonella infection included lymphadenopathy adjacent to an Ixodes scapularis tick bite and a rash in the remaining patients. In order to function normally, all three patients required very high doses of antidepressants, benzodiazepines, or antipsychotics. Medication doses were lowered following antibiotic treatment and all three patients returned to their previously healthy or near-normal baseline mental health status. The authors reported that diagnostic and therapeutic management of these patients did not offer certain proof of Bartonella infection, but that patients infected with Bartonella might have a variety of mental health symptoms.
In 2011, Breitschwerdt et al. reported B. koehlerae bacteremia in a young woman experiencing depression, anxiety, mood swings, severe headaches, muscle spasms, interphalangeal joint stiffness, decreased peripheral vision, diminished tactile sensation, and hallucinations. When tested against five Bartonella spp. antigens, the patient was only B. koehlerae seroreactive. Before serological and BAPGM (Bartonella alpha Proteobacteria growth medium) enrichment culture/PCR diagnosis, the patient had a slowly progressive history of neurological and neurocognitive abnormalities. Following the initial course of antibiotics (doxycycline and rifampin), there was a decrease in hallucination frequency while receiving a stable dose of antipsychotic medication. Despite an initial treatment failure, total resolution of hallucinations and visual field deficits followed a third course of antibiotics (rifampin and azithromycin). Following treatment, B. koehlerae antibodies and Bartonella DNA were no longer detectable, all symptoms resolved and antipsychotic drugs were sequentially withdrawn. On the basis of serological and PCR findings (Tables 2 and 3), and the resolution of symptoms following antibiotic treatment; prospective studies are needed to clarify the extent to which Bartonella spp. bacteremia might contribute to neurocognitive abnormalities.
| Date(mo/day/yr) | Bartonella IFA reciprocal titer | PCR/DNA sequencing resultc | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B. koehlerae | B. henselae | B. vinsoniisubsp. berkhoffiigenotype | DNA extraction | BAPGM enrichment culture at day: | |||||
| I | II | III | Blood | Serum | 7 | 14 | |||
| 1/13/09 | 64b | < 16 | < 16 | < 16 | < 16 | Neg | Neg | Neg | Bartonella sp.aand B. koehleraeb |
| 3/10/09 | 256b | < 16 | 64 | < 16 | < 16 | Neg | Neg | Neg | Neg |
| 5/1/09 | 256b | < 16 | < 16 | < 16 | < 16 | B. koehleraeb | Neg | Neg | Neg |
| 11/4/09 | 256 | < 16 | < 16 | < 16 | 64 | Neg | Neg | B. koehlerae | Neg |
| 2/23/10 | 64 | < 16 | < 16 | < 16 | < 16 | B. koehlerae | Neg | Neg | Neg |
| 6/7/10 | 128 | < 16 | < 16 | < 16 | < 16 | Neg | Neg | Neg | B. koehlerae |
| 8/30/10 | < 16 | < 16 | < 16 | < 16 | < 16 | Neg | Neg | Neg | Neg |
| 9/1/10 | < 16 | < 16 | < 16 | < 16 | < 16 | Neg | Neg | Neg | Neg |
| 9/2/10 | < 16 | < 16 | < 16 | < 16 | < 16 | Neg | Neg | Neg | Neg |
| 1/4/11 | < 16 | < 16 | < 16 | < 16 | < 16 | Neg | Neg | Neg | Neg |
| 1/5/11 | < 16 | < 16 | < 16 | < 16 | < 16 | Neg | Neg | Bartonella sp. | Neg |
| 3/28/11 | < 16 | < 16 | < 16 | < 16 | < 16 | Neg | Neg | Neg | Neg |
| 3/29/11 | < 16 | < 16 | < 16 | < 16 | < 16 | Neg | Neg | Neg | Neg |
| 3/30/11 | < 16 | < 16 | < 16 | < 16 | < 16 | Neg | Neg | Neg | Neg |
a Bartonella sp., amplicon obtained using Bartonella genus ITS primers, but sequencing failed to confirm the species.
b Retrospective testing was performed using stored frozen serum, blood, or BAPGM enrichment culture samples after the development of a B. koehlerae IFA serological assay and a B. koehlerae-specific ITS PCR assay.
c Neg, DNA was not amplified using B. koehlerae 16S-23S ITS primers. The identities of all B. koehlerae PCR amplicons were confirmed by DNA sequencing.
Table 3: Serological, BAPGM enrichment blood culture, and PCR/DNA sequencing test results for an 18-year-old woman with hallucinations and peripheral visual deficits [19].
Neurological diseases with Bartonella spp. bacteremia
During the past decade, a number of Bartonella research laboratories validated the utility of using an insect cell culture medium in conjunction with PCR amplification to enhance the diagnostic sensitivity of confirming Bartonella spp. bacteremia in human patients [44,61]. In the Intracellular Pathogens Research Laboratory (IPRL) at the College of Veterinary Medicine, North Carolina State University, Bartonella alpha Proteobacteria Growth Medium (BAPGM) was used as an enrichment culture step in conjunction with Bartonella sp. PCR. Initially, due to frequent exposure to bacteremic animals and their arthropod vectors, blood or cerebrospinal fluid samples from veterinarians and other animal health professionals with historical animal and arthropod exposures were tested using the BAPGM enrichment culture platform. As a result, it appears that these individuals, a subset of whom report various neurological complaints, are at risk for persistent Bartonella spp. bacteremia [12]. Subsequently, the use of the BAPGM platform has resulted in documentation of Bartonella spp. bacteremia or cerebrospinal fluid infection in individual patients and small case series summarized below. Clearly there is the need for future case control studies to define the neuropathogenic potential of this genus and to clarify mechanisms of neural injury or dysfunction.
In 2008, Breitschwerdt et al. reported infection with B. vinsonii subsp. berkhoffii and B. henselae in six immunocompetent individuals with symptoms including ataxia, tremors, memory loss, and seizures. In one patient, B. henselae bacteremia was associated with recent onset of migraines with historical support for an acute infection following tick attachment, whereas the remaining five patients were symptomatic for at least one year. Subsequently, the IPRL research group described B. vinsonii subsp. berkhoffii and B. henselae bacteremia in a father and daughter from the same household [14]. The father, a veterinarian, developed progressive weight loss, muscle weakness, and lack of coordination over a one-year time frame. An MRI of the father’s brain was notable for an increase in signal intensity throughout the pons and upper medulla lateralizing to left of the midline. Subsequently, the daughter developed headaches, muscle pain, and insomnia. Both patients were being evaluated by a neurologist at the time of initial testing for evidence of Bartonella infection. Intravascular infection with B. vinsonii subsp. berkhoffii genotype II or B. henselae (Houston strain 1) was repeatedly identified in father and daughter’s blood by enrichment blood culture followed by PCR and DNA sequencing (Tables 2, 5, and 6). Bartonella vinsonii subsp. berkhoffii DNA was also PCR amplified and sequenced from a BAPGM enrichment CSF culture from the father, with bacteremia concurrently documented. Multiple courses of antibiotics (two courses of doxycycline and rifampin for the father, azithromycin and later doxycycline for the daughter) were administered before both patients’ clinical status improved and before there was no longer microbiological, serological and molecular evidence of infection. Four pet cats in the household were B. vinsonii subsp. berkhoffii and B. henselae seroreactive by IFA testing, whereas antibodies were not detectable in serum samples from the two newly acquired dogs, which were also negative by BAPGM enrichment blood culture/PCR testing. Identical B. henselae (ITS Houston 1 strain) DNA sequences (the same ITS strain found in the father and daughter) were found in samples from three of four cats.
Diagnosis of neurobartonellosis
In recent years, an increasing number of Bartonella spp. have been identified as zoonotic pathogens that are transmitted by animal bites or scratches or by arthropods. Although historically the term bartonellosis was attributed to infections with B. bacilliformis, transmitted by sandflies in the Peruvian Andes, a more inclusive medical use of this term now includes infections caused by any Bartonella sp., anywhere in the world. Importantly, cat scratch disease represents only a subset of patients with bartonellosis. Potentially, because Bartonella spp. can infect erythrocytes, endothelial cells, and various macrophage-type cells, including brain derived dendritic cells in vitro [54], the symptoms and pathological manifestations of bartonellosis appear to be extremely diverse among patients.
The numerous species within the genus Bartonella, antigenic and virulence differences among strains and subspecies, the diverse cell tropism, the ability to induce persistent occult infections in both reservoir and non-reservoir hosts, and the extraordinarily low levels of bacteremia found in accidentally-infected hosts, all contribute to the clinical, microbiological, and pathological complexities associated with the diagnosis of bartonellosis. The use of serology for the diagnosis of Bartonella infection is further complicated by the fact that seropositivity is common in some populations. A study of Sicilian blood donors and children demonstrated that 25.1% of children and 11.4% of healthy blood donors had IgG antibodies to B. henselae [48]. None of these patients had recent clinical evidence of infection. All were IgM negative. Another project to evaluate Bartonella seroprevalence in rural Thailand demonstrated positive IgG antibodies to at least one of four Bartonella species in 9.9% of febrile and 19% of afebrile patients [5]. Seroreactivity to Bartonella was measured in a population of patients in the Dominican Republic of the Congo, and 4.5% of the samples were found to be positive for B. henselae, B. quintana, or B. clarridgeiae [42]. A recent report of zoonotic infections in employees from Great Smoky Mountains and Rocky Mountain National Parks in 2008 and 2009 found that 26.7% of park employees had serological evidence of past infection with B. henselae, and in a 1 year period of observation 5.7% of employees had incident infections [1]. Some of the patients in the above reports may have had mild or asymptomatic infections that went unnoticed, and if detected, the presence of Bartonella spp. antibodies can only be used to infer prior exposure. It is important that positive serologic results be interpreted with care and in clinical context. Follow-up titers may also be useful to document infection (seroconversion) or support elimination of the infection following treatment (a decrease in antibody titer, often to undetectable levels).
Culture of blood or other diagnostic specimens (cerebrospinal fluid, joint fluid or cavity effusions) onto blood agar plates has proven to be very insensitive [44,61]. For reasons that remain unclear, Bartonella antibodies are not detected in a subset of bacteremic human patients [47]. Therefore, conventional culture approaches and antibody testing are diagnostically insensitive, particularly in persistently-infected immunocompetent patients. Similar to culture onto blood agar plates, unless the patient is immunocompromised, PCR amplification of Bartonella spp. DNA from patient blood or CSF samples is also relatively insensitive due to the low level of bactermia.
Bartonella enrichment culture
Ideally, the diagnosis of Bartonella infection should be confirmed by culturing the organism from blood, CSF, lymph node or heart valve (patients with endocarditis) or by amplifying DNA from tissues using PCR. In 2005, a novel, chemically modified, insect-based liquid culture medium (Bartonella/alpha-Proteobacteria growth media, BAPGM) that supports the growth of at least seven Bartonella species was described [45]. This medium also supported co-cultures consisting of different Bartonella species. Subsequently, a unique approach, which combines enrichment culture in BAPGM, followed by a highly sensitive PCR assay targeting the 16S-23S ITS region, was developed to characterize and quantify Bartonella infection in blood and CSF samples. When compared with more traditional methods, this combined approach has facilitated the microbiological diagnosis of bacteremia with B. henselae, B. quintana, B. vinsonii subsp. berkhoffii, and a B. volans-like organism [13,14,16,23]. Table 2 summarizes the tests performed to diagnose the cases summarized in this review.
It is important that the medical history include questions related to arthropod (lice, fleas, and ticks) and animal exposures. If these questions are not asked, the patient may be subjected to extensive testing prior to consideration of bartonellosis as a differential diagnosis. In neurological cases with arthropod and animal exposure histories, there should be a high index of suspicion for Bartonella infection. In patients with suspected autoimmune neuropathy, tests for infectious agents, in addition to an immunological panel, would be indicated. Historically, a serological diagnosis has most often been based upon B. henselae and B. quintana serology (Table 2). In a case of traverse myelitis [4], a serological diagnosis was obtained using B. quintana and B. henselae antigens; however, cross reaction between the two antigens did not allow for determination of the infecting species. Mantadakis et al., 2007, also reported the use of two antigens to differentiate B. henselae infection from B. quintana infection [49]. There was sharp decrease in the B. quintana IgM titer after antibiotic treatment, but no decrease in B. henselae titer and PCR analysis supported B. quintana as the causative agent as B. quintana DNA was amplified from CSF.
Breitschwerdt et al., 2011, reported the use of an expanded panel of antigens including B. henselae, B. koehlerae, and B. vinsonii subsp. berkhoffii genotypes I, II, and III [19]. Using this panel, a species or subspecies specific serological response was found in some patients infected with B. koehlerae or B. vinsonii subsp. berkhoffii bacteremia, respectively [16,56] (Tables 3 and 4). As the number of recognized zoonotic Bartonella sp. that infect human patients expands, the limitations of serology as a sole diagnostic test become much more obvious. Also, as a subset of patients with neurological disease can be coinfected with more than one Bartonella sp. [13,14], caution should be exercised when attributing dual seroreactivity (antibodies to more than one antigen) in a patient to non-specific cross reactivity. When used in conjunction with PCR analyses and an enrichment culture approach, serological results can be more accurately interpreted. For example, as illustrated in Tables 5 and 6, a father and daughter tested because of neurological disease had B. henselae and B. vinsonii subsp. berkhoffii genotypes II and III antibodies [14]. Based upon PCR from blood, CSF or enrichment blood cultures, both patients were infected with B. henselae and B. vinsonii subsp. berkhoffii, thus co-infection rather than serological cross reactivity was most likely responsible for the antibodies detected in these patients. Following antibiotic treatment, there was resolution in neurological signs accompanied by a sequential decline in antibodies over a one-year period to undetectable levels. Also, Bartonella sp. DNA was no longer amplified from blood or enrichment blood cultures. These two cases illustrate the utility of repeated sampling along with serological and enrichment culturing followed by PCR analyses to enhance patient management decisions. Optimally, serology and enrichment culture/PCR should be performed prior to antibiotics and should be sequentially followed to document therapeutic elimination of the infection. Diagnostic use of enrichment culture is expanding in other laboratories, which should facilitate diagnostic confirmation of neurobartonellosis by physicians throughout the world. Unfortunately, it is rare to obtain subculture isolates following enrichment culture, thus determining antibiotic sensitivity and resistance patterns is often not possible.
| PID | Ehrlichia canis | Bartonella IFA reciprocal titers | PCR/DNA sequencing results | |||||
|---|---|---|---|---|---|---|---|---|
| Bartonella vinsonii subsp. berkhoffii | ||||||||
| Genotype I | Genotype II | Genotype III | B. henselae | Directextraction* | BAPGM plateformenrichment culture** | Subcultureisolate*** | ||
| 5 | <16 | 16 | <16 | 16 | <16 | Neg | Neg | Neg |
| 34 | <16 | 16 | <16 | 16 | <16 | Bvb I | Neg | Neg |
| 81 | <16 | 32 | <16 | 32 | <16 | Neg | Bvb I | Neg |
| 97 | <16 | 64 | <16 | 64 | 32 | Neg | Neg | Neg |
| 123 | <16 | 64 | <16 | 64 | 32 | Neg | Neg | Neg |
| 144 | <16 | 128 | <16 | 64 | 32 | Neg | Neg | Neg |
| 180 | <16 | 64 | <16 | 64 | <16 | Neg | Neg | Neg |
| 206 | <16 | 32 | <16 | <16 | <16 | Neg | Neg | Neg |
| 234 | <16 | 16 | <16 | <16 | <16 | Neg | Neg | Neg |
Antibiotic treatment was initiated on PID 138.
aBoth blood and serum were extracted for PCR.
bBAPGM enrichment cultures were extracted at 7 and at 14 days for PCR.
cAgar plate subcultures were generated from the enrichment cultures at 7 and 14 days.
d16S-23S intergenic spacer PCR results were confirmed by sequencing of the amplicon.
PCR, polymerase chain reaction; BAPGM, Bartonella alpha Protecobacteria growth medium; IFA, immunofluorescence antibody assays; Neg, negative; PID, postinoculation day.
Table 4: Serological, blood culture, and PCR test results for veterinarian who was infected with Bartonella vinsonii subsp. berkhoffii [56].
| Date/sample (father) | Bartonella IFA reciprocal titers | PCR/DNA sequencing results | ||||
|---|---|---|---|---|---|---|
| B. henselae | Bvb Genotype II | Bvb Genotype III | Direct extraction | BAPGM enrichment culture | Subculture isolate | |
| 10-18-07 Blood | 512 | 32 | 256 | Bvb TII | Neg | Neg |
| 11-02-07 Blood | 8192 | 64 | 128 | Neg | Bvb TII | Neg |
| 11-05-07 CSF | NT | NT | NT | Neg | Bvb TII | Neg |
| 12-11-07 Oral swab | NT | NT | NT | Bvb TII | N/A | N/A |
| 1-18-08 Blood | 1024 | 32 | 128 | Neg | Bh H1 | Neg |
| 5-27-08 Blood | < 16 | 16 | 16 | Neg | Neg | Neg |
| 11-04-08 Blood | < 16 | < 16 | < 16 | Neg | Neg | Neg |
Table 5: Serological, culture, and molecular test results for a 50-year-old veterinarian (the father) with chronic weight loss and progressive neurological dysfunction [14].
| Date/sample (father) | Bartonella IFA reciprocal titers | PCR/DNA sequencing results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B. henselae | Bvb Genotype II | Bvb Genotype III | Direct extraction | BAPGM enrichment culture | Subculture isolate | ||||
| 11-07-07 | Blood | 256 | <16 | 128 | Neg | Bvb TII | Neg | ||
| 11-28-07 | Blood | 256 | <16 | 64 | Neg | Bvb TII | Neg | ||
| 1-11-08 | Blood | 64 | 32 | 32 | Neg | Bvb TII | Bvb TII | ||
| 3-20-08 | Blood | 64 | 32 | 32 | Neg | Neg | Neg | ||
| 5-08-08 | Blood | 64 | 16 | 16 | Neg | Neg | Ochrobactrum sp. | ||
| 6-24-08 | Blood | 64 | 32 | 16 | Neg | Neg | Neg | ||
| 7-31-08 | Blood | 16 | 32 | 64 | Neg | Neg | Neg | ||
| 10-13-08 | Blood | <16 | <16 | <16 | Neg | Bh H1 | Neg | ||
| 12-01-08 | Blood | <16 | <16 | <16 | Neg | Neg | Neg | ||
Neg = DNA was not amplified using Bartonella 16S-23S intergenic spacer (ITS) primers.
Table 6: Serological, culture, and molecular test results for a 7.5-year-old girl (the daughter) with progressive neurological dysfunction [14].
Treatment challenges
For severe or persistent neurobartonellosis, there is minimal evidence upon which to base treatment decisions. Expert opinion often recommends a combination of doxycycline plus rifampin for 10 to 14 days [8,62]. Based upon in vitro testing, numerous antibiotics appear to be effective for the treatment of Bartonella infections [8]. However, as Bartonella spp. induce both intracellular, as well as extracellular infection, in vitro test results can identify antibiotics that are not effective and can also be used to select antibiotics that should be tested in clinical trials of in vivo efficacy. Doxycycline, erythromycin, and rifampin are the most frequently recommended antibiotics for treating Bartonella spp. infection in people, but clinical improvement has been reported following the use of penicillin, gentamicin, ceftriaxone, ciprofloxacin, and azithromycin [30,40,58,62]. Treatment for 2 weeks in immunocompetent and 6 weeks in immunocompromised people is generally recommended for treating bartonellosis. However, relapses or ongoing bacteremia have been reported in patients treated with longer courses of antibiotics (three months) [15]. Evolving evidence, although meager, suggests that neurobartonellosis should be treated with two antibiotics that maintain high plasma and high intracellular concentrations for months. It is likely that bartonellosis should be treated like brucellosis, which is caused by a closely related alpha Proteobacteria.
In the cases in this review, third generation cephalosporins, macrolides, and the tetracycline family were often used. Within each antibiotic class, individual antibiotics have different pharmacokinetic characteristics, with some antibiotics within a class eliciting therapeutic improvement in one Bartonella patient whereas another antibiotic within the class failing to elicit improvement in another patient. For example, a 6-year-old Hispanic boy died after treatment with a third generation cephalosporin [33], while other patients treated with cephalosporins, often along with other antibiotics such as netilmicin, recovered [4]. Obviously, recovery also depends upon the host immune response and potentially the virulence of the infecting Bartonella sp. or strain. However, based upon case data, caution should be used if a cephalosporin is the sole treatment for neurobartonellosis. Another patient had persistent symptoms despite 14 days of erythromycin and corticosteroids, whereas improvement was noted following administration of a combination of doxycycline and rifampin. Macrolides, such as azithromycin, have been used effectively for the treatment of neurobartonellosis patients, but close monitoring is important due to the potential for development of resistance.
In several patients described in this review, combinations of antibiotics were used to eliminate neurological abnormalities, however, for many patients the antibiotic combination was changed when the patient’s clinical status deteriorated. Biswas et al., 2010, have reported that resistance to azithromycin evolves more rapidly than drugs from floxacin family [6]. It has also been demonstrated that cat and human B. henselae isolates are more susceptible to pradofloxacin than azithromycin and enrofloxacin [7]. Similar in vitro studies are needed to characterize the resistance of doxycycline and rifampin antibiotics when used in combination and alone. There is a paucity of data regarding the appropriate length of treatment for patients with neurobartonellosis, and further study is needed to identify optimal treatments to effectively treat these infections.
Future perspective
It is now well known that B. quintana and B. henselae can cause vasoproliferative lesions such as bacillary angiomatosis or peliosis hepatis in immunosuppressed individuals and that B. henselae is the primary, if not the sole, cause of CSD. In immunocompetent patients, including those with neurological disease, angiomatosis is rarely reported. It seems likely that expression of those factors responsible for vasoproliferation and neoangiogenesis is immunologically blocked or suppressed by the immunocompetent host, at least at the gross anatomic level. The extent to which chronic intravascular or endotheliotropic infection contributes to immunemediated or autoimmune (molecular mimicry) neurological phenomena in neurobartonellosis patients has not been investigated to any degree. The mechanisms by which some B. henselae strains cause aphasia, encephalopathy, neuropathy, seizures or transverse myelitis should be studied. Based upon limited case data, Bartonella sp. can be isolated from the CSF of patients with minimal pathological evidence (i.e., increased protein and cell counts) to support inflammation or infection [10,14,15].
Finally, it is critical for the physician to consider each patient’s travel history, exposures to domestic animals and wildlife, exposure to arthropod or insect vectors, and potentially whether there is a history of blood product administration. For future cases, diagnosis can be enhanced by using a panel of serological assays in conjunction with enrichment culture/PCR from blood, serum, tissues or cerebrospinal fluid [15,47]. Efforts to standardize antibiotic dose and duration treatment regimens, based upon both in vitro antibiotic susceptibility testing and patient outcome assessments are critically needed to effectively manage patients with neurobartonellosis. There is also a critical need for research to elucidate the mechanism(s) by which chronic interplay between the host and bacteria ultimately leads to neurological manifestations. In addition, research is needed to determine whether antibiotic resistance or other factors contribute to treatment failures in some patients with neurobartonellosis [10].
Disclaimer
In conjunction with Dr. Sushama Sontakke and North Carolina State University, Dr. Breitschwerdt holds U.S. Patent No. 7,115,385; Media and Methods for cultivation of microorganisms, which was issued October 3, 2006. He is the chief scientific officer for Galaxy Diagnostics, a newly formed company that provides diagnostic testing for the detection of Bartonella species infection in animals and in human patient samples. Dr. Sontakke is not affiliated with Galaxy Diagnostics and Dr. Hopkins has no conflicts of interest relative to this review.
Acknowledgments
The authors thank Mrs. Tonya Lee for editorial assistance and Drs Ricardo Maggi, Patricia Mascarelli, and Nandhu Blakrishnan, andMs. Julie Bradley and Barbara Hegarty for generating the data in Tables 3, 4, 5, and 6 and the images provided in Figure 1.
References
- J. Adjemian, I. B. Weber, J. McQuiston, K. S. Griffith, P. S. MeadNicholson, et al., Zoonotic infections among employees from great smoky mountains and rocky mountain national parks, 2008-2009, Vector Borne Zoonotic Dis, 12 (2012), 922–931.
- B. Avidor, M. Graidy, G. Efrat, C. Leibowitz, G. ShapiraSchattner, et al., Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis, J Clin Microbiol,42 (2004), 3462–3468.
- Y. Bai, M. Kosoy, S. Recuenco, D. Alvarez, D. MoranTurmelle, et al., Bartonella spp. in Bats, Guatemala, Emerg Infect Dis, 17 (2011), 1269–1272.
- P. Baylor, A. Garoufi, T. Karpathios, J. Lutz, J. Mogelof, and Moseley, Transverse myelitis in 2 patients with Bartonella henselae infection (cat scratch disease), Clin Infect Dis, 45(2007), e42–e45.
- S. Bhengsri, H. C. Baggett, L. F. Peruski, C. Morway, Y. BaiL. Fisk, et al., Bartonella seroprevalence in rural Thailand, Southeast Asian J Trop Med Public Health, 42 (2011), 687–692.
- S. Biswas, R. G. Maggi, M. G. Papich, and E. B. Breitschwerdt, Molecular mechanisms of Bartonella henselae resistance to azithromycin, pradofloxacin and enrofloxacin, J AntimicrobChemother, 65 (2010), 581–582.
- S. Biswas, R. G. Maggi, M. G. Papich, D. Keil, and E. B. Bre-itschwerdt, Comparative activity of pradofloxacin, enrofloxacin, and azithromycin against Bartonella henselae isolates collected from cats and a human, J Clin Microbiol, 48 (2010), 617–618.
- S. Biswas and J. M. Rolain, Bartonella infection: treatment and drug resistance, Future Microbiol, 5 (2010), 1719–1731.
- H. J. Boulouis, C. C. Chang, J. B. Henn, R. W. Kasten, and B. B. Chomel, Factors associated with the rapid emergence of zoonotic Bartonella infections, Vet Res, 36 (2005), 383–410.
- E. B. Breitschwerdt, R. G. Maggi, M. B. Cadenas, and P. P. de Paiva Diniz, A groundhog, a novel Bartonella sequence, and my father’s death, Emerg Infect Dis, 15 (2009), 2080–2086.
- E. B. Breitschwerdt, R. G. Maggi, B. B. Chomel, and M. R. Lappin, Bartonellosis: an emerging infectious disease of zoonotic importance to animals and human beings, J Vet Emerg Crit Care(San Antonio), 20 (2010), 8–30.
- E. B. Breitschwerdt, R. G. Maggi, A. W. Duncan, W. L. Nicholson, B. C. Hegarty, and C. W. Woods, Bartonella species in blood of immunocompetent persons with animal and arthropod contact, Emerg Infect Dis, 13 (2007), 938–941.
- E. B. Breitschwerdt, R. G. Maggi, P. Farmer, and P. E. Mascarelli, Molecular evidence of perinatal transmission of Bartonella vinsonii subsp. berkhoffii and Bartonella henselae to a child, JClin Microbiol, 48 (2010), 2289–2293.
- E. B. Breitschwerdt, R. G. Maggi, P. M. Lantos, C. W. WoodsC. Hegarty, and J. M. Bradley, Bartonella vinsonii subsp. berkhoffii and Bartonella henselae bacteremia in a father and daughter with neurological disease, Parasit Vectors, 3 (2010), 29.
- E. B. Breitschwerdt, R. G. Maggi, W. L. Nicholson, N. A. Cherry, and C. W. Woods, Bartonella sp. bacteremia in patients with neurological and neurocognitive dysfunction, J Clin Microbiol,46 (2008), 2856–2861.
- E. B. Breitschwerdt, R. G. Maggi, B. Robert Mozayeni, B. C. Hegarty, J. M. Bradley, and P. E. Mascarelli, Pcr amplification of Bartonella koehlerae from human blood and enrichment blood cultures, Parasit Vectors, 3 (2010), 76.
- E. B. Breitschwerdt, R. G. Maggi, B. Sigmon, and W. L. Nicholson, Isolation of Bartonella quintana from a woman and a cat following putative bite transmission, J Clin Microbiol, 45(2007), 270–272.
- E. B. Breitschwerdt, R. G. Maggi, M. Varanat, K. E. Linder, and Weinberg, Isolation of Bartonella vinsonii subsp. berkhoffii genotype II from a boy with epithelioid hemangioendothelioma and a dog with hemangiopericytoma, J Clin Microbiol, 47(2009), 1957–1960.
- E. B. Breitschwerdt, P. E. Mascarelli, L. A. Schweickert, R. G. Maggi, B. C. Hegarty, J. M. Bradley, et al., Hallucinations, sensory neuropathy, and peripheral visual deficits in a young woman infected with Bartonella koehlerae, J Clin Microbiol, 49(2011), 3415–3417.
- C. Brenneis, C. Scherfler, K. Engelhardt, R. Helbok, G. Brossner, Beer, et al., Encephalitis lethargica following Bartonella henselae infection, J Neurol, 254 (2007), 546–547.
- V. V. Brinar and M. Habek, Rare infections mimicking MS, Clin Neurol Neurosurg, 112 (2010), 625–628.
- T. C. Chen, W. R. Lin, P. L. Lu, C. Y. Lin, and Y. H. Chen, Cat scratch disease from a domestic dog, J Formos Med Assoc, 106(2007), S65–S68.
- N. A. Cherry, R. G. Maggi, J. H. Rossmeisl, B. C. Hegarty, and B. Breitschwerdt, Ecological diversity of Bartonella species infection among dogs and their owner in Virginia, Vector BorneZoonotic Dis, 11 (2011), 1425–1432.
- B. B. Chomel, H. J. Boulouis, and E. B. Breitschwerdt, Cat scratch disease and other zoonotic Bartonella infections, J AmVet Med Assoc, 224 (2004), 1270–1279.
- B. B. Chomel, H. J. Boulouis, E. B. Breitschwerdt, R. W. Kasten, Vayssier-Taussat, R. J. Birtles, et al., Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors, Vet Res, 40 (2009), 29.
- B. B. Chomel and R. W. Kasten, Bartonellosis, an increasingly recognized zoonosis, J Appl Microbiol, 109 (2010), 743–750.
- V. Cotte,´ S. Bonnet, D. Le Rhun, E. Le Naour, A. Chauvin, H. J. Boulouis, et al., Transmission of Bartonella henselae by Ixodes ricinus, Emerg Infect Dis, 14 (2008), 1074–1080.
- R. Dale, S. Irani, F. Brilot, S. Pillai, R. Webster, D. Gill, et al., N-methyl-D-aspartate receptor antibodies in pediatric dyskinetic encephalitis lethargica, Ann Neurol, 66 (2009), 704–709.
- F. Dietrich, T. Schmidgen, R. G. Maggi, D. Richter, F. R. Matuschka, R. Vonthein, et al., Prevalence of Bartonella hense-lae and Borrelia burgdorferi sensu lato DNA in ixodes ricinus ticks in Europe, Appl Environ Microbiol, 76 (2010), 1395–1398.
- C. Dorbecker,¨ A. Sander, K. Oberle, and T. Schulin¨-Casonato, In vitro susceptibility of Bartonella species to 17 antimicrobial compounds: comparison of Etest and agar dilution, J AntimicrobChemother, 58 (2006), 784–788.
- A. W. Duncan, R. G. Maggi, and E. B. Breitschwerdt, Bartonella DNA in dog saliva, Emerg Infect Dis, 13 (2007), 1948–1950.
- P. Dyachenko, M. Ziv, R. Raz, B. Chazan, A. Lev, and Rozenman, Cat scratch disease encephalopathy in an immunocompetent patient, Eur J Intern Med, 16 (2005), 610–611.
- B. Fouch and S. Coventry, A case of fatal disseminated Bar-tonella henselae infection (cat-scratch disease) with encephalitis,Arch Pathol Lab Med, 131 (2007), 1591–1594.
- J. W. Fox, J. K. Studley, and D. M. Cohen, Recurrent expressive aphasia as a presentation of cat-scratch encephalopathy, Pedi-atrics, 119 (2007), e760–e763.
- L. Guptill, Bartonellosis, Vet Microbiol, 140 (2010), 347–359.
- K. Holden, J. T. Boothby, R. W. Kasten, and B. B. Chomel, Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus ticks from California, USA, Vector Borne Zoonotic Dis, 6 (2006), 99–102.
- P. O. Kaiser, T. Riess, F. O’Rourke, D. Linke, and V. A. Kempf, Bartonella spp.: throwing light on uncommon human infections,Int J Med Microbiol, 301 (2011), 7–15.
- C. Kalogeropoulos, I. Koumpoulis, A. Mentis, C. Pappa, Zafeiropoulos, and M. Aspiotis, Bartonella and intraocular inflammation: a series of cases and review of literature, ClinOphthalmol, 5 (2011), 817–829.
- P. Kelly, J. M. Rolain, R. Maggi, S. Sontakke, B. Keene, Hunter, et al., Bartonella quintana endocarditis in dogs, Emerg Infect Dis, 12 (2006), 1869–1872.
- D. L. Kordick, M. G. Papich, and E. B. Breitschwerdt, Efficacy of enrofloxacin or doxycycline for treatment of Bartonella henselae or Bartonella clarridgeiae infection in cats, Antimicrob AgentsChemother, 41 (1997), 2448–2455.
- M. Kosoy, C. Morway, K. W. Sheff, Y. Bai, J. ColbornChalcraft, et al., Bartonella tamiae sp. nov., a newly recognized pathogen isolated from three human patients from Thailand, JClin Microbiol, 46 (2008), 772–775.
- A. Laudisoit, J. Iverson, S. Neerinckx, J. C. Shako, J. M. Nsabimana, G. Kersh, et al., Human seroreactivity against Bartonella species in the Democratic Republic of Congo, AsianPac J Trop Med, 4 (2011), 320–322.
- J. W. Lin, C. M. Chen, and C. C. Chang, Unknown fever and back pain caused by Bartonella henselae in a veterinarian after a needle puncture: a case report and literature review, VectorBorne Zoonotic Dis, 11 (2011), 589–591.
- T. Lynch, J. Iverson, and M. Kosoy, Combining culture tech-niques for Bartonella: the best of both worlds, J Clin Microbiol,49 (2011), 1363–1368.
- R. Maggi, A. Duncan, and E. Breitschwerdt, Novel chemically modified liquid medium that will support the growth of seven Bartonella species, J Clin Microbiol, 43 (2005), 2651–2655.
- R. Maggi, M. Kosoy, M. Mintzer, and E. Breitschwerdt, Isolation of Candidatus Bartonella melophagi from human blood, EmergInfect Dis, 15 (2009), 66–68.
- R. G. Maggi, P. E. Mascarelli, E. L. Pultorak, B. C. Hegarty, M. Bradley, B. R. Mozayeni, et al., Bartonella spp. bacteremia in high-risk immunocompetent patients, Diagn Microbiol InfectDis, 71 (2011), 430–437.
- P. Mansueto, I. Pepe, E. Cillari, F. Arcoleo, A. Micalizzi, Bonura, et al., Prevalence of antibodies anti-Bartonella henselae in western Sicily: children, blood donors, and cats, JImmunoassay Immunochem, 33 (2012), 18–25.
- E. Mantadakis, A. M. Spanaki, A. Psaroulaki, D. Fitrolaki, Minadakis, E. Michaeloudi, et al., Encephalopathy com-plicated by Guillain-Barre syndrome and hydrocephalus and associated with acute Bartonella quintana infection, PediatrInfect Dis J, 26 (2007), 860–862.
- C. B. Marienfeld, D. B. Dicapua, G. K. Sze, and J. M. Goldstein, Expressive aphasia as a presentation of encephalitis with Bartonella henselae infection in an immunocompetent adult,Yale J Biol Med, 83 (2010), 67–71.
- P. E. Mascarelli, J. R. Iredell, R. G. Maggi, G. Weinberg, and E. B. Breitschwerdt, Bartonella species bacteremia in two patients with epithelioid hemangioendothelioma, J ClinMicrobiol, 49 (2011), 4006–4012.
- F. Massei, L. Gori, G. Taddeucci, P. Macchia, and G. Maggiore, Bartonella henselae infection associated with Guillain-Barre syndrome, Pediatr Infect Dis J, 25 (2006), 90–91.
- M. E. Mosbacher, S. Klotz, J. Klotz, and J. L. Pinnas, Bartonella henselae and the potential for arthropod vector-borne transmission, Vector Borne Zoonotic Dis, 11 (2011), 471–477.
- K. R. Munana,˜ S. M. Vitek, B. C. Hegarty, D. L. Kordick, and B. Breitschwerdt, Infection of fetal feline brain cells in culture with Bartonella henselae, Infect Immun, 69 (2001), 564–569.
- N. Nishio, T. Kubota, Y. Nakao, and H. Hidaka, Cat scratch disease with encephalopathy in a 9-year-old girl, Pediatr Int, 50(2008), 823–824.
- A. M. Oliveira, R. G. Maggi, C. W. Woods, and E. B. Breitschwerdt, Suspected needle stick transmission of Bartonella vinsonii subspecies berkhoffii to a veterinarian, J Vet Intern Med,24 (2010), 1229–1232.
- W. Probert, J. K. Louie, J. R. Tucker, R. Longoria, R. HogueMoler, et al., Meningitis due to a “Bartonella washoensis”-like human pathogen, J Clin Microbiol, 47 (2009), 2332–2335.
- D. Raoult, P. E. Fournier, F. Vandenesch, J. L. Mainardi, S. J. Eykyn, J. Nash, et al., Outcome and treatment of Bartonella endocarditis, Arch Intern Med, 163 (2003), 226–230.
- D. Raoult, F. Roblot, J. M. Rolain, J. M. Besnier, J. Loulergue, Bastides, et al., First isolation of Bartonella alsatica from a valve of a patient with endocarditis, J Clin Microbiol, 44 (2006),278–279.
- C. Reis, M. Cote, D. Le Rhun, B. Lecuelle, M. L. Levin, Vayssier-Taussat, et al., Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii, PLoS Negl TropDis, 5 (2011), e1186.
- T. Riess, F. Dietrich, K. V. Schmidt, P. O. Kaiser, H. Schwarz, Schafer,¨ et al., Analysis of a novel insect cell culture medium-based growth medium for Bartonella species, Appl EnvironMicrobiol, 74 (2008), 5224–5227.
- J. M. Rolain, P. Brouqui, J. E. Koehler, C. Maguina, M. J. Dolan, and D. Raoult, Recommendations for treatment of human infections caused by Bartonella species, Antimicrob AgentsChemother, 48 (2004), 1921–1933.
- J. M. Rolain, M. Franc, B. Davoust, and D. Raoult, Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France, Emerg Infect Dis, 9 (2003), 338–342.
- J. L. Schaller, G. A. Burkland, and P. J. Langhoff, Do bar-tonella infections cause agitation, panic disorder, and treatment-resistant depression?, MedGenMed, 9 (2007), 54.
- S. R. Telford 3rd and G. P. Wormser, Bartonella spp. transmis-sion by ticks not established, Emerg Infect Dis, 16 (2010), 379–384.
- Y. L. Tsai, C. C. Chang, S. T. Chuang, and B. B. Chomel, Bar-tonella species and their ectoparasites: selective host adaptation or strain selection between the vector and the mammalian host?,Comp Immunol Microbiol Infect Dis, 34 (2011), 299–314.
- T. R. VanderHeyden, S. L. Yong, E. B. Breitschwerdt, R. G. Maggi, A. R. Mihalik, J. P. Parada, et al., Granulomatous hepati-tis due to Bartonella henselae infection in an immunocompetent patient, BMC Infect Dis, 12 (2012), 17.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 21336
- [From(publication date):
December-2012 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 16612
- PDF downloads : 4724